Protective Effects of Dietary Grape on UVB-Mediated Cutaneous Damages and Skin Tumorigenesis in SKH-1 Mice
Abstract
1. Introduction
2. Results and Discussion
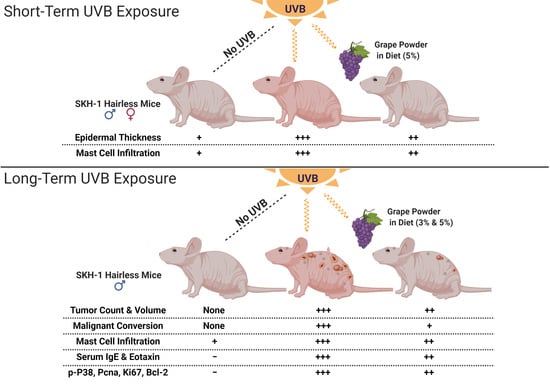
2.1. Dietary GP Consumption Decreases Epidermal Thickening and Mast Cell Counts in SKH-1 Hairless Mouse Skin in a Short-Term UVB Exposure Protocol
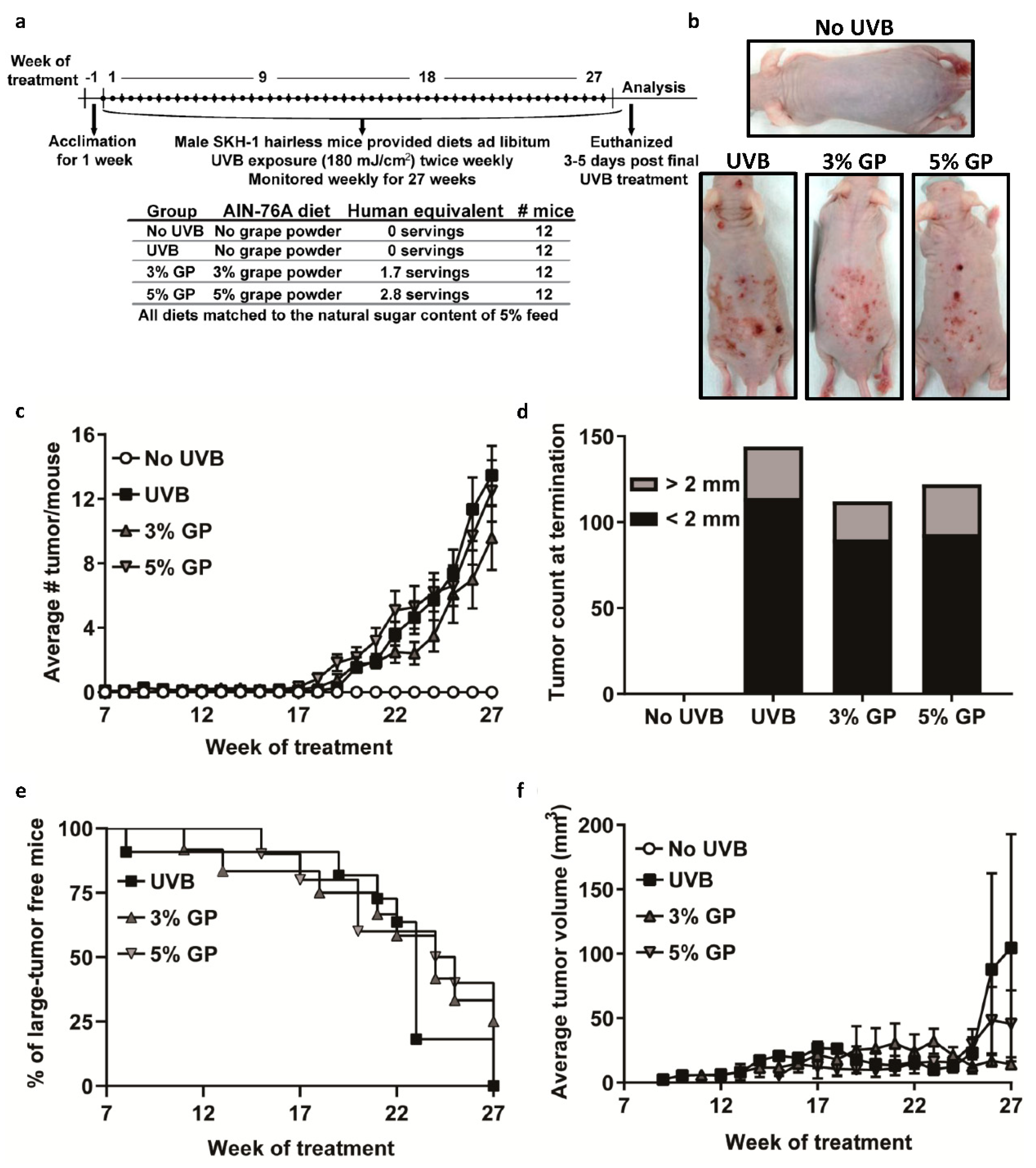
2.2. Dietary GP Consumption Reduced Chronic UVB Exposure-Mediated Skin Tumorigenesis in Male SKH-1 Hairless Mice
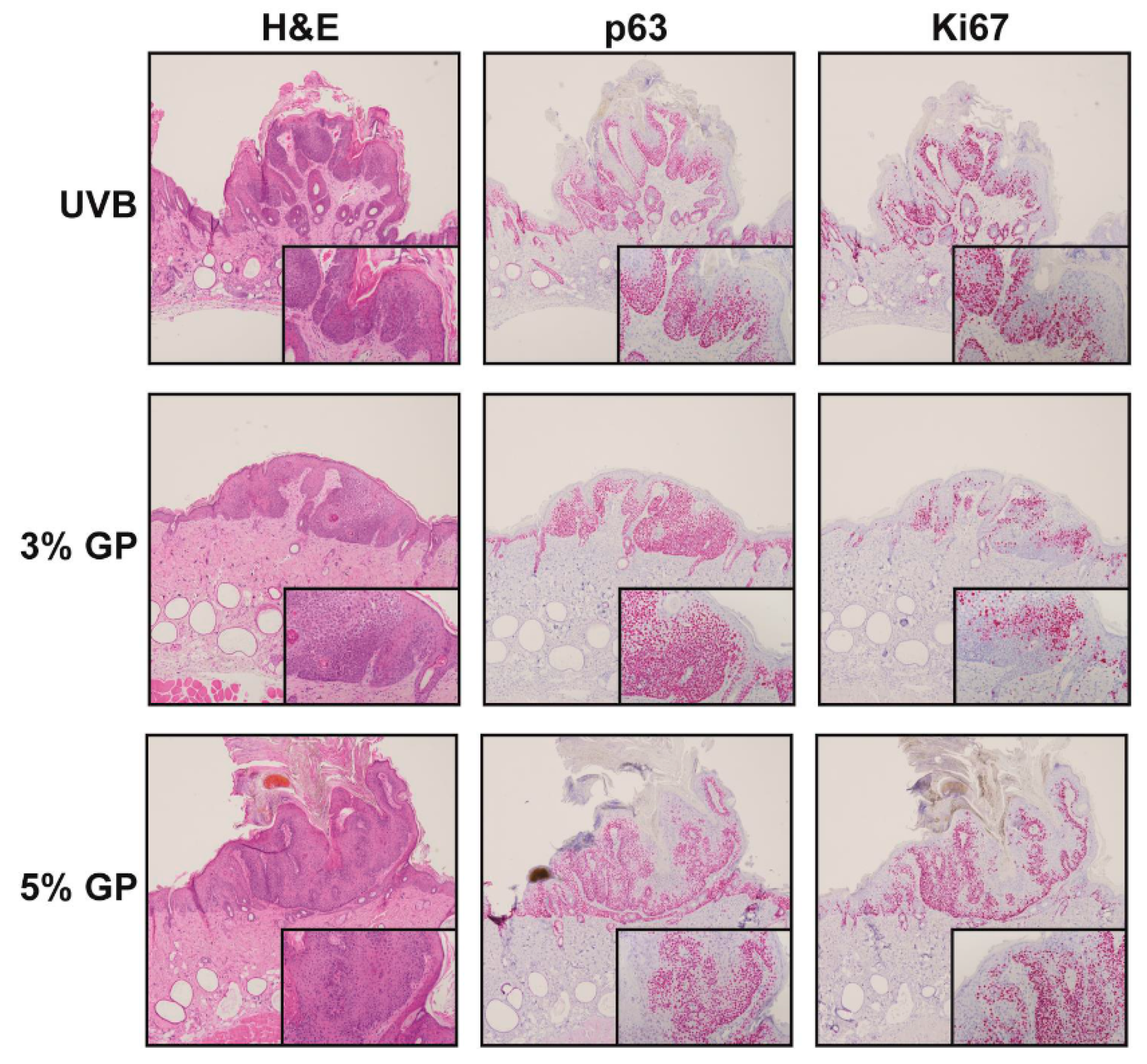
2.3. Dietary GP Consumption Reduces the Malignant Conversion of UVB-Mediated Tumors in SKH-1 Hairless Mice
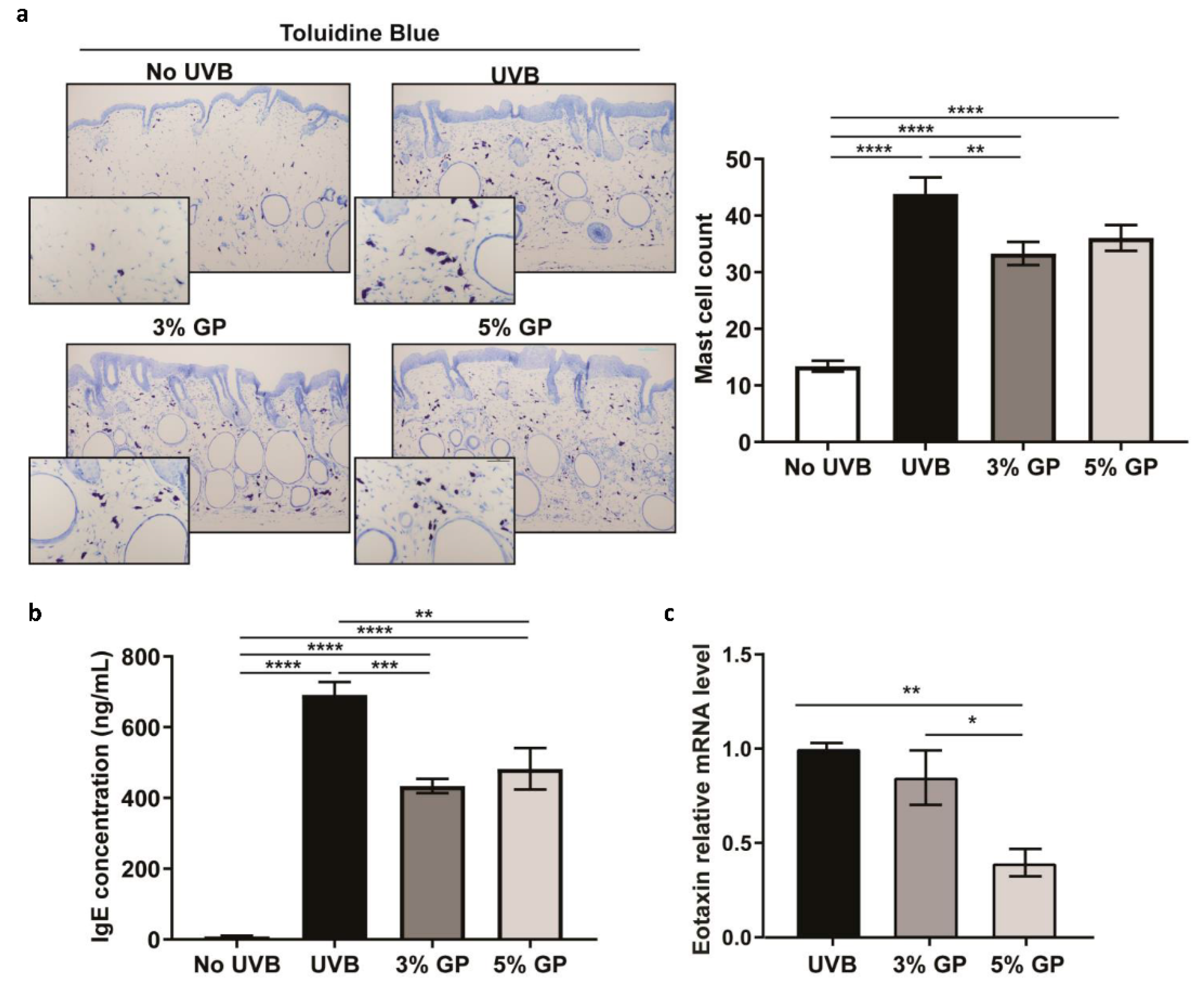
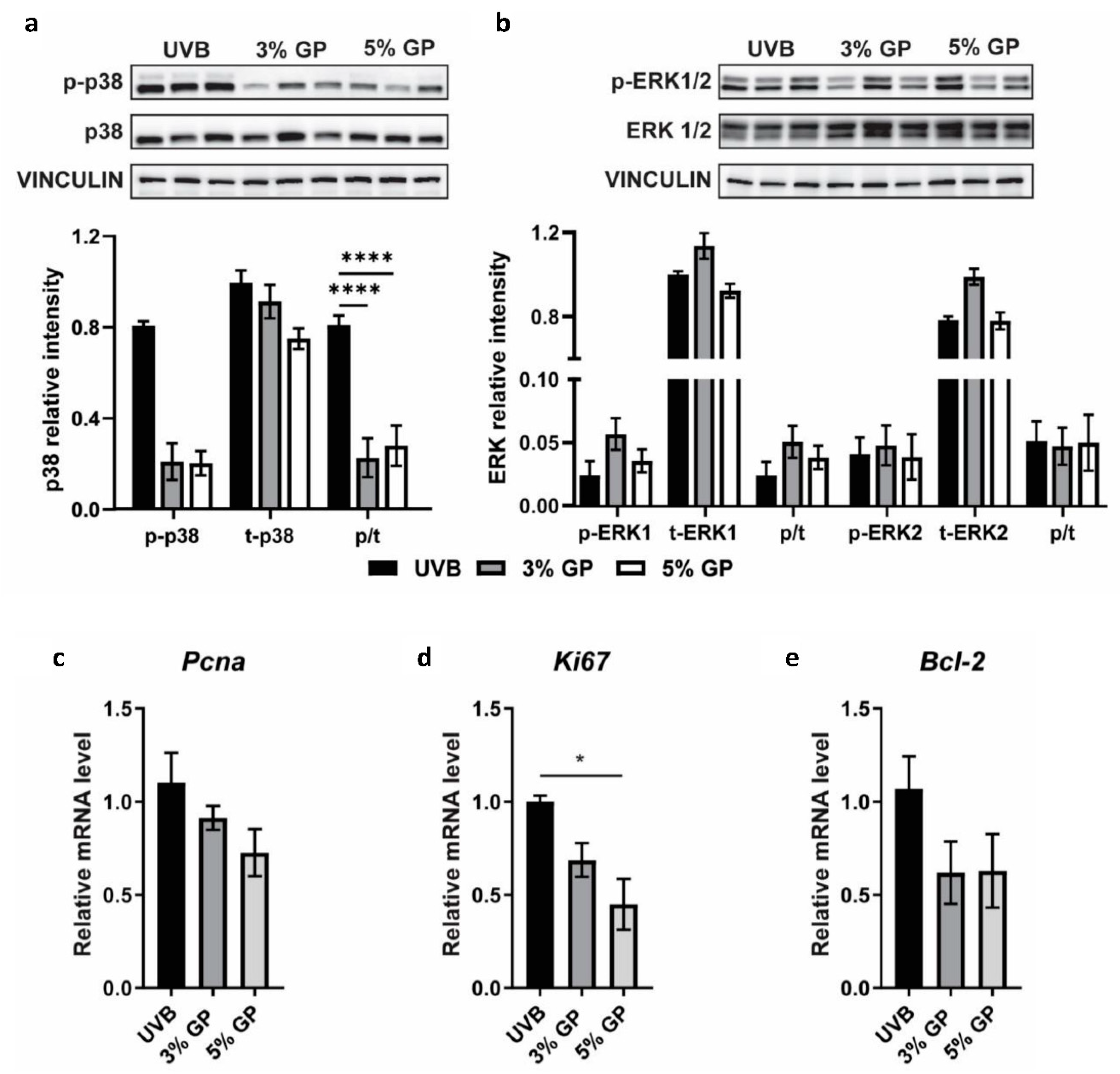
2.4. Dietary GP Consumption Imparts Anti-Inflammatory Effects in Long-Term UVB-Treated SKH-1 Hairless Mice
3. Materials and Methods
3.1. Materials and Animal Care
3.2. UVB-Mediated Cutaneous Damage
3.3. Histology and Epidermal Thickness
3.4. Toluidine Blue Staining
3.5. UVB-Mediated Carcinogenesis
3.6. Lesion Scoring
3.7. Immunohistochemistry (IHC)
3.8. Protein and RNA Isolation
3.9. RT-qPCR Analysis
3.10. IgE ELISA
3.11. Cytokine Array
3.12. Immunoblot Analysis
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Chren, M.M.; Torres, J.S.; Stuart, S.E.; Bertenthal, D.; Labrador, R.J.; Boscardin, W.J. Recurrence after treatment of nonmelanoma skin cancer: A prospective cohort study. Arch. Dermatol. 2011, 147, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef]
