Digital Droplet PCR is a Specific and Sensitive Tool for Detecting IDH2 Mutations in Acute Myeloid LeuKemia Patients
Abstract
1. Introduction
2. Results
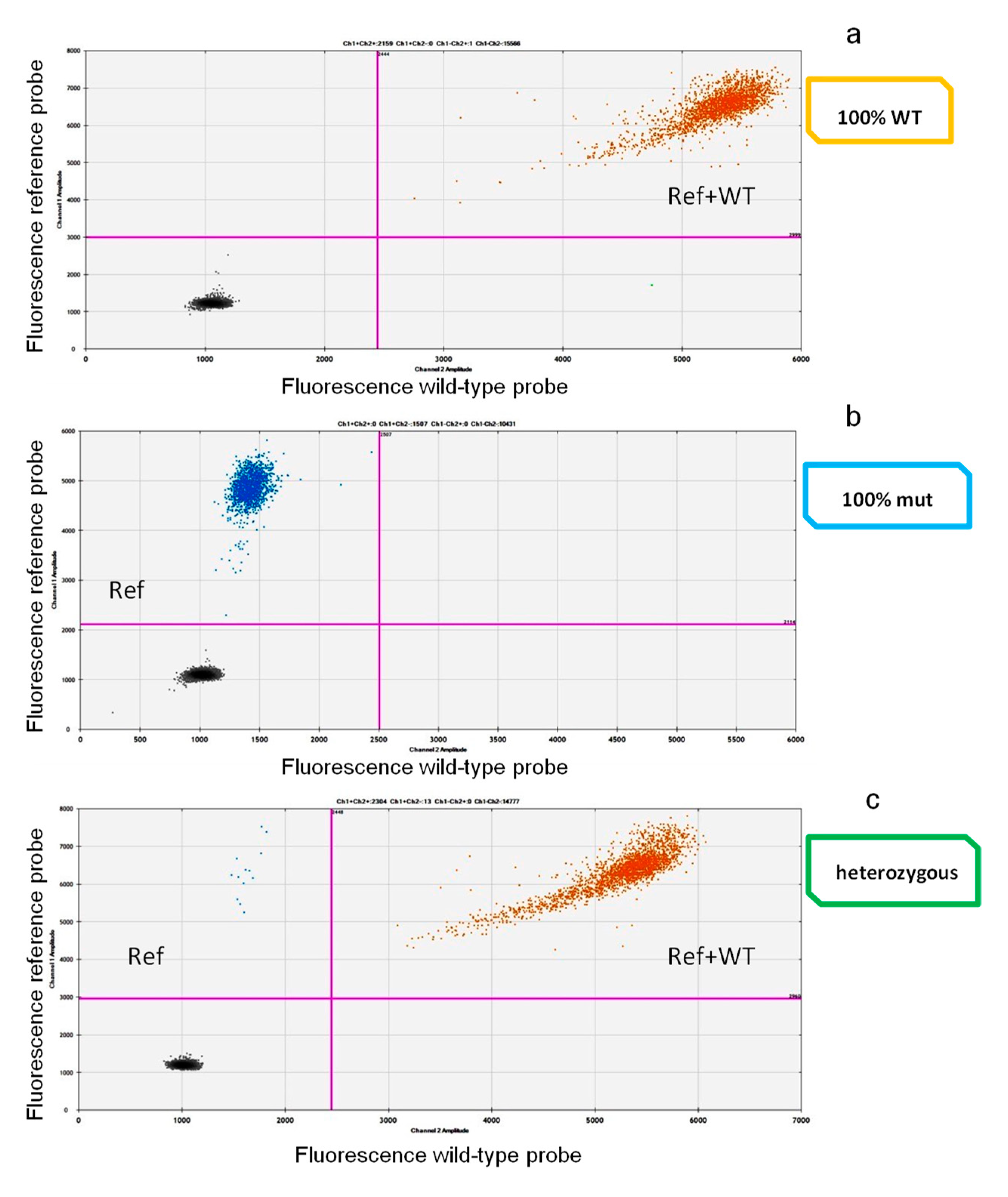
2.1. Drop-Off ddPCR Setting and Validation
2.2. Assessment of IDH2 Mutations and Comparison among ddPCR, ARMS PCR, and Sanger
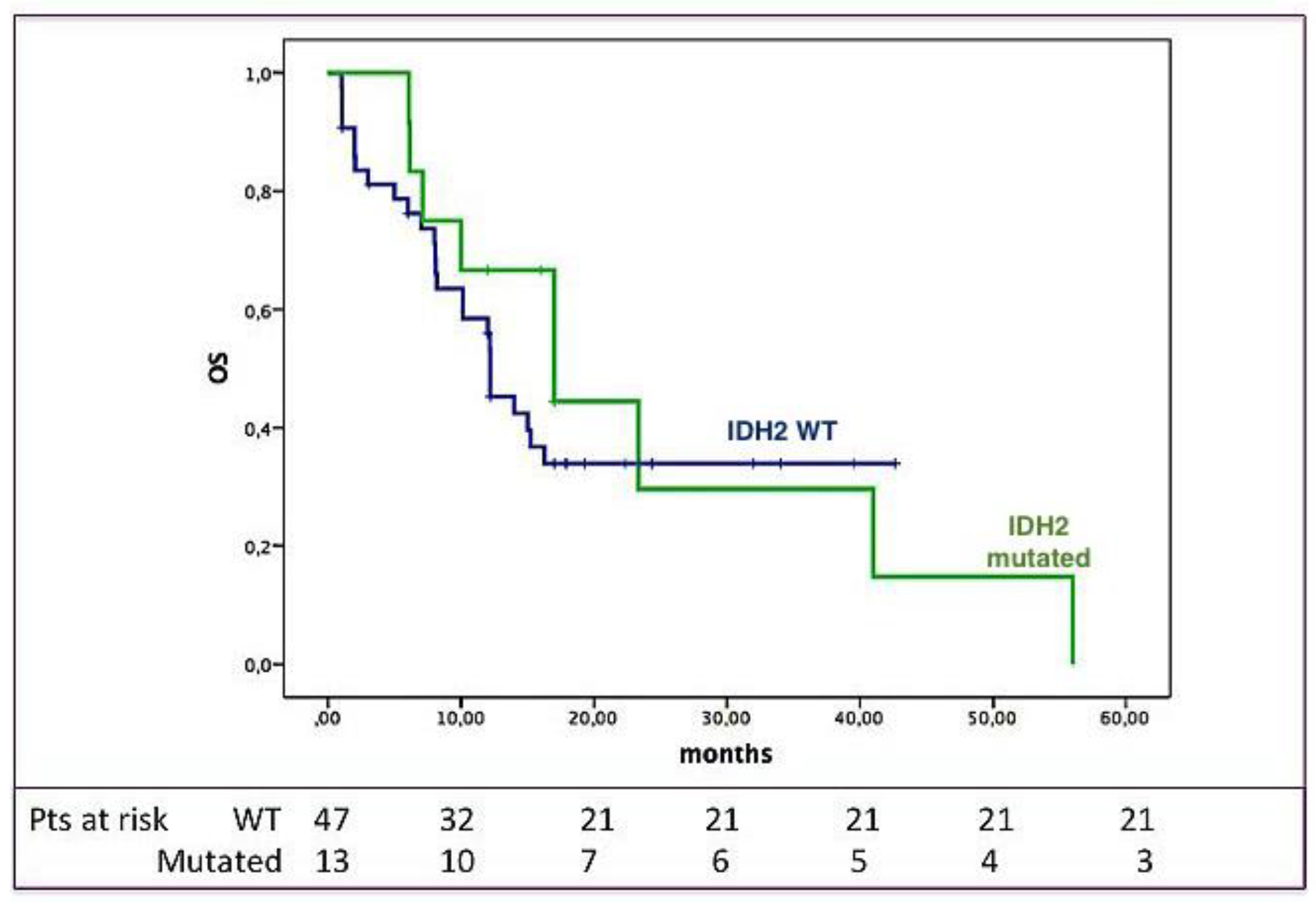
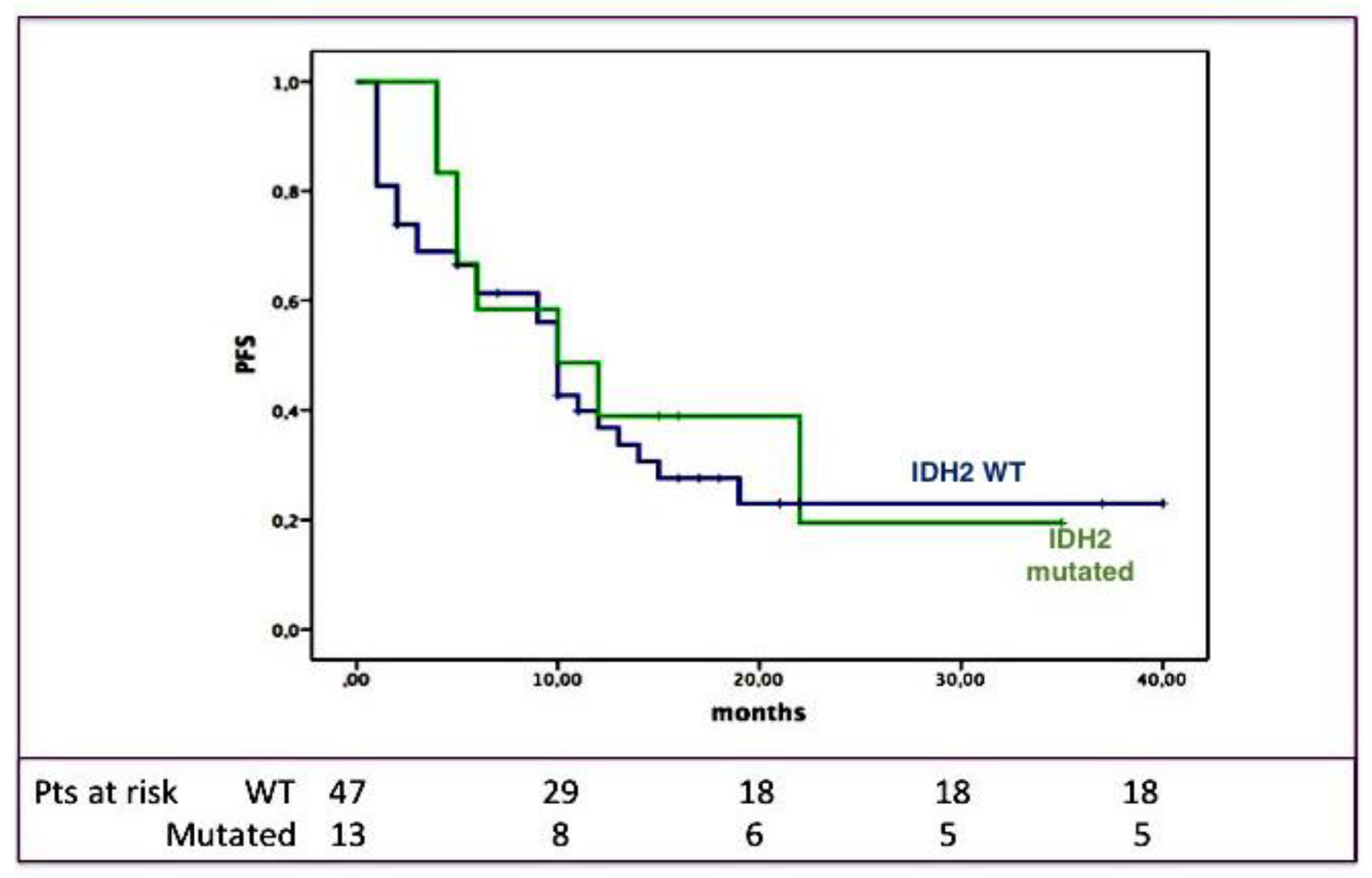
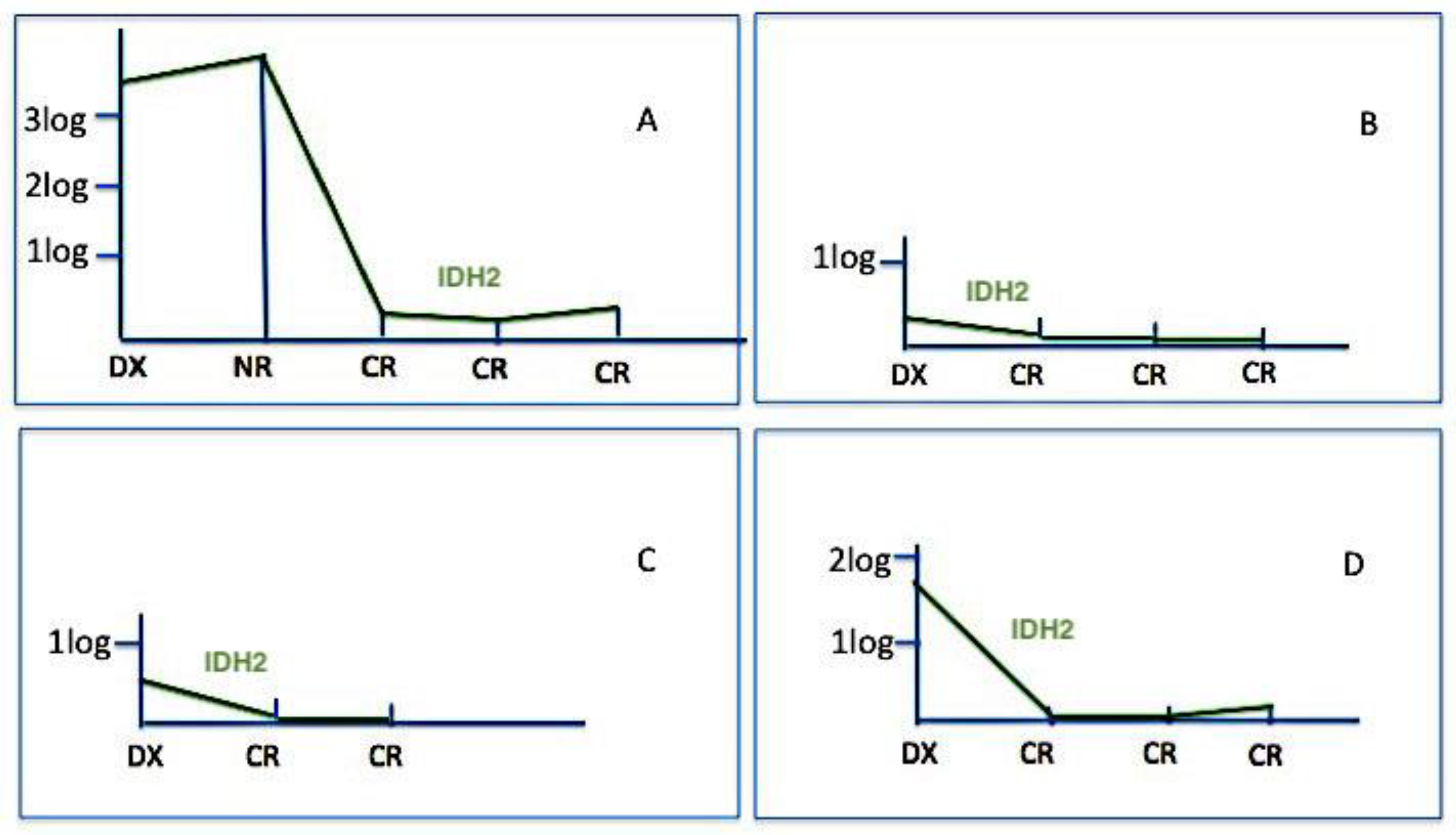
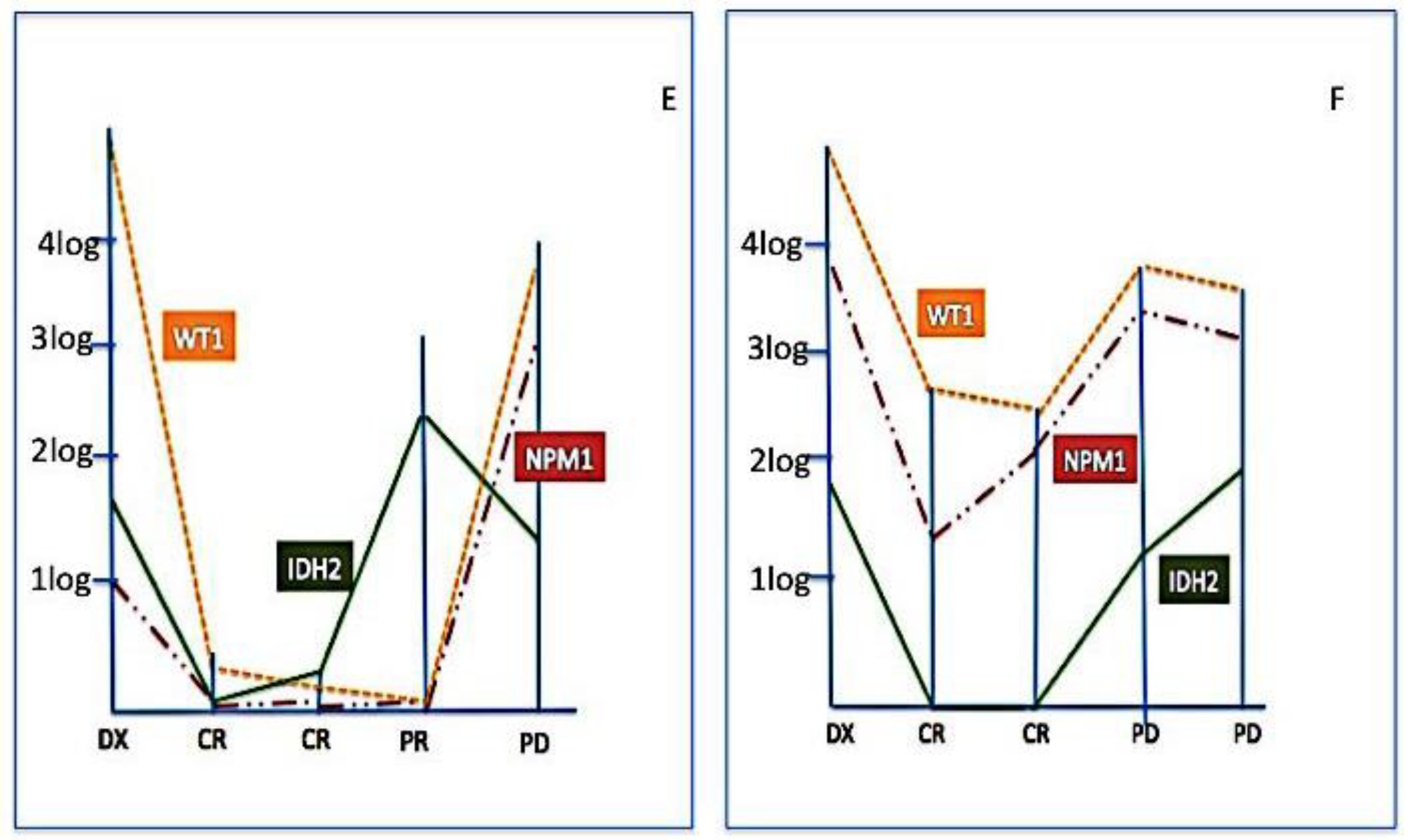
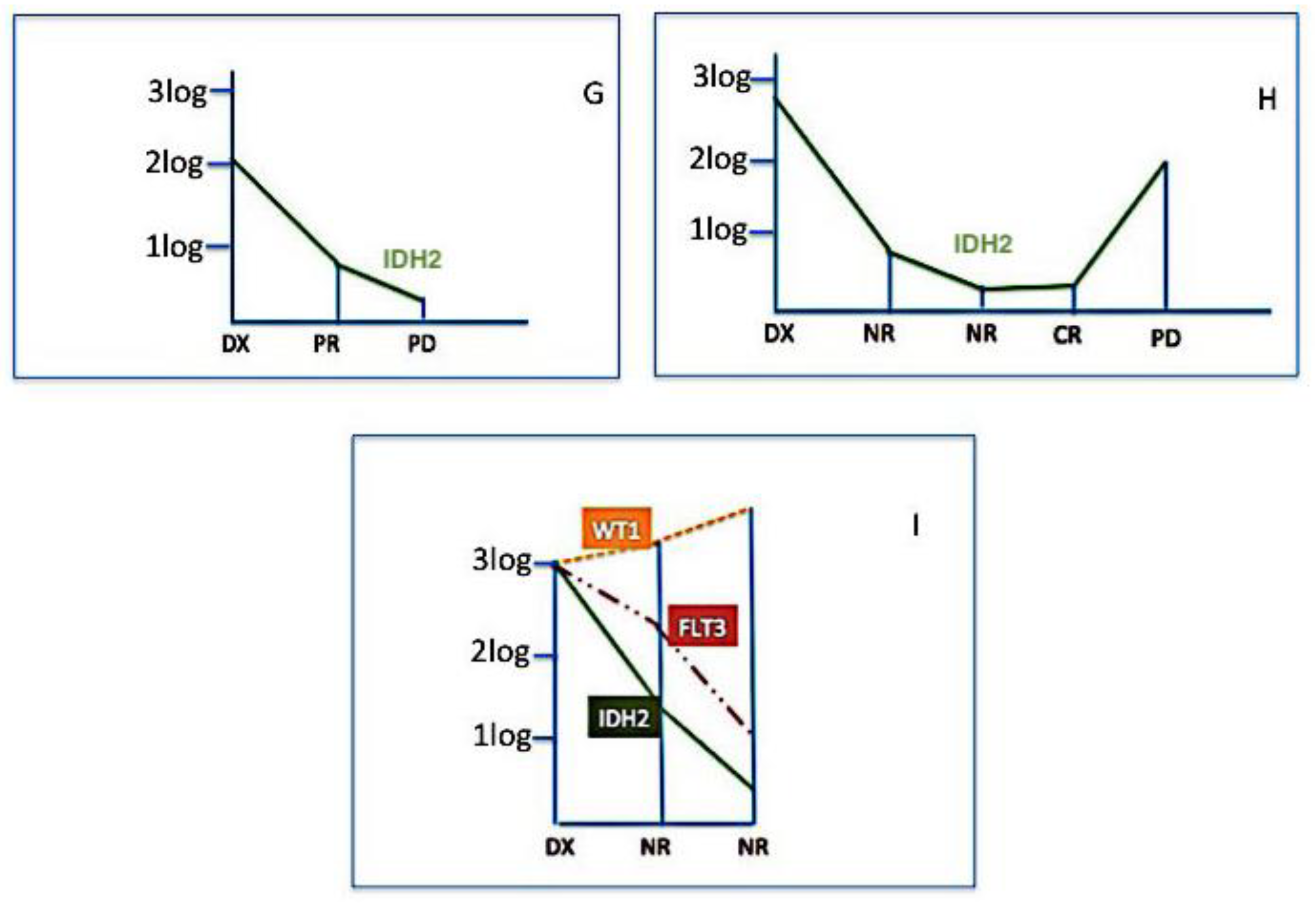
2.3. Clinical Impact of IDH2 Mutations
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
4.2.1. DNA Extraction
4.2.2. Sanger Sequencing of IDH2 R140 and R172 Mutations
4.2.3. IDH2 R140 and R172 Mutations and Real-Time ARMS PCR
4.2.4. IDH2 R140 and R172 Analysis by the Drop-Off ddPCR
4.2.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K.; Koboldt, D.C.; Fulton, R.S.; Delehaunty, K.D.; McGrath, S.D.; et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Lasho, T.L.; Finke, C.M.; Mai, M.; McClure, R.F.; Tefferi, A. IDH1 and IDH2 mutation analysis in chronic- and blast-phase myeloproliferative neoplasms. Leukemia 2010, 24, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Kosmider, O.; Gelsi-Boyer, V.; Slama, L.; Dreyfus, F.; Beyne-Rauzy, O.; Quesnel, B.; Hunault-Berger, M.; Slama, B.; Vey, N.; Lacombe, C.; et al. Mutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasms. Leukemia 2010, 24, 1094–1096. [Google Scholar] [CrossRef]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzymatic activity that converts α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Ravandi, F.; Agresta, S.; Konopleva, M.; Takahashi, K.; Kadia, T.; Routbort, M.; Patel, K.P.; Mark, B.; Pierce, S.; et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am. J. Hematol. 2015, 90, 732–736. [Google Scholar] [CrossRef]
