Hereditary Gastric and Breast Cancer Syndromes Related to CDH1 Germline Mutation: A Multidisciplinary Clinical Review
Abstract
1. Introduction
2. Hereditary Diffuse Gastric Cancer
2.1. Environmental Factors and GC
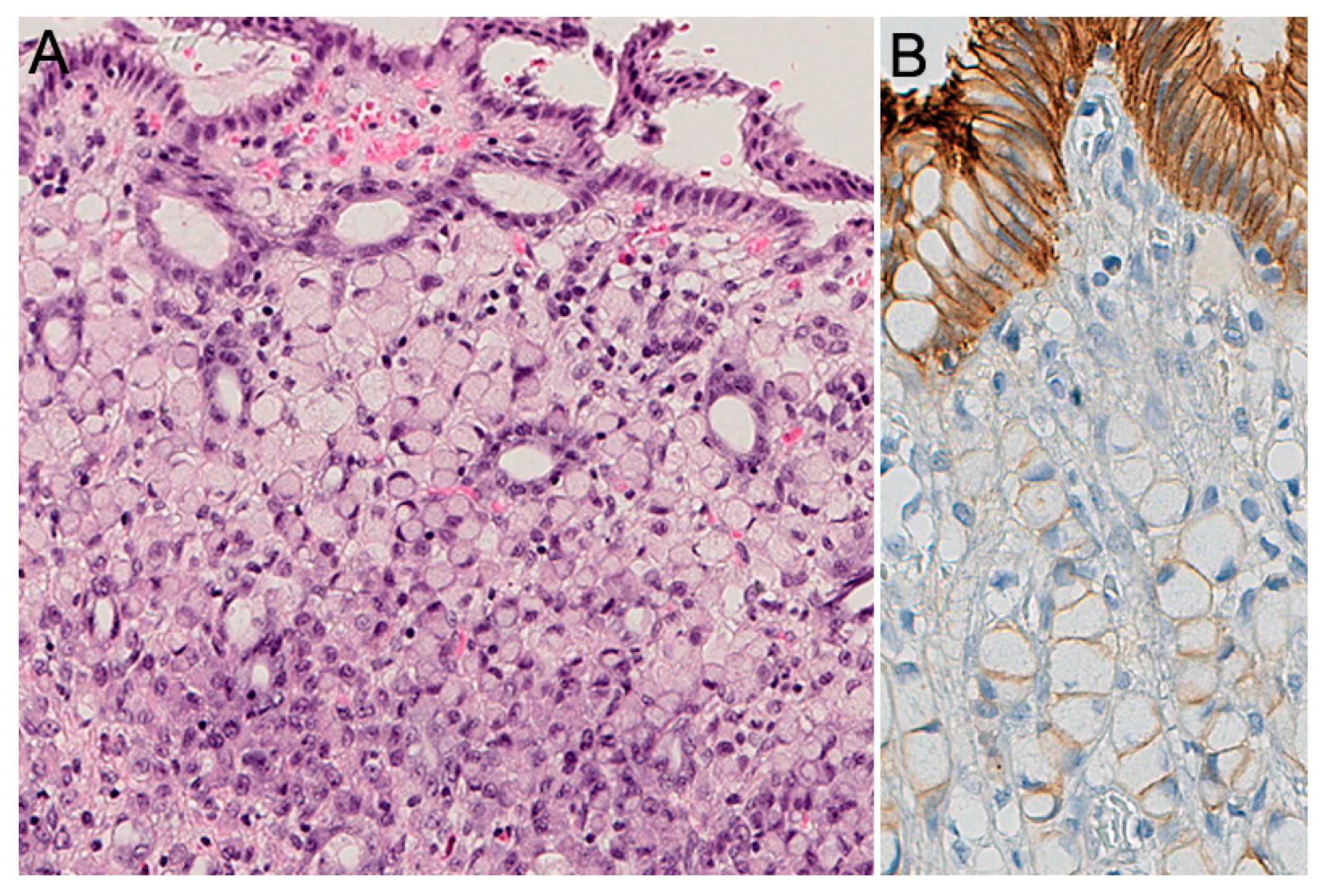
2.2. Pathology of HDGC
2.3. Histopathology of Prophylactic Gastrectomy
2.4. Histopathology: Advanced HDGC
2.5. Histochemical and Immunohistochemical Stains
2.6. Endoscopy
2.7. Prophylactic Gastrectomy
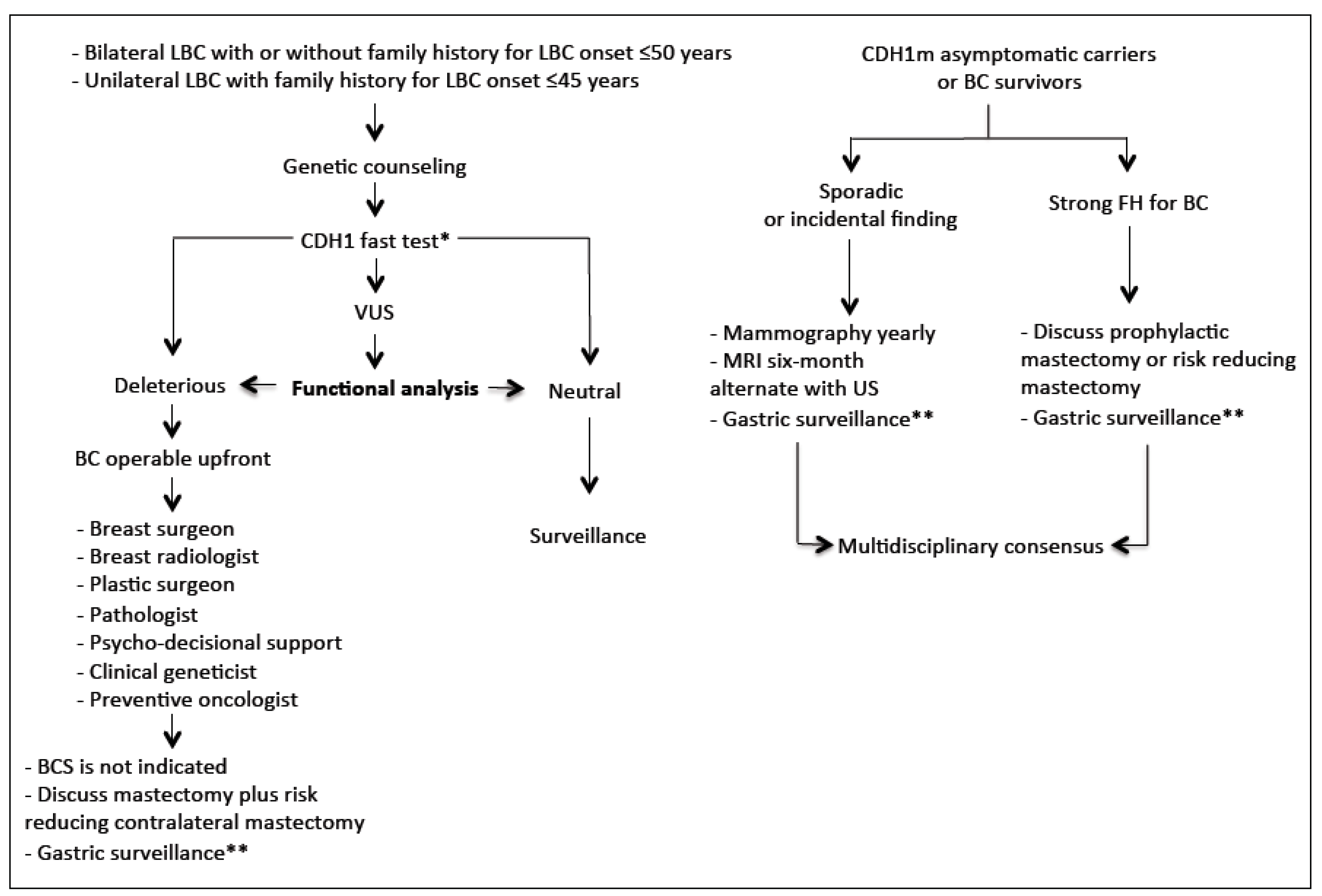
3. Hereditary Lobular Breast Cancer (HLBC)
3.1. Definition
3.2. CDH1 Screening: Preliminary Considerations
3.3. Pathology
3.4. Breast Imaging
3.5. Surgical Management
3.6. Post-Mastectomy Breast Reconstruction
4. Common Managements
4.1. Genetic Counseling
4.2. Psychological Counseling
4.3. CDH1 Missense Variants: Challenging Routine Laboratory Tests
4.4. Bioimaging Strategies to Identify Aberrant E-Cadherin Expression Signatures
5. Others
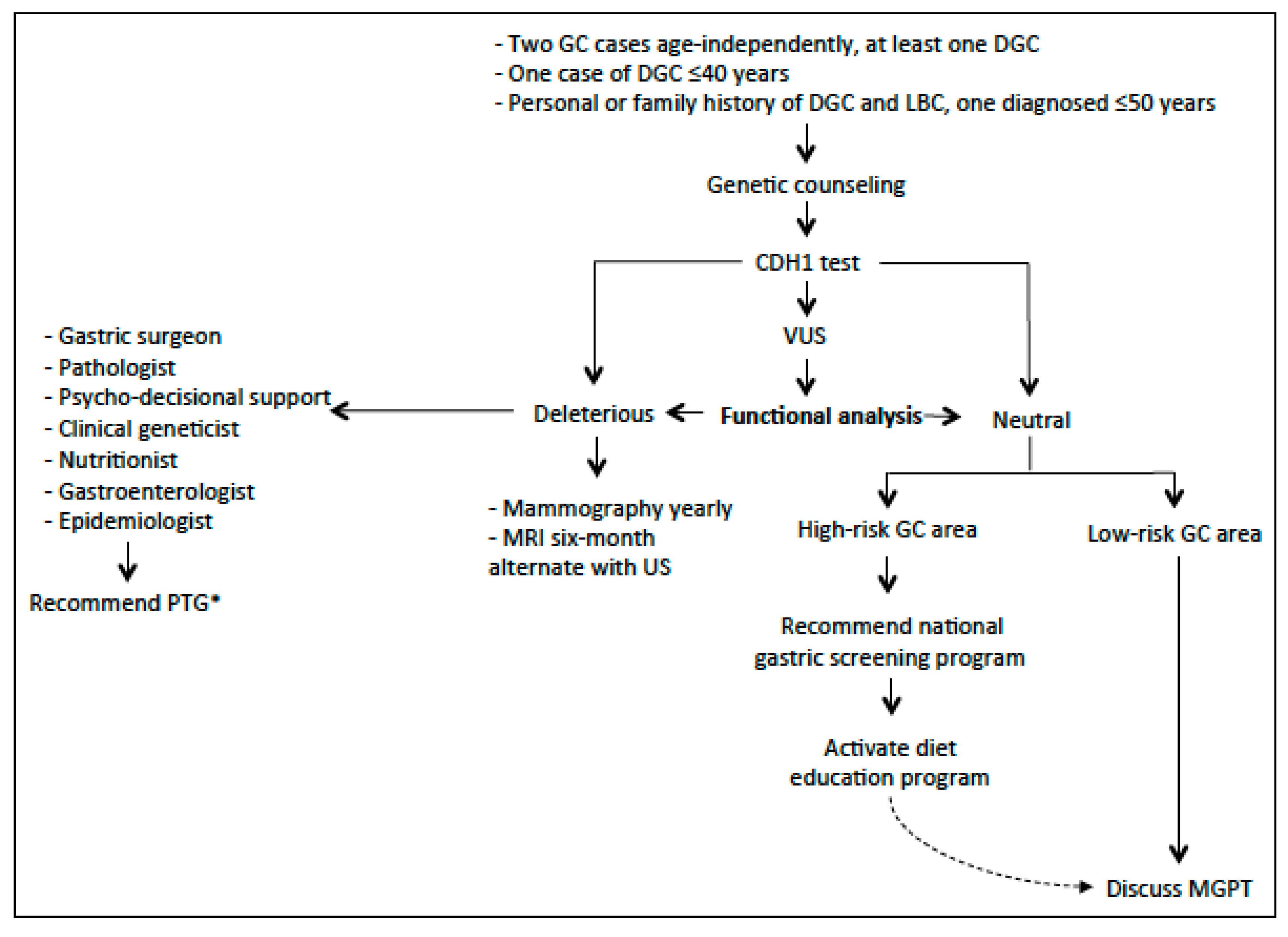
5.1. Clinical Management of CDH1 Carriers without a Family History of GC and LBC
5.2. High and Low-Risk Geographical Regions for GC: Impact on Clinical Management
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.G. Familial gastric cancer. N. Z. Med. J. 1964, 63, 287–296. [Google Scholar]
- Caldas, C.; Carneiro, F.; Lynch, H.T.; Yokota, J.; Wiesner, G.L.; Powell, S.M.; Lewis, F.R.; Huntsman, D.G.; Pharoah, P.D.; Jankowski, J.; et al. Familial gastric cancer: Overview and guidelines for management. J. Med. Genet. 1999, 36, 873–880. [Google Scholar]
- Kaurah, P.; Macmillan, A.; Boyd, N.; Senz, J.; De Luca, A.; Chun, N.; Suriano, G.; Zaor, S.; Van Manen, L.; Gilpin, C.; et al. Founder and Recurrent CDH1 Mutations in Families with Hereditary Diffuse Gastric Cancer. JAMA 2007, 297, 2360–2372. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Wilson, A.R.; Kaurah, P.; Suriano, G.; Leach, S.; Senz, J.; Grehan, N.; Butterfield, Y.S.N.; Jeyes, J.; Schinas, J.; Bacani, J.; et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: Assessment of 42 new families and review of genetic screening criteria. J. Med. Genet. 