- Foote, J.A.; Harris, R.B.; Giuliano, A.R.; Roe, D.J.; Moon, T.E.; Cartmel, B.; Alberts, D.S. Predictors for cutaneous basal- and squamous-cell carcinoma among actinically damaged adults. Int. J. Cancer 2001, 95, 7–11. [Google Scholar] [CrossRef]
- Guy, G.P., Jr.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB light stimulates production of reactive oxygen species: Unexpected role for catalase. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol. Appl. Pharmacol. 2003, 186, 28–37. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Taguchi, M.; Watanabe, S.; Yashima, K.; Murakami, Y.; Sekiya, T.; Ikedat, S. Aberrations of the tumor suppressor p53 gene and p53 protein in solar keratosis in human skin. J. Investig. Dermatol. 1994, 103, 500–503. [Google Scholar] [CrossRef]
- Melnikova, V.O.; Pacifico, A.; Chimenti, S.; Peris, K.; Ananthaswamy, H.N. Fate of UVB-induced p53 mutations in SKH-hr1 mouse skin after discontinuation of irradiation: Relationship to skin cancer development. Oncogene 2005, 24, 7055–7063. [Google Scholar] [CrossRef]
- Wan, Y.S.; Wang, Z.Q.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation activates PI 3-kinase/AKT survival pathway via EGF receptors in human skin in vivo. Int. J. Oncol. 2001, 18, 461–466. [Google Scholar] [CrossRef]