- Liu, X.; Gong, Y. Isocitrate dehydrogenase inhibitors in acute myeloid leukemia. Biomark. Res. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Petrova, L.; Vrbacky, F.; Lanska, M.; Zavrelova, A.; Zak, P.; Hrochova, K. IDH1 and IDH2 mutations in patients with acute myeloid leukemia: Suitable targets for minimal residual disease monitoring? Clin. Biol. Chem. 2018, 61, 34–39. [Google Scholar] [CrossRef]
- Wiseman, D.H.; Somervaille, T.C.P. Nano fluidic Allele-Specific Digital PCR Method for Quantifying IDH1 and IDH2 Mutation Burden in Acute Myeloid Leukemia. In Acute Myeloid Leukemia: Methods and Protocols, Methods in Molecular Biology; Paolo, F., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1633. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Fathi, A.T.; DiNardo, C.D.; Pollyea, D.A.; Chan, S.M.; Swords, R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia 2017, 31, 272–281. [Google Scholar] [CrossRef]
- Salehzadeh, S.; Guerrini, F.; Pizzano, U.; Grassi, S.; Ciabatti, E.; Iovino, L.; Buda, G.; Caracciolo, F.; Benedetti, E.; Orciuolo, E.; et al. The assessment of minimal residual disease versus that of somatic mutations for predicting the outcome of acute myeloid leukemia patients. Cancer Cell Int. 2019, 19, 83. [Google Scholar] [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Dogra, R.; Bhatia, R.; Shankar, R.; Bansal, P.; Rawal, R.K. Enasidenib: First Mutant IDH2 Inhibitor for the Treatment of Refractory and Relapsed Acute Myeloid Leukemia. Anticancer Agents Med. Chem. 2018, 18, 1936–1951. [Google Scholar] [CrossRef]