2004, 41, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Van Der Post, R.S.; Vogelaar, I.; Carneiro, F.; Guilford, P.; Huntsman, D.; Hoogerbrugge, N.; Caldas, C.; Schreiber, K.E.C.; Hardwick, R.H.; Ausems, M.G.E.M.; et al. Hereditary diffuse gastric cancer: Updated clinical guidelines with an emphasis on germlineCDH1mutation carriers. J. Med. Genet. 2015, 52, 361–374. [Google Scholar] [CrossRef]
- Corso, G.; Figueiredo, J.; La Vecchia, C.; Veronesi, P.; Pravettoni, G.; Macis, D.; Karam, R.; Gullo, R.L.; Provenzano, E.; Toesca, A.; et al. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J. Med. Genet. 2018, 55, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Hansford, S.; Kaurah, P.; Li-Chang, H.; Woo, M.; Senz, J.; Pinheiro, H.; A Schrader, K.; Schaeffer, D.F.; Shumansky, K.; Zogopoulos, G.; et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015, 1, 23–32. [Google Scholar] [CrossRef]
- Roberts, M.E.; Ranola, J.M.O.; Marshall, M.L.; Susswein, L.R.; Graceffo, S.; Bohnert, K.; Tsai, G.; Klein, R.T.; Hruska, K.S.; Shirts, B.H. Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol. 2019, 5, 1325. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Lyons, K.; Le, L.C.; Pham, Y.T.-H.; Borron, C.; Park, J.Y.; Tran, C.T.; Tran, T.V.; Tran, H.T.-T.; Vu, K.T.; Do, C.D.; et al. Gastric cancer: Epidemiology, biology, and prevention: A mini review. Eur. J. Cancer Prev. 2019, 28, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Lunet, N.; Adany, S.B.C.R.; Zhang, Z.; Praud, D.; Boffetta, P.; Levi, F.; Matsuo, K.; Ito, H.; Hu, J.; et al. The stomach cancer pooling (StoP) project: Study design and presentation. Eur. J. Cancer Prev. 2015, 24, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Praud, D.; Rota, M.; Pelucchi, C.; Bertuccio, P.; Rosso, T.; Galeone, C.; Zhang, Z.; Matsuo, K.; Ito, H.; Hu, J.; et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur. J. Cancer Prev. 2018, 27, 124–133. [Google Scholar] [CrossRef]
