Ceramide Synthase 5 Deficiency Aggravates Dextran Sodium Sulfate-Induced Colitis and Colon Carcinogenesis and Impairs T-Cell Activation
Abstract
1. Introduction
2. Results
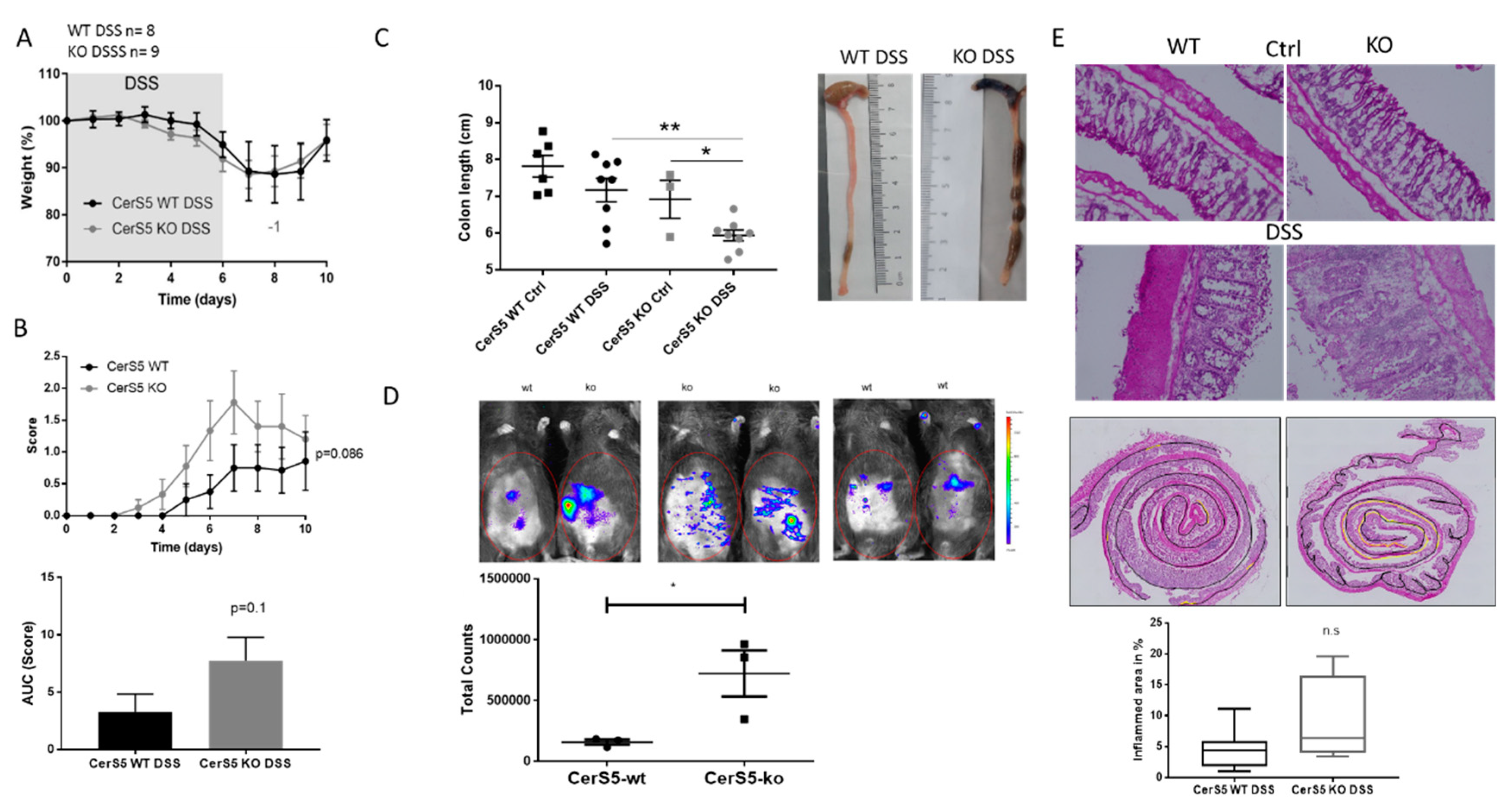
2.1. CerS5-Deficient Mice Were More Susceptible to DSS-Induced Acute Colitis
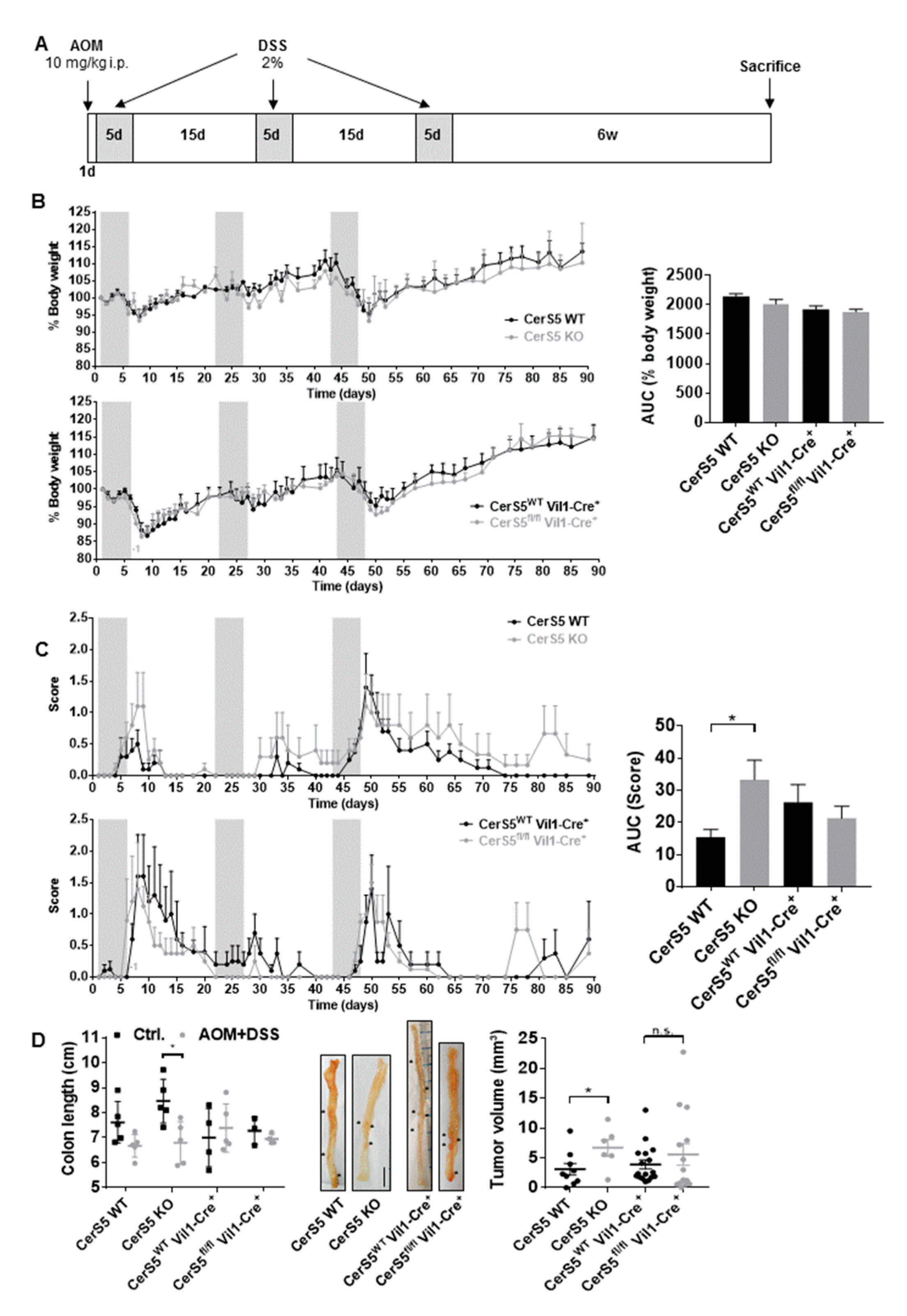
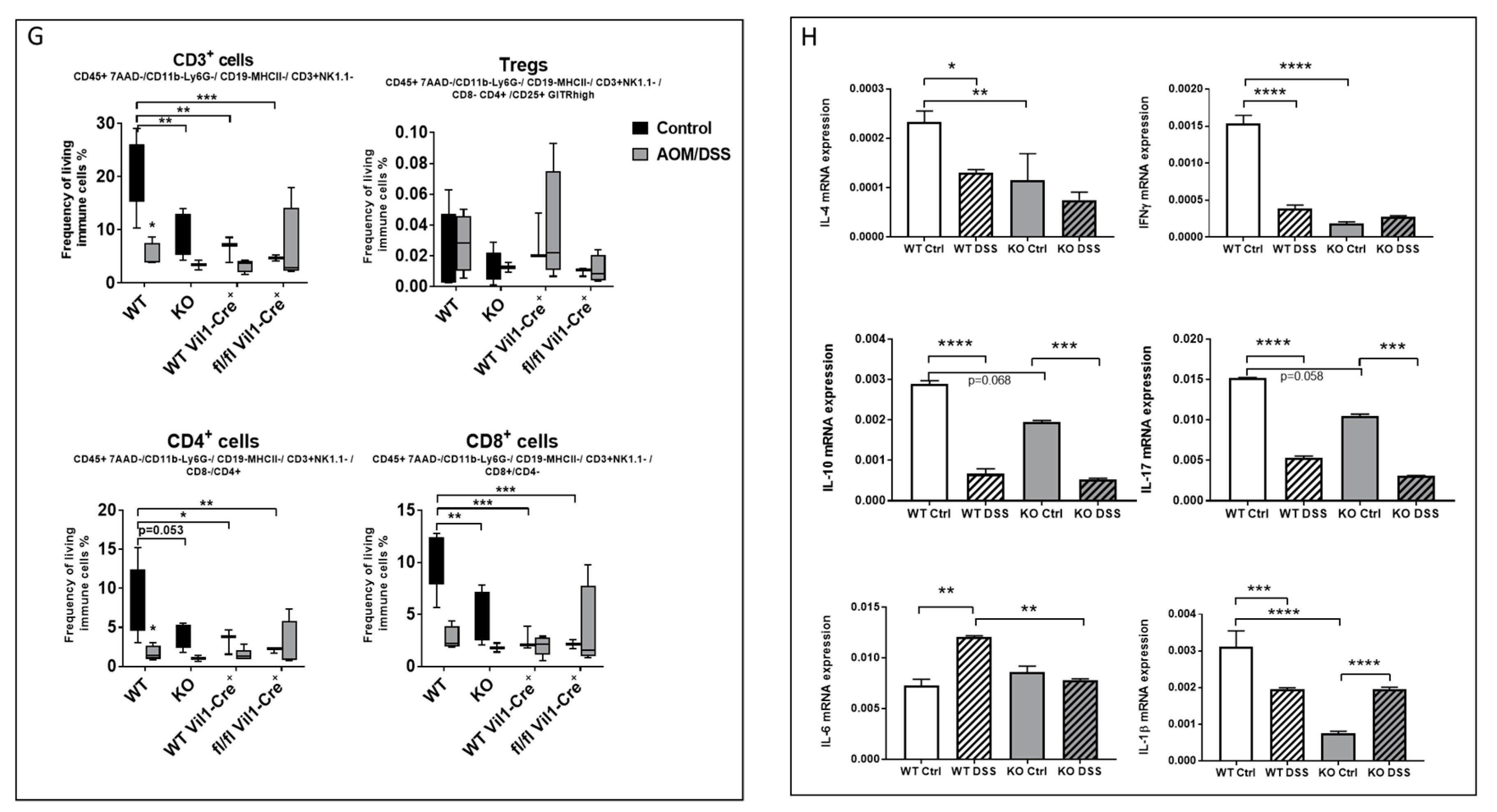
2.2. CerS5-ko Increased Tumor Development in the AOM/DSS CAC Model
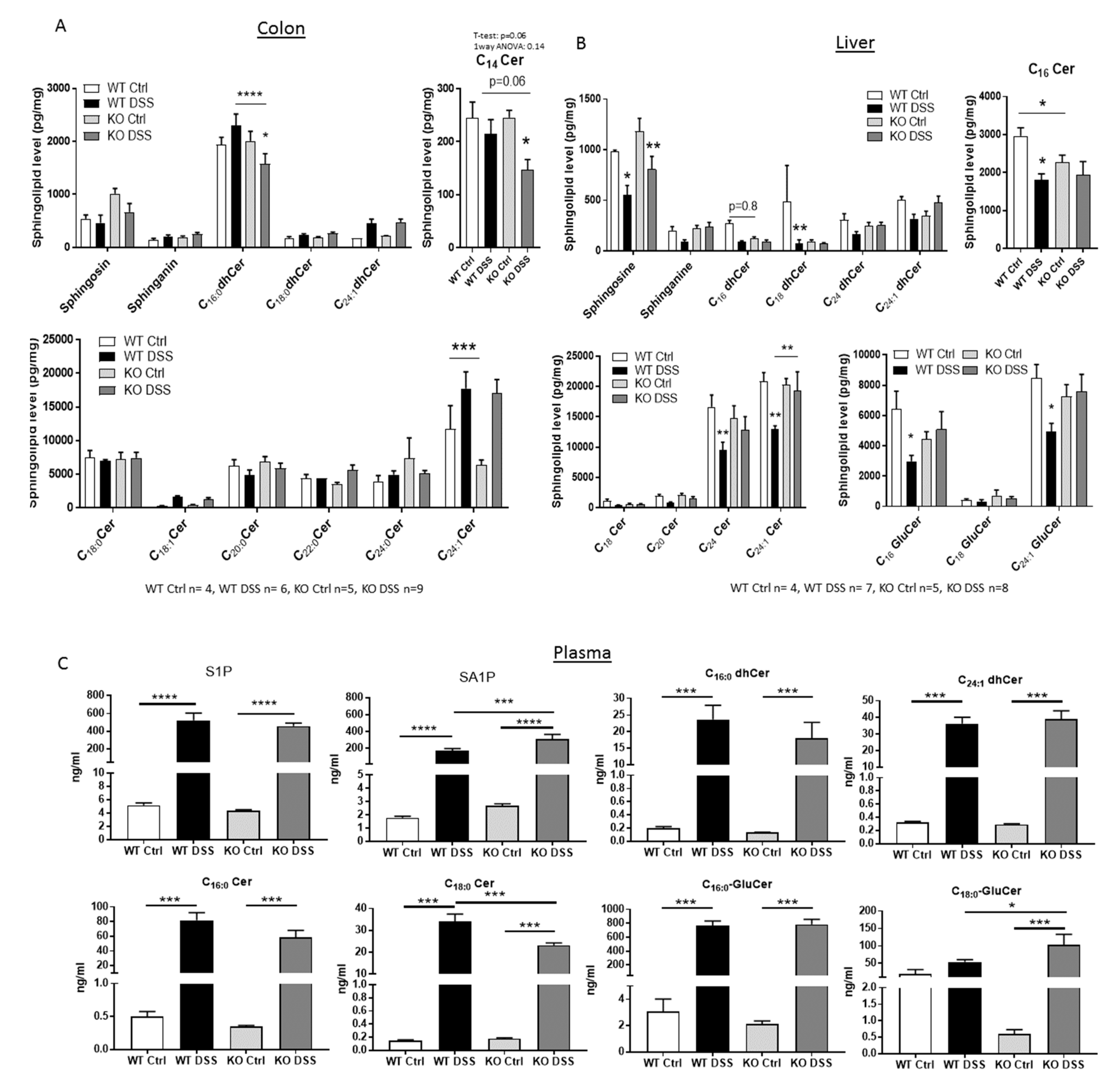
2.3. Sphingolipid Status in CerS5-ko Mice after DSS Treatment
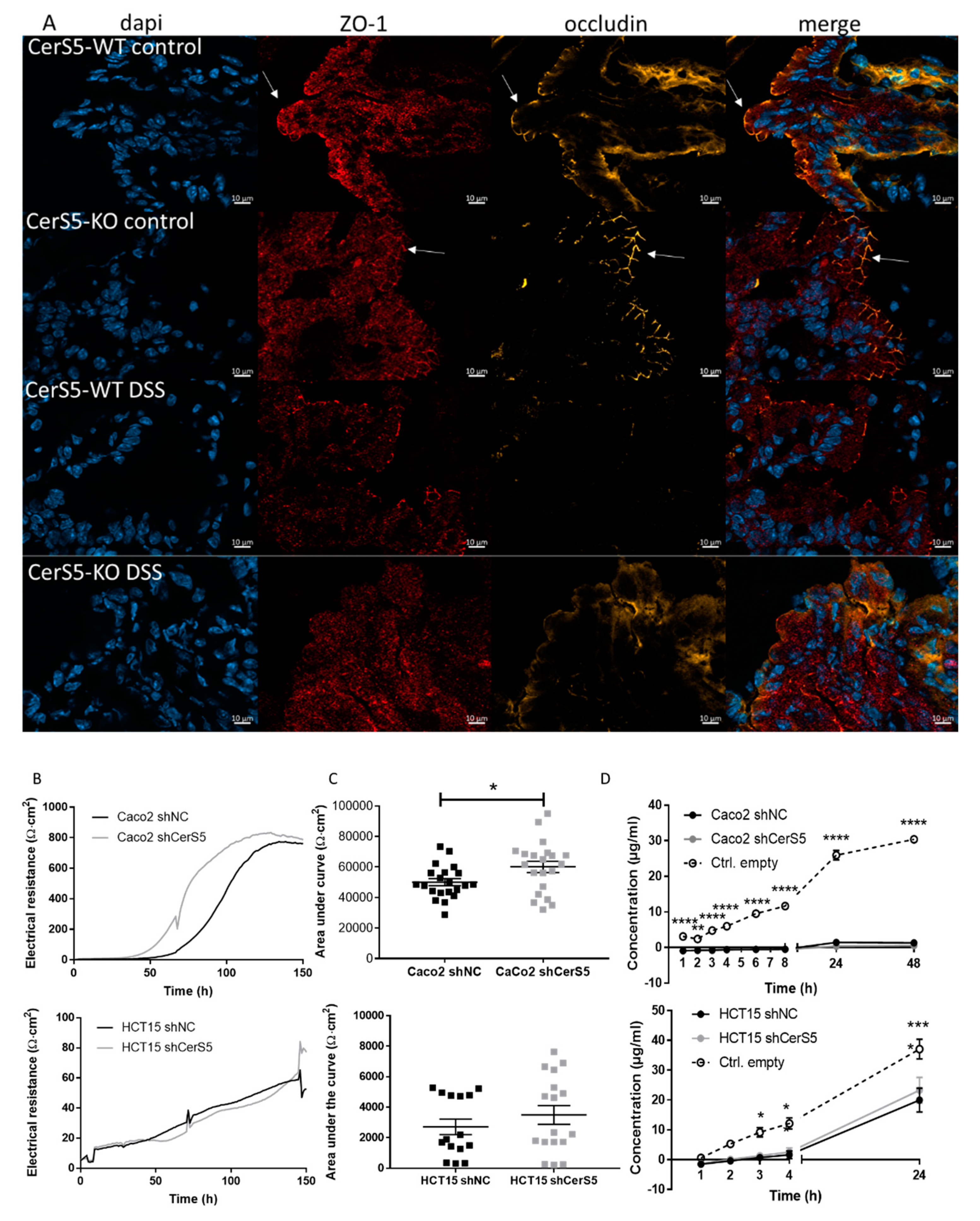
2.4. Increased Sensitivity of CerS5-ko Mice to DSS-Induced Colitis Was Not Based on Defects in Barrier Function
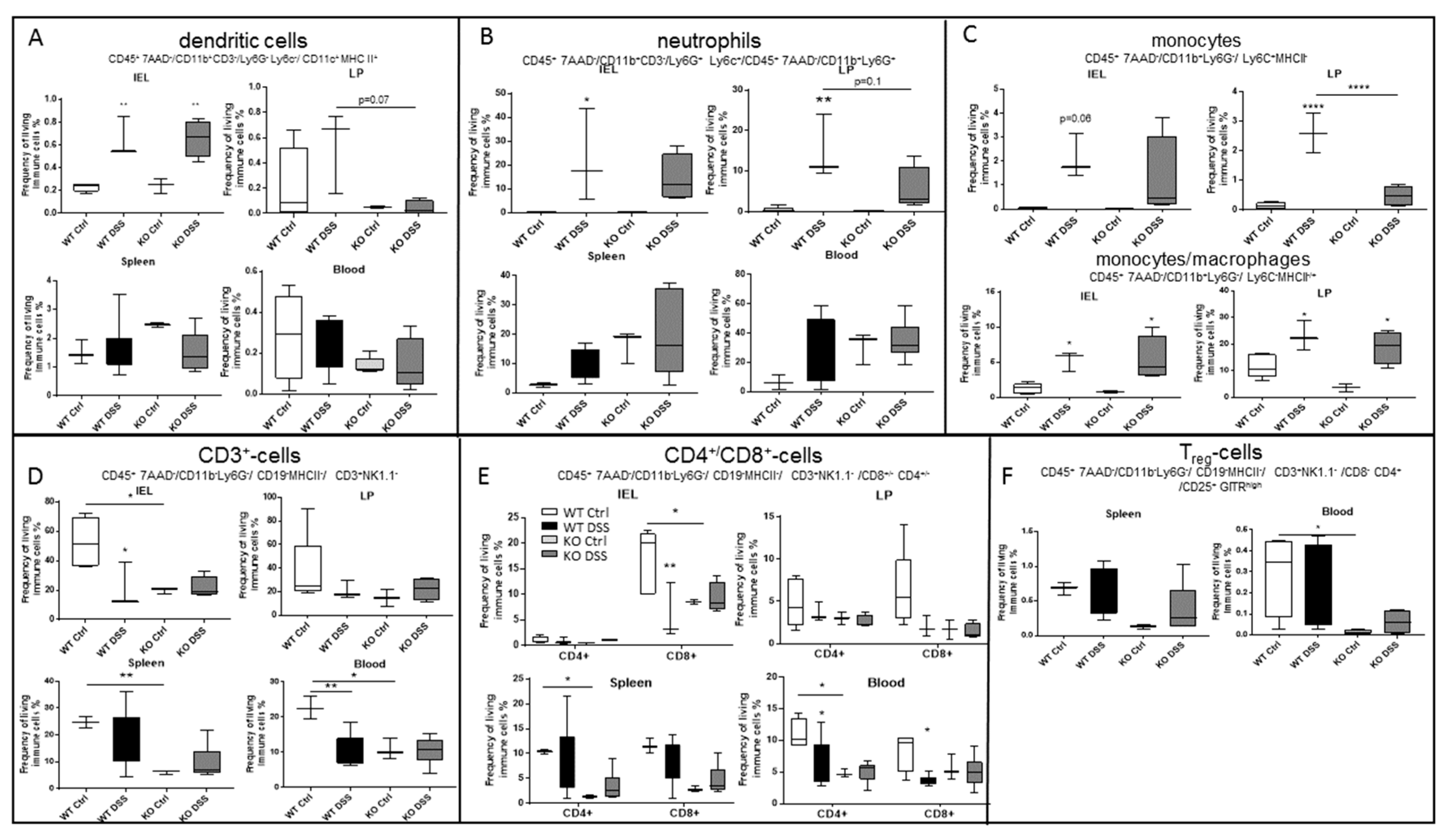
2.5. CerS5-ko Mice Differed in Their T-cell Status
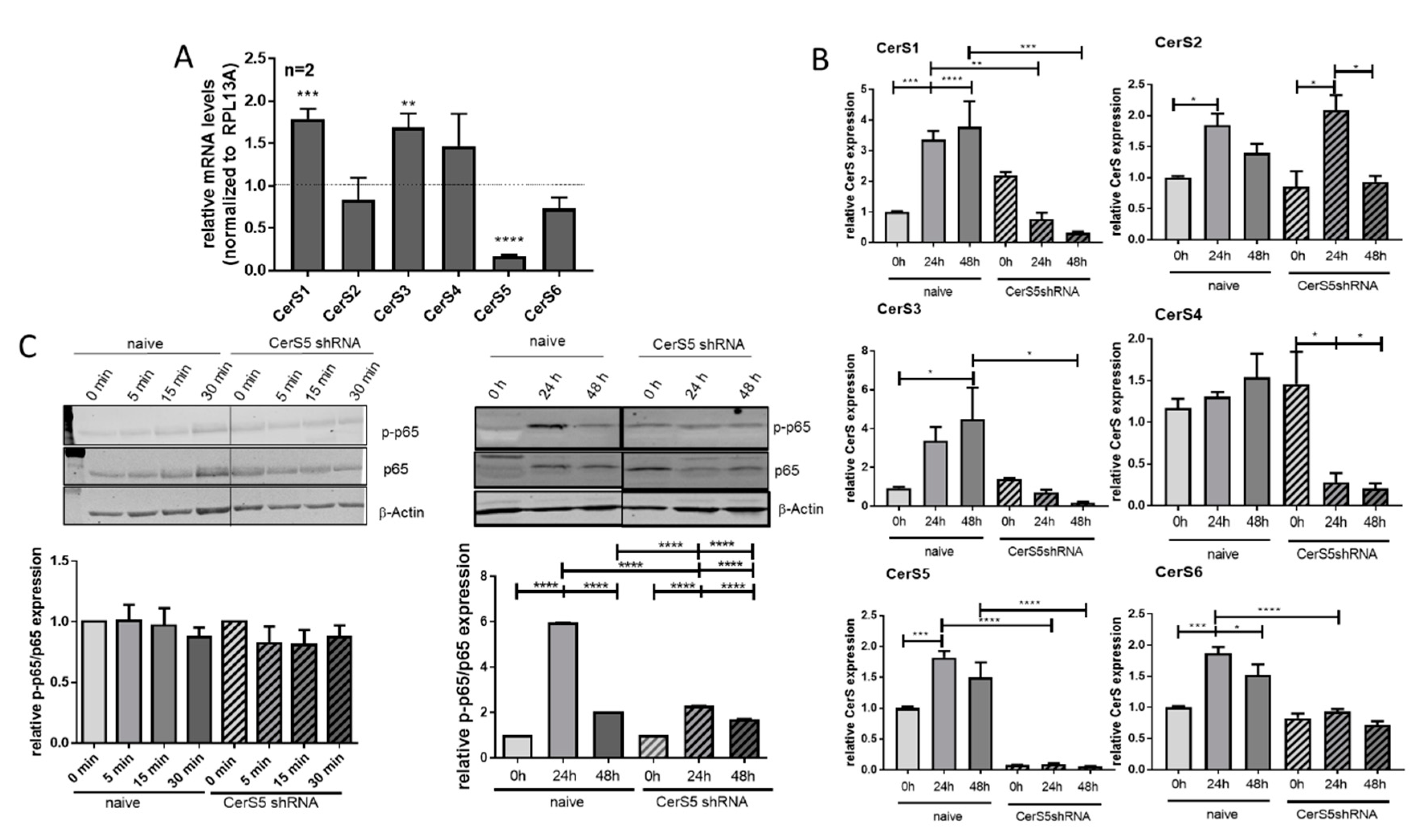
2.6. CerS5 Deficiency Impaired T-cell Signaling in Human T-cells
3. Discussion
4. Materials and Methods
4.1. Cell and Reagents
4.2. Generation of CerS5 Knockdown Cells
4.3. Animal Models
- CerS5FWD: 5′CAACATGATTCCAGTCTGTTCC3’
- CerS5Int4_6: 5′ GGCACGAAGAAAGTCTGGAG3’
- CerS5floxREV: 5′CTCACTATGTAACCATGCTG3’
4.4. In Vivo Imaging of Colon Inflammation
4.5. Tissue Preparation for Molecular and Histological Studies
4.6. Preparation for FACS
4.7. Eosin–Hematoxylin Staining
4.8. Immunohistochemistry
4.9. RNA Isolation and qRT-PCR
4.10. LC–MS/MS
4.11. TEER Measurements
4.12. Permeability Assay
4.13. Detection of Proteins by Western Blot