- Debarri, H.; Lebon, D.; Roumier, C.; Cheok, M.; Marceau-Renaut, A.; Nibourel, O.; Geffroy, S.; Helevaut, N.; Rousselot, P.; Gruson, B.; et al. IDH1/2 but not DNMT3A mutations are suitable targets for minimal residual disease monitoring in acute myeloid leukemia patients: A study by the Acute Leukemia French Association. Oncotarget 2015, 6, 42345–42353. [Google Scholar] [CrossRef] [PubMed]
- Petiti, J.; Rosso, V.; Croce, E.; Franceschi, V.; Andreani, G.; Dragani, M.; DeGobbi, M.; Lunghi, M.; Saglio, G.; Fava, C.; et al. Highly Sensitive Detection of IDH2 Mutations in Acute Myeloid Leukemia. J. Clin. Med. 2020, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Guolo, F.; Minetto, P.; Fianchi, L.; Rondoni, M.; Daghia, G.; D’Ardia, S.; Morselli, M.; Pasciolla, C.; Pavesi, F.; Scappini, B.; et al. Preliminary Results from CPX-351 Italian Compassionate Use Program Show High Response Rate and Good Tolerability in Poor Prognosis AML Patients. Blood 2019, 134 (Suppl. 1), 1363. [Google Scholar] [CrossRef]
- Talati, C.; Goldberg, A.D.; Desai, P.; Chan, O.; Famulare, C.; Komrokji, R.S.; Kuykendall, A.T.; Fernandez, H.F.; Deutsch, Y.E.; Padron, E.; et al. Genomic Landscape Impacts Induction Outcome with CPX-351 in Patients with Acute Myeloid Leukemia. Blood 2018, 132 (Suppl. 1), 2741. [Google Scholar] [CrossRef]
- Chiche, E.; Bertoli, S.; Rahmé, R.; Micol, J.B.; Pasquier, F.; Peterlin, P.; Chevallier, P.; Thomas, X.; Loschi, M.; Genthon, A.; et al. CPX-351 Induces Deep Response and Suppress the Impact of Poor Prognosis Mutations (TP53, ASXL1, RUNX1 and EVI1) Defined By ELN-2017 in t-AML and MRC AML: A Report from a Multicentric French Cohort. Blood 2019, 134 (Suppl. 1), 1355. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Schuh, A.C.; Stein, E.M.; Fernandez, P.M.; Wei, A.; DeBotton, S.; Zeidan, A.M.; Fathi, A.T.; Quek, L.; Kantarjian, H.M.; et al. Enasidenib Plus Azacitidine Significantly Improves Complete Remission and Overall Response Compared with Azacitidine Alone in Patients with Newly Diagnosed Acute Myeloid Leukemia (AML) with Isocitrate Dehydrogenase 2 (IDH2) Mutations: Interim Phase II Results from an Ongoing, Randomized Study. Blood 2019, 134 (Suppl. 1), 643. [Google Scholar] [CrossRef]
- Klco, J.M.; Miller, C.A.; Griffith, M.; Petti, A.; Spencer, D.H.; Ketkar-Kulkarni, S.; Wartman, L.D.; Christopher, M.; Lamprecht, T.L.; Helton, N.M.; et al. Association between mutation clearance after induction therapy and outcomes in acute myeloid leukemia. JAMA 2015, 314, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Pløen, G.G.; Nederby, L.; Guldberg, P.; Hansen, M.; Ebbesen, L.H.; Jensen, U.B.; Hokland, P.; Aggerholm, A. Persistence of DNMT3A mutations at long- term remission in adult patients with AML. Br. J. Haematol. 2014, 167, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Gaidzik, V.I.; Weber, D.; Paschka, P.; Kaumanns, A.; Krieger, S.; Corbacioglu, A.; Krönke, J.; Kapp-Schwoerer, S.; Krämer, D.; Horst, H.A.; et al. DNMT3A mutant transcript levels persist in remission and do not predict outcome in patients with acute myeloid leukemia. Leukemia 2018, 32, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gales, J.; McKinney, M.S.; Erba, H.P.; McCall, C.M. Real World Use of IDH2- Targeted Inhibitors in a Single Academic Medical Center Experience Since Enasidenib FDA-Approval. Blood 2019, 134 (Suppl. 1), 5131. [Google Scholar] [CrossRef]