- Rota, M.; Pelucchi, C.; Bertuccio, P.; Matsuo, K.; Zhang, Z.; Ito, H.; Hu, J.; Johnson, K.C.; Palli, D.; Ferraroni, M.; et al. Alcohol consumption and gastric cancer risk-A pooled analysis within the StoP project consortium. Int. J. Cancer 2017, 141, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.; Alicandro, G.; Pelucchi, C.; Bonzi, R.; Bertuccio, P.; Hu, J.; Zhang, Z.; Johnson, K.C.; Palli, D.; Ferraroni, M.; et al. Education and gastric cancer risk—An individual participant data meta-analysis in the StoP project consortium. Int. J. Cancer 2019, 146, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Rosato, V.; Rota, M.; Costa, A.R.; Morais, S.; Pelucchi, C.; Johnson, K.C.; Hu, J.; Palli, D.; Ferraroni, M.; et al. Meat intake and risk of gastric cancer in the Stomach cancer Pooling (StoP) project. Int. J. Cancer 2019, 147, 45–55. [Google Scholar] [CrossRef]
- Bertuccio, P.; Alicandro, G.; Rota, M.; Pelucchi, C.; Bonzi, R.; Galeone, C.; Bravi, F.; Johnson, K.C.; Hu, J.; Palli, D.; et al. Citrus fruit intake and gastric cancer: The stomach cancer pooling (StoP) project consortium. Int. J. Cancer 2019, 144, 2936–2944. [Google Scholar] [CrossRef]
- Carneiro, F.; Huntsman, D.G.; Smyrk, T.C.; A Owen, D.; Seruca, R.; Pharoah, P.; Caldas, C.; Sobrinho-Simões, M. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J. Pathol. 2004, 203, 681–687. [Google Scholar] [CrossRef]
- Carneiro, F.; Guilford, P.; Oliveira, C.; van der Post, R.S. Hereditary Diffuse Gastric Cancer. In WHO Classification of Tumours Editorial Board. Digestive System Tumours, 5th ed.; WHO classification of tumours series; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1. [Google Scholar]
- Gullo, I.; Devezas, V.; Baptista, M.; Garrido, L.; Castedo, S.; Morais, R.; Wen, X.; Rios, E.; Pinheiro, J.; Pinto-Ribeiro, I.; et al. Phenotypic heterogeneity of hereditary diffuse gastric cancer: Report of a family with early-onset disease. Gastrointest. Endosc. 2018, 87, 1566–1575. [Google Scholar] [CrossRef]
- Thompson, I.W.; Day, D.W.; A Wright, N. Subnuclear vacuolated mucous cells: A novel abnormality of simple mucin-secreting cells of non-specialized gastric mucosa and Brunner’s glands. Histopathology 1987, 11, 1067–1081. [Google Scholar] [CrossRef]