- Chen, W.; Tang, Q.; Gonzales, M.S.; Bowden, G.T. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene 2001, 20, 3921. [Google Scholar] [CrossRef] [PubMed]
- Kabuyama, Y.; Hamaya, M.; Homma, Y. Wavelength specific activation of PI 3-kinase by UVB irradiation. FEBS Lett. 1998, 441, 297–301. [Google Scholar] [CrossRef]
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Jurgensen, J.S.; Degen, A.; Hackethal, M.; Ulrich, M.; Patel, M.J.; Eberle, J.; Terhorst, D.; Sterry, W.; Stockfleth, E. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: A 24 months, prospective, case-control study. Br. J. Dermatol. 2009, 161 (Suppl. 3), 78–84. [Google Scholar] [CrossRef]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2013, 1290, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta 2015, 1852, 1178–1185. [Google Scholar] [CrossRef]
- Singh, C.K.; Liu, X.; Ahmad, N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann. N. Y. Acad. Sci. 2015, 1348, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Siddiqui, I.A.; El-Abd, S.; Mukhtar, H.; Ahmad, N. Combination chemoprevention with grape antioxidants. Mol. Nutr. Food Res. 2016, 60, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Mintie, C.A.; Singh, C.K.; Ahmad, N. Whole fruit phytochemicals combating skin damage and carcinogenesis. Transl. Oncol. 2020, 13, 146–156. [Google Scholar] [CrossRef]