- van Dongen, J.J.M.; Macintyre, E.A.; Gabert, J.A.; Delabesse, E.; Rossi, V.; Saglio, G.; Gottardi, E.; Rambaldi, A.; Dotti, G.; Griesinger, F.; et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: Investigation of minimal residual disease in acute leukemia. Leukemia 1999, 13, 1901–1928. [Google Scholar] [CrossRef]
- Chen, W.; Jones, D.; Medeiros, L.J.; Luthra, R.; Lin, P. Acute myeloid leukaemia with FLT3 gene mutations of both internal tandem duplication and point mutation type. Br. J. Haematol. 2005, 130, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Noguera, N.I.; Ammatuna, E.; Zangrilli, D.; Lavorgna, S.; Divona, M.; Buccisano, F.; Amadori, S.; Mecucci, C.; Falini, B.; Lo-Coco, F. Simultaneous detection of NPM1 and FLT3-ITD mutations by capillary electrophoresis in acute myeloid leukemia. Leukemia 2005, 19, 1479–1482. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef]
- Marcucci, G.; Maharry, K.; Wu, Y.Z.; Radmacher, M.D.; Mrozek, K.; Margeson, D.; Holland, K.B.; Whitman, S.P.; Becker, H.; Schwind, S.; et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J. Clin. Oncol. 2010, 28, 2348–2355. [Google Scholar] [CrossRef]
| Sanger | ARMS PCR | Drop-Off ddPCR | ||
|---|---|---|---|---|
| Positive | R140Q | 6 | 10 | 10 |
| R172K | 2 | 3 | 3 | |
| TOTAL (%) | 8 (13.3%) | 13 (21.7%) | 13 (21.7%) | |
| Negative | 52 (86.7%) | 47 (78.3%) | 47 (78.3%) |
| Test A | ||||
|---|---|---|---|---|
| + | – | Total | ||
| Test B | + | 8 | 0 | 8 |
| - | 5 | 47 | 52 | |
| Total | 13 | 47 | 60 | |
| Concordance = 0.917 | ||||
| K = 0.715 | ||||
| Clinical Features | IDH2-WT n. (%) | IDH2-MUTATED n. (%) | p |
|---|---|---|---|
| SEX | |||
| M | 25 (53%) | 7 (54%) | ns |