4.14. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Castaño-Milla, C.; Chaparro, M.; Gisbert, J.P. Systematic review with meta-analysis: The declining risk of colorectal cancer in ulcerative colitis. Aliment. Pharm. Ther. 2014, 39, 645–659. [Google Scholar] [CrossRef]
- Phatak, U.P.; Alper, A.; Pashankar, D.S. Complementary and alternative medicine use in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, Q.; Sun, L.; Ye, Y.; Ji, G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 2018, 57, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary bile acids and short chain fatty acids in the colon: A focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef] [PubMed]
- Tylichova, Z.; Slavík, J.; Ciganek, M.; Ovesná, P.; Krčmář, P.; Strakova, N.; Machala, M.; Kozubík, A.; Hofmanova, J.; Vondráček, J. Butyrate and docosahexaenoic acid interact in alterations of specific lipid classes in differentiating colon cancer cells. J. Cell. Biochem. 2018, 119, 4664–4679. [Google Scholar] [CrossRef] [PubMed]
- Bazarganipour, S.; Hausmann, J.; Oertel, S.; El-Hindi, K.; Brachtendorf, S.; Blumenstein, I.; Kubesch, A.; Sprinzl, K.; Birod, K.; Hahnefeld, L.; et al. The lipid status in patients with ulcerative colitis: Sphingolipids are disease-dependent regulated. J. Clin. Med. 2019, 8, 971. [Google Scholar] [CrossRef]
- Brachtendorf, S.; El-Hindi, K.; Grosch, S. Ceramide synthases in cancer therapy and chemoresistance. Prog. Lipid Res. 2019, 74, 160–185. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Helke, K.; Angel, P.; Lu, P.; Garrett-Mayer, E.; Ogretmen, B.; Drake, R.; Voelkel-Johnson, C. Ceramide synthase 6 deficiency enhances inflammation in the dss model of colitis. Sci. Rep. 2018, 8, 1627. [Google Scholar] [CrossRef]