- Singh, C.K.; Mintie, C.A.; Ndiaye, M.A.; Chhabra, G.; Dakup, P.P.; Ye, T.; Yu, M.; Ahmad, N. Chemoprotective effects of dietary grape powder on UVB radiation-mediated skin carcinogenesis in SKH-1 hairless mice. J. Investig. Dermatol. 2019, 139, 552–561. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Benjamin, C.L.; Ullrich, S.E.; Kripke, M.L.; Ananthaswamy, H.N. p53 tumor suppressor gene: A critical molecular target for UV induction and prevention of skin cancer. Photochem. Photobiol. 2008, 84, 55–62. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Siiskonen, H.; Smorodchenko, A.; Krause, K.; Maurer, M. Ultraviolet radiation and skin mast cells: Effects, mechanisms and relevance for skin diseases. Exp. Dermatol. 2018, 27, 3–8. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Granata, F.; Borriello, F.; Marone, G. Controversial role of mast cells in skin cancers. Exp. Dermatol. 2017, 26, 11–17. [Google Scholar] [CrossRef]
- Che, D.N.; Xie, G.H.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Protective effects of grape stem extract against UVB-induced damage in C57BL mice skin. J. Photochem. Photobiol. B 2017, 173, 551–559. [Google Scholar] [CrossRef]
- Thomas-Ahner, J.M.; Wulff, B.C.; Tober, K.L.; Kusewitt, D.F.; Riggenbach, J.A.; Oberyszyn, T.M. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res. 2007, 67, 3468–3474. [Google Scholar] [CrossRef] [PubMed]