| F | 22 (47%) | 6 (46%) | |
| AGE | |||
| < 65y | 31 (66%) | 9 (69%) | ns |
| ≥65 y | 16 (34%) | 4 (31%) | |
| WHO classification | |||
| AML with recurrent abnormalities | 17 (37%) | 4 (31%) | ns |
| Post-myelodysplasia | 6 (12%) | 3 (23%) | |
| Therapy-related | 3 (6%) | 0 (0%) | |
| NOS | 21 (45%) | 6 (46%) | |
| CYTOGENETIC score | |||
| Good | 8 (17%) | 3 (23%) | ns |
| Intermediate | 22 (47%) | 7 (54%) | |
| Poor | 17 (36%) | 3 (23%) | |
| ELN score | |||
| Good | 4 (9%) | 1 (8%) | ns |
| Intermediate | 27 (57%) | 6 (46%) | |
| Poor | 16 (34%) | 6 (46%) | |
| RAS mutations | |||
| No | 36 (77%) | 11 (85%) | ns |
| Yes | 11 (23%) | 2 (15%) | |
| c-KIT mutations | |||
| No | 42 (89%) | 12 (92%) | ns |
| Yes | 5 (11%) | 1 (8%) | |
| FLT3 mutations | |||
| No | 36 (77%) | 11 (85%) | ns |
| Yes | 11 (23%) | 2 (15%) | |
| NPM1 mutations | |||
| No | 37 (78%) | 11 (85%) | ns |
| Yes | 10 (22%) | 2 (15%) | |
| CBF mutations | |||
| No | 40 (85%) | 13 (100%) | ns |
| Yes | 7 (15%) | 0 (0%) | |
| WBC, median, × 109/L | 21.8 | 20.1 | ns |
| Hb, median, g/dL | 10.2 | 10.4 | ns |
| PLT, median, × 109/L | 937 | 756 | ns |
| Blasts %, median | 40 | 34 | ns |
| IDH2 Positive Pts | Additional Mutations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NPM1 | Ckit | FLT3-ITD | INV16 | Runx1 | IDH1 | NRas | None | |||
| 1 | R140Q | + | ||||||||
| 2 | R140Q | + | + | + | ||||||
| 3 | R140Q | + | + | + | ||||||
| 4 | R172K | + | ||||||||
| 5 | R140Q | + | + | |||||||
| 6 | R172K | + | ||||||||
| 7 | R140Q | + | + | |||||||
| 8 | R140Q | + | ||||||||
| 9 | R140Q | + | ||||||||
| 10 | R140Q | + | ||||||||
| 11 | R140Q | + | ||||||||
| 12 | R172K | + | ||||||||
| 13 | R140Q | + | ||||||||