- Scheffel, M.J.; Helke, K.; Lu, P.; Bowers, J.S.; Ogretmen, B.; Garrett-Mayer, E.; Paulos, C.M.; Voelkel-Johnson, C. Adoptive transfer of ceramide synthase 6 deficient splenocytes reduces the development of colitis. Sci. Rep. 2017, 7, 15552. [Google Scholar] [CrossRef]
- Oertel, S.; Scholich, K.; Weigert, A.; Thomas, D.; Schmetzer, J.; Trautmann, S.; Wegner, M.-S.; Radeke, H.H.; Filmann, N.; Brüne, B.; et al. Ceramide synthase 2 deficiency aggravates aom-dss-induced colitis in mice: Role of colon barrier integrity. Cell. Mol. Life Sci. 2017, 74, 3039–3055. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, P.; Ostkotte, D.; Nolte, H.; Gerl, M.J.; Jais, A.; Brunner, H.L.; Sprenger, H.-G.; Awazawa, M.; Nicholls, H.T.; Turpin-Nolan, S.M.; et al. CerS6-derived sphingolipids interact with mff and promote mitochondrial fragmentation in obesity. Cell 2019, 177, 1536–1552.e23. [Google Scholar] [CrossRef] [PubMed]
- Machala, M.; Procházková, J.; Hofmanová, J.; Králiková, L.; Slavík, J.; Tylichová, Z.; Ovesná, P.; Kozubík, A.; Vondráček, J. Colon cancer and perturbations of the sphingolipid metabolism. Int. J. Mol. Sci. 2019, 20, 6051. [Google Scholar] [CrossRef]