- Wang, K.; Weinrach, D.; Lal, A.; Musunuri, S.; Ramirez, J.; Ozer, O.; Keh, P.; Rao, M.S. Signet-ring cell change versus signet-ring cell carcinoma: A comparative analysis. Am. J. Surg. Pathol. 2003, 27, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.P.; Gullo, I.; Wen, X.; Devezas, V.; Baptista, M.; Oliveira, C.; Carneiro, F. Pathological features of total gastrectomy specimens from asymptomatic hereditary diffuse gastric cancer patients and implications for clinical management. Histopathology 2018, 73, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Wickremeratne, T.; Lee, C.H.; Kirk, J.; Charlton, A.; Thomas, G.; John, G.K. Prophylactic gastrectomy in a 16-year-old. Eur. J. Gastroenterol. Hepatol. 2014, 26, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.; Murrell, A.; Ito, Y.; Maia, A.-T.; Hyland, S.; Oliveira, C.; Save, V.; Carneiro, F.; Paterson, A.; Grehan, N.; et al. Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer. J. Pathol. 2008, 216, 295–306. [Google Scholar] [CrossRef]
- Blair, V.; Martin, I.; Shaw, D.; Winship, I.; Kerr, D.; Arnold, J.; Harawira, P.; McLeod, M.; Parry, S.; Charlton, A.; et al. Hereditary Diffuse Gastric Cancer: Diagnosis and Management. Clin. Gastroenterol. Hepatol. 2006, 4, 262–275. [Google Scholar] [CrossRef]
- Charlton, A.; Blair, V.; Shaw, D.; Parry, S.; Guilford, P.; Martin, I.G. Hereditary diffuse gastric cancer: Predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut 2004, 53, 814–820. [Google Scholar] [CrossRef]
- Chun, Y.S.; Lindor, N.M.; Smyrk, T.C.; Petersen, B.T.; Burgart, L.J.; Guilford, P.J.; Donohue, J.H. Germline E-cadherin gene mutations: Is prophylactic total gastrectomy indicated? Cancer 2001, 92, 181–187. [Google Scholar] [CrossRef]
- Huntsman, D.G.; Carneiro, F.; Lewis, F.R.; MacLeod, P.M.; Hayashi, A.; Monaghan, K.G.; Maung, R.; Seruca, R.; Jackson, C.E.; Caldas, C. Early Gastric Cancer in Young, Asymptomatic Carriers of Germ-Line E-Cadherin Mutations. N. Engl. J. Med. 2001, 344, 1904–1909. [Google Scholar] [CrossRef]