| n°pts (%) | 3 (23%) | 2 (15%) | 1 (7%) | 1 (7%) | 2 (15%) | 1 (7%) | 5 (38%) | 4 (31%) | ||
| Clinical Features | PTS N. (%) |
|---|---|
| Sex | |
| M | 32 (53%) |
| F | 28 (47%) |
| Age | |
| <65 y | 40 (66%) |
| ≥65 y | 20 (34%) |
| WHO classification | |
| AML with recurrent abnormalities | 21 (35%) |
| Post-myelodysplasia | 9 (15%) |
| Therapy-related | 3 (5%) |
| NOS | 27 (45%) |
| Cytogenetic score | |
| Good | 11 (18%) |
| Intermediate | 29 (48%) |
| Poor | 20 (34%) |
| ELN score | |
| Good | 5 (8%) |
| Intermediate | 33 (55%) |
| Poor | 22 (36%) |
| WBC, median (range) × 109/L | 6.05 (1–118) |
| Hb, median (range) g/dL | 9.7 (5–13) |
| PLT, median (range) × 109/L | 59.5 (10–344) |
| Blasts %, median (range) | 45 (20–85%) |
| RAS mutations | |
| No | 47 (78%) |
| Yes | 13 (22%) |
| c-KIT mutations | |
| No | 54 (90%) |
| Yes | 6 (10%) |
| FLT3 mutations | |
| No | 47 (78%) |
| Yes | 13 (22%) |
| NPM1 mutations | |
| No | 48 (80%) |
| Yes | 12 (20%) |
| CBF mutations | |
| No | 53 (88%) |
| Yes | 7 (12%) |
| Gene | COSMIC ID | Nucleotide Change | Acid Change | Assay Catalog |
|---|---|---|---|---|
| IDH2 | 41877 | c.418C>T | R140W | SMPH025023A |
| IDH2 | 41590 | c.419G>A | R140Q | SMPH025021A |
| IDH2 | 41875 | c.419G>T | R140L | SMPH025022A |
| IDH2 | 34039 | c.514A>T | R172W | SMPH006598A |
| IDH2 | 33733 | c.515G>A | R172K | SMPH006597A |
| Primers | Probes | |
|---|---|---|
| IDH2 R140 REFERENCE | Fwd [9 µM] GATGGGCTTGGTCCAG Rvs [9 µM] AAGAAGATGTGGAAAAGTCC | Probe [5 µM] 6-FAM ACATCCCACGCCTAGT |
| IDH2 R140 WT | Fwd [9 µM] GATGGGCTTGGTCCAG Rvs [9 µM] AAGAAGATGTGGAAAAGTCC | Probe [5 µM] HEX CCAGGATGTTCCGGATAG |
| IDH2 R172 REFERENCE | Fwd [9 µM] CTCCACCCTGGCCTA Rvs [9 µM] CTGGACCAAGCCCATC | Probe [5 µM] 6-FAM TCGCCATGGGCGTGCC |