- Jang, S.W.; Park, W.J.; Min, H.; Kwon, T.K.; Baek, S.K.; Hwang, I.; Kim, S.; Park, J.W. Altered mrna expression levels of the major components of sphingolipid metabolism, ceramide synthases and their clinical implication in colorectal cancer. Oncol. Rep. 2018, 40, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Laviad, E.L.; Albee, L.; Pankova-Kholmyansky, I.; Epstein, S.; Park, H.; Merrill, A.H., Jr.; Futerman, A.H. Characterization of ceramide synthase 2: Tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 2008, 283, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, S.; Birod, K.; Männich, J.; Eberle, M.; Wegner, M.-S.; Wanger, R.; Hartmann, D.; Ferreirós, N.; Geisslinger, G.; Grösch, S. Ceramide metabolism in mouse tissue. Int. J. Biochem. Cell Biol. 2013, 45, 1886–1894. [Google Scholar] [CrossRef]
- Lutter, L.; Van Konijnenburg, D.P.H.; Brand, E.C.; Oldenburg, B.; van Wijk, F. The elusive case of human intraepithelial t cells in gut homeostasis and inflammation. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 637–649. [Google Scholar] [CrossRef]
- Hirata, I.; Berrebi, G.; Austin, L.L.; Keren, D.F.; Dobbins, W.O., 3rd. Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig. Dis. Sci. 1986, 31, 593–603. [Google Scholar] [CrossRef]
- Courtney, A.H.; Lo, W.-L.; Weiss, A. Tcr signaling: Mechanisms of initiation and propagation. Trends Biochem. Sci. 2018, 43, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Thaker, Y.R.; Schneider, H.; Rudd, C.E. TCR and CD28 activate the transcription factor NF- κB in T-cells via distinct adaptor signaling complexes. Immunol. Lett. 2015, 163, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.H.; Heinrichs, J.; Dany, M.; Nguyen, H.; Dai, M.; Bastian, D.; Schutt, S.; Wu, Y.; Daenthanasanmak, A.; Gencer, S.; et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight 2017, 2, e91701. [Google Scholar] [CrossRef] [PubMed]