- Rogers, W.M.; Dobo, E.; Norton, J.A.; Van Dam, J.; Jeffrey, R.B.; Huntsman, D.G.; Kingham, K.; Chun, N.; Ford, J.M.; Longacre, T.A. Risk-reducing Total Gastrectomy for Germline Mutations in E-cadherin (CDH1): Pathologic Findings with Clinical Implications. Am. J. Surg. Pathol. 2008, 32, 799–809. [Google Scholar] [CrossRef]
- Fujita, H.; Lennerz, J.K.; Chung, D.C.; Patel, D.; Deshpande, V.; Yoon, S.S.; Lauwers, G.Y. Endoscopic Surveillance of Patients with Hereditary Diffuse Gastric Cancer: Biopsy recommendations after topographic distribution of cancer foci in a series of 10 CDH1-mutated gastrectomies. Am. J. Surg. Pathol. 2012, 36, 1709–1717. [Google Scholar] [CrossRef]
- Pandalai, P.K.; Lauwers, G.Y.; Chung, D.C.; Patel, D.; Yoon, S.S. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery 2011, 149, 347–355. [Google Scholar] [CrossRef]
- Bardram, L.; Hansen, T.V.O.; Gerdes, A.-M.; Timshel, S.; Friis-Hansen, L.; Federspiel, B. Prophylactic total gastrectomy in hereditary diffuse gastric cancer: Identification of two novel CDH1 gene mutations—A clinical observational study. Fam. Cancer 2014, 13, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F.; Oliveira, C.; Suriano, G.; Seruca, R. Molecular pathology of familial gastric cancer, with an emphasis on hereditary diffuse gastric cancer. J. Clin. Pathol. 2007, 61, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Di Pietro, M.; O’Donovan, M.; Richardson, S.; Debiram, I.; Dwerryhouse, S.; Hardwick, R.H.; Tischkowitz, M.; Caldas, C.; Ragunath, K.; et al. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest. Endosc. 2014, 80, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Frebourg, T.; Oliveira, C.; Hochain, P.; Karam, R.; Manouvrier, S.; Graziadio, C.; Vekemans, M.; Hartmann, A.; Baert-Desurmont, S.; Aandrelex, C.; et al. Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J. Med. Genet. 2006, 43, 138–142. [Google Scholar] [CrossRef]
- Kluijt, I.; Siemerink, E.J.; Ausems, M.G.; Van Os, T.A.; De Jong, D.; Correia, J.S.; Van Krieken, J.H.J.; Ligtenberg, M.J.; Figueiredo, J.; Van Riel, E.; et al. CDH1-related hereditary diffuse gastric cancer syndrome: Clinical variations and implications for counseling. Int. J. Cancer 2011, 131, 367–376. [Google Scholar] [CrossRef]