| IDH2 R172 WT | Fws [9 µM] CTCCACCCTGGCCTA Rvs [9 µM] CTGGACCAAGCCCATC | Probe [5 µM] HEX ATTGGCAGGCACGCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, S.; Guerrini, F.; Ciabatti, E.; Puccetti, R.; Salehzadeh, S.; Metelli, M.R.; Di Vita, A.; Domenichini, C.; Caracciolo, F.; Orciuolo, E.; et al. Digital Droplet PCR is a Specific and Sensitive Tool for Detecting IDH2 Mutations in Acute Myeloid LeuKemia Patients. Cancers 2020, 12, 1738. https://doi.org/10.3390/cancers12071738
Grassi S, Guerrini F, Ciabatti E, Puccetti R, Salehzadeh S, Metelli MR, Di Vita A, Domenichini C, Caracciolo F, Orciuolo E, et al. Digital Droplet PCR is a Specific and Sensitive Tool for Detecting IDH2 Mutations in Acute Myeloid LeuKemia Patients. Cancers. 2020; 12(7):1738. https://doi.org/10.3390/cancers12071738
Chicago/Turabian StyleGrassi, Susanna, Francesca Guerrini, Elena Ciabatti, Riccardo Puccetti, Serena Salehzadeh, Maria Rita Metelli, Alessia Di Vita, Cristiana Domenichini, Francesco Caracciolo, Enrico Orciuolo, and et al. 2020. "Digital Droplet PCR is a Specific and Sensitive Tool for Detecting IDH2 Mutations in Acute Myeloid LeuKemia Patients" Cancers 12, no. 7: 1738. https://doi.org/10.3390/cancers12071738
APA StyleGrassi, S., Guerrini, F., Ciabatti, E., Puccetti, R., Salehzadeh, S., Metelli, M. R., Di Vita, A., Domenichini, C., Caracciolo, F., Orciuolo, E., Pelosini, M., Mazzantini, E., Rossi, P., Mazziotta, F., Petrini, M., & Galimberti, S. (2020). Digital Droplet PCR is a Specific and Sensitive Tool for Detecting IDH2 Mutations in Acute Myeloid LeuKemia Patients. Cancers, 12(7), 1738. https://doi.org/10.3390/cancers12071738