- Carol, M.; Lambrechts, A.; Van Gossum, A.; Libin, M.; Goldman, M.; Mascart-Lemone, F. Spontaneous secretion of interferon gamma and interleukin 4 by human intraepithelial and lamina propria gut lymphocytes. Gut 1998, 42, 643–649. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Jutz, S.; Leitner, J.; Schmetterer, K.; Doel-Perez, I.; Majdic, O.; Grabmeier-Pfistershammer, K.; Paster, W.; Huppa, J.B.; Steinberger, P. Assessment of costimulation and coinhibition in a triple parameter T cell reporter line: Simultaneous measurement of NF-κB, NFAT and AP-1. J. Immunol. Methods 2016, 430, 10–20. [Google Scholar] [CrossRef]
- Zech, T.; Ejsing, C.S.; Gaus, K.; de Wet, B.; Shevchenko, A.; Simons, K.; Harder, T. Accumulation of raft lipids in t-cell plasma membrane domains engaged in tcr signalling. EMBO J. 2009, 28, 466–476. [Google Scholar] [CrossRef]
- Habtezion, A.; Nguyen, L.P.; Hadeiba, H.; Butcher, E.C. Leukocyte trafficking to the small intestine and colon. Gastroenterol. 2016, 150, 340–354. [Google Scholar] [CrossRef]
- Ge, Y.; Gao, J.; Jordan, R.; Naumann, C.A. Changes in cholesterol level alter integrin sequestration in raft-mimicking lipid mixtures. Biophys. J. 2018, 114, 158–167. [Google Scholar] [CrossRef]