- Jacobs, M.F.; Dust, H.; Koeppe, E.S.; Wong, S.; Mulholland, M.; Choi, E.-Y.; Appelman, H.; Stoffel, E.M. Outcomes of Endoscopic Surveillance in Individuals with Genetic Predisposition to Hereditary Diffuse Gastric Cancer. Gastroenterology 2019, 157, 87–96. [Google Scholar] [CrossRef]
- Mills, S.E. Histology for Pathologists, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Van Der Kaaij, R.T.; Van Kessel, J.P.; Van Dieren, J.M.; Snaebjörnsson, P.; Balagué, O.; Van Coevorden, F.; Van Der Kolk, L.E.; Sikorska, K.; Cats, A.; Van Sandick, J.W. Outcomes after prophylactic gastrectomy for hereditary diffuse gastric cancer. Br. J. Surg. 2018, 105, e176–e182. [Google Scholar] [CrossRef]
- Lee, A.F.; Rees, H.; Owen, D.A.; Huntsman, D.G. Periodic Acid-Schiff Is Superior to Hematoxylin and Eosin for Screening Prophylactic Gastrectomies from CDH1 Mutation Carriers. Am. J. Surg. Pathol. 2010, 34, 1007–1013. [Google Scholar] [CrossRef]
- Van Der Post, R.S.; Gullo, I.; Oliveira, C.; Tang, L.H.; Grabsch, H.I.; O’Donovan, M.; Fitzgerald, R.C.; Van Krieken, J.H.J.; Carneiro, F. Histopathological, Molecular, and Genetic Profile of Hereditary Diffuse Gastric Cancer: Current Knowledge and Challenges for the Future. Adv. Exp. Med. Biol. 2016, 908, 371–391. [Google Scholar] [CrossRef]
- Lee, H.E.; Smyrk, T.C.; Zhang, L. Histologic and immunohistochemical differences between hereditary and sporadic diffuse gastric carcinoma. Hum. Pathol. 2018, 74, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Godwin, T.D.; Kelly, S.T.; Brew, T.P.; Bougen-Zhukov, N.M.; Single, A.B.; Chen, A.; Stylianou, C.E.; Harris, L.D.; Currie, S.K.; Telford, B.J.; et al. E-cadherin-deficient cells have synthetic lethal vulnerabilities in plasma membrane organisation, dynamics and function. Gastric Cancer 2018, 22, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Van Der Post, R.S.; Oliveira, C.; Guilford, P.; Carneiro, F. Hereditary gastric cancer: What’s new? Update 2013–2018. Fam. Cancer 2019, 18, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Van Dieren, J.M.; Kodach, L.L.; Cats, A. Targeted vs Random Biopsies in Surveillance Endoscopy in Hereditary Diffuse Gastric Cancer Syndrome. Clin. Gastroenterol. Hepatol. 