- Benavides, F.; Oberyszyn, T.M.; VanBuskirk, A.M.; Reeve, V.E.; Kusewitt, D.F. The hairless mouse in skin research. J. Dermatol. Sci. 2009, 53, 10–18. [Google Scholar] [CrossRef]
- Missero, C.; Antonini, D. p63 in squamous cell carcinoma of the skin: More than a stem cell/progenitor marker. J. Investig. Dermatol. 2017, 137, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Keyes, W.M.; Pecoraro, M.; Aranda, V.; Vernersson-Lindahl, E.; Li, W.; Vogel, H.; Guo, X.; Garcia, E.L.; Michurina, T.V.; Enikolopov, G.; et al. ΔNp63α is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell 2011, 8, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Mintie, C.A.; Ahmad, N. Grape chemopreventive agents against angiogenesis and metastasis. In Natural Products for Cancer Chemoprevention: Single Compounds and Combinations; Pezzuto, J.M., Vang, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 375–400. [Google Scholar] [CrossRef]
- Mintie, C.A.; Singh, C.K.; Ndiaye, M.A.; Barrett-Wilt, G.A.; Ahmad, N. Identification of molecular targets of dietary grape-mediated chemoprevention of ultraviolet B skin carcinogenesis: A comparative quantitative proteomics analysis. J. Proteome Res. 2019, 18, 3741–3751. [Google Scholar] [CrossRef]