- Badawy, S.M.M.; Okada, T.; Kajimoto, T.; Hirase, M.; Matovelo, S.A.; Nakamura, S.; Yoshida, D.; Ijuin, T.; Nakamura, S.I. Extracellular α-synuclein drives sphingosine 1-phosphate receptor subtype 1 out of lipid rafts, leading to impaired inhibitory G-protein signaling. J. Biol. Chem. 2018, 293, 8208–8216. [Google Scholar] [CrossRef]
- Karuppuchamy, T.; Behrens, E.H.; González-Cabrera, P.; Sarkisyan, G.; Gima, L.; Boyer, J.D.; Bamias, G.; Jedlicka, P.; Veny, M.; Clark, D.; et al. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol. 2017, 10, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Argollo, M.; Furfaro, F.; Gilardi, D.; Roda, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Modulation of sphingosine-1-phosphate in ulcerative colitis. Expert Opin. Biol. Ther. 2020, 20, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.E.; Cruickshank, S.M.; Egan, C.E.; Mears, R.; Newton, D.J.; Andrew, E.M.; Lawrence, B.; Howell, G.; Else, K.J.; Gubbels, M.J.; et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterol. 2006, 131, 818–829. [Google Scholar] [CrossRef]
- Brachtendorf, S.; Wanger, R.A.; Birod, K.; Thomas, D.; Trautmann, S.; Wegner, M.S.; Fuhrmann, D.C.; Brüne, B.; Geisslinger, G.; Grosch, S. Chemosensitivity of human colon cancer cells is influenced by a p53-dependent enhancement of ceramide synthase 5 and induction of autophagy. Biochim. Biophys. Acta Mol. Cell Boil. Lipids 2018, 1863, 1214–1227. [Google Scholar] [CrossRef] [PubMed]
- Tosetti, B.; Brodesser, S.; Brunn, A.; Deckert, M.; Blüher, M.; Doehner, W.; Anker, S.D.; Wenzel, D.; Fleischmann, B.; Pongratz, C.; et al. A tissue-specific screen of ceramide expression in aged mice identifies ceramide synthase-1 and ceramide synthase-5 as potential regulators of fiber size and strength in skeletal muscle. Aging Cell 2020, 19, e13049. [Google Scholar] [CrossRef] [PubMed]