- Sarchio, S.N.E.; Kok, L.-F.; O’Sullivan, C.; Halliday, G.M.; Byrne, S.N. Dermal mast cells affect the development of sunlight-induced skin tumours. Exp. Dermatol. 2012, 21, 241–248. [Google Scholar] [CrossRef]
- Falcone, F.H.; Haas, H.; Gibbs, B.F. The human basophil: A new appreciation of its role in immune responses. Blood 2000, 96, 4028–4038. [Google Scholar] [CrossRef]
- Lupu, M.; Caruntu, A.; Caruntu, C.; Papagheorghe, L.M.L.; Ilie, M.A.; Voiculescu, V.; Boda, D.; Constantin, C.; Tanase, C.; Sifaki, M.; et al. Neuroendocrine factors: The missing link in nonmelanoma skin cancer (Review). Oncol. Rep. 2017, 38, 1327–1340. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Wiemels, J.L.; Wiencke, J.K.; Li, Z.; Ramos, C.; Nelson, H.H.; Karagas, M.R. Risk of squamous cell carcinoma of the skin in relation to IgE: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Bae, J.Y.; Park, S.H.; Kim, Y.H.; Park, J.H.; Kang, Y.H. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J. Nutr. 2013, 143, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, K.; Amornphimoltham, P.; Molinolo, A.A.; Basile, J.R.; Koontongkaew, S.; Gutkind, J.S. A role for p38 MAPK in head and neck cancer cell growth and tumor-induced angiogenesis and lymphangiogenesis. Mol. Oncol. 2014, 8, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.Y.; Michaud, M.; Tsai, K.Y.; Oh, J.; Sundberg, J.P. Differential hairless mouse strain-specific susceptibility to skin cancer and sunburn. J. Investig. Dermatol. 