- Turpin-Nolan, S.M.; Hammerschmidt, P.; Chen, W.; Jais, A.; Timper, K.; Awazawa, M.; Brodesser, S.; Brüning, J.C. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 2019, 26, 1–10.e7. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Primer | Forward 5′–3′ | Reverse 5′–3′ |
|---|---|---|
| RLP37A | ATT GAA ATCA GCC AGC ACG C | AGG AAC CAC AGT GCC AGA TCC |
| RLP13A (Jurkat) | CTCAAGGTGTTTGACGGCATCC | TACTTCCAGCCAACCTCGTGAG |
| hLass1 | CCT CCA GCC CAG AGA T | AGA AGG GGT AGT CGG TG |
| hLass2 | CCA GGT AGA GCG TTG GTT | CCA GGG TTT ATC CAC AAT GAC |
| hLass3 | CCT GGC TGCTAT TAG TCT GAT | TCA CGA GGG TCC CAC T |
| hLass4 | CTG GTG GTA CCT CTT GGA GC | CGT CGC ACA CTT GCT GAT AC |
| hLass5 | CAA GTA TCA GCG GCT CTG T | ATT ATC TCC CAA CTC TCA AAG A |
| hLass6 | AAG CAA CTG GAC TGG GAT GTT | AAT CTG ACT CCG TAG GTA AAT ACA |
| mIL-4 | GTC ATC CTG CTC TTC TTT CTC G | CTG TGG TGT TCT TCG TTG CTG |
| mIL-1ß | TCC AGG ATG AGG ACA TGA G | GAG CCT GTA GTG CAG TTG |
| mIL-6 | TAG TCC TTC CTA CCC CAA TTT CC | TTG GTC CTT AGC CAC TCC TTC |
| mIL-10 | TGC CAA GCC TTA TCG GAA ATG | ACT CTT CAC CTG CTC CAC TGCC |
| mIL-17 | TCT GTG TCT CTG ATG CTG TTG CTG | CAG GGT CTT CAT TGC GGT GG |
| mINF-y | CAC GGC ACA GTC ATT GAA AGC | CAC CAT CCT TTT GCC AGT TCC |
| mGAPDH (MP205604, ORIGENE) | CAT CAC TGC CAC CCA GAA GAC TG | ATG CCA GTG AGC TTC CCG TTC AG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hindi, K.; Brachtendorf, S.; Hartel, J.C.; Oertel, S.; Birod, K.; Trautmann, S.; Thomas, D.; Ulshöfer, T.; Weigert, A.; Utermöhlen, O.; et al. Ceramide Synthase 5 Deficiency Aggravates Dextran Sodium Sulfate-Induced Colitis and Colon Carcinogenesis and Impairs T-Cell Activation. Cancers 2020, 12, 1753. https://doi.org/10.3390/cancers12071753
El-Hindi K, Brachtendorf S, Hartel JC, Oertel S, Birod K, Trautmann S, Thomas D, Ulshöfer T, Weigert A, Utermöhlen O, et al. Ceramide Synthase 5 Deficiency Aggravates Dextran Sodium Sulfate-Induced Colitis and Colon Carcinogenesis and Impairs T-Cell Activation. Cancers. 2020; 12(7):1753. https://doi.org/10.3390/cancers12071753
Chicago/Turabian StyleEl-Hindi, Khadija, Sebastian Brachtendorf, Jennifer Christina Hartel, Stephanie Oertel, Kerstin Birod, Sandra Trautmann, Dominique Thomas, Thomas Ulshöfer, Andreas Weigert, Olaf Utermöhlen, and et al. 2020. "Ceramide Synthase 5 Deficiency Aggravates Dextran Sodium Sulfate-Induced Colitis and Colon Carcinogenesis and Impairs T-Cell Activation" Cancers 12, no. 7: 1753. https://doi.org/10.3390/cancers12071753
APA StyleEl-Hindi, K., Brachtendorf, S., Hartel, J. C., Oertel, S., Birod, K., Trautmann, S., Thomas, D., Ulshöfer, T., Weigert, A., Utermöhlen, O., Krönke, M., & Grösch, S. (2020). Ceramide Synthase 5 Deficiency Aggravates Dextran Sodium Sulfate-Induced Colitis and Colon Carcinogenesis and Impairs T-Cell Activation. Cancers, 12(7), 1753. https://doi.org/10.3390/cancers12071753