2019, 139, 1837–1840.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seed, B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003, 31, e154. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mintie, C.A.; Musarra, A.K.; Singh, C.K.; Ndiaye, M.A.; Sullivan, R.; Eickhoff, J.C.; Ahmad, N. Protective Effects of Dietary Grape on UVB-Mediated Cutaneous Damages and Skin Tumorigenesis in SKH-1 Mice. Cancers 2020, 12, 1751. https://doi.org/10.3390/cancers12071751
Mintie CA, Musarra AK, Singh CK, Ndiaye MA, Sullivan R, Eickhoff JC, Ahmad N. Protective Effects of Dietary Grape on UVB-Mediated Cutaneous Damages and Skin Tumorigenesis in SKH-1 Mice. Cancers. 2020; 12(7):1751. https://doi.org/10.3390/cancers12071751
Chicago/Turabian StyleMintie, Charlotte A., Anna K. Musarra, Chandra K. Singh, Mary A. Ndiaye, Ruth Sullivan, Jens C. Eickhoff, and Nihal Ahmad. 2020. "Protective Effects of Dietary Grape on UVB-Mediated Cutaneous Damages and Skin Tumorigenesis in SKH-1 Mice" Cancers 12, no. 7: 1751. https://doi.org/10.3390/cancers12071751
APA StyleMintie, C. A., Musarra, A. K., Singh, C. K., Ndiaye, M. A., Sullivan, R., Eickhoff, J. C., & Ahmad, N. (2020). Protective Effects of Dietary Grape on UVB-Mediated Cutaneous Damages and Skin Tumorigenesis in SKH-1 Mice. Cancers, 12(7), 1751. https://doi.org/10.3390/cancers12071751