2020, 18, 1647–1648. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Adar, T.; Patel, D.; Lauwers, G.Y.; Yoon, S.S.; Mullen, J.T.; Chung, D.C. Surveillance Endoscopy in the Management of Hereditary Diffuse Gastric Cancer Syndrome. Clin. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef]
- Mi, E.Z.; di Pietro, M.; O’Donovan, M.; Mi, E.M.; Hardwick, R.; Ziauddeen, H.; Fletcher, P.; Caldas, C.; Ragunath, K. A comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest. Endosc. 2018, 8, 408–418. [Google Scholar] [CrossRef]
- Artifon, E.L.D.A.; Marinho, F.R.T. Endoscopic screening for hereditary diffuse gastric cancer: One size does not fit all. Gastrointest. Endosc. 2018, 87, 405–407. [Google Scholar] [CrossRef]
- Van Dieren, J.M.; Kodach, L.L.; Hartog, P.D.; Van Der Kolk, L.E.; Sikorska, K.; Van Velthuysen, M.-L.F.; Van Sandick, J.W.; Koemans, W.J.; Snaebjornsson, P.; Cats, A. Gastroscopic surveillance with targeted biopsies compared with random biopsies in CDH1 mutation carriers. Endoscopy 2020. [Google Scholar] [CrossRef]
- Goetz, M. Characterization of lesions in the stomach: Will confocal laser endomicroscopy replace the pathologist? Best Pract. Res. Clin. Gastroenterol. 2015, 29, 589–599. [Google Scholar] [CrossRef]
- Laszkowska, M.; Silver, E.R.; Schrope, B.; Kastrinos, F.; Wang, T.C.; Hur, C. Optimal Timing of Total Gastrectomy to Prevent Diffuse Gastric Cancer in Individuals with Pathogenic Variants in CDH1. Clin. Gastroenterol. Hepatol. 2020, 18, 822–829. [Google Scholar] [CrossRef]
- Xicola, R.M.; Li, S.; Rodriguez, N.; Reinecke, P.; Karam, R.; Speare, V.; Black, M.H.; LaDuca, H.; Llor, X. Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J. Med. Genet. 2019, 56, 838–843. [Google Scholar] [CrossRef]
- Kumar, S.; Long, J.M.; Ginsberg, G.G.; Katona, B.W. The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome. World J. Gastroenterol. 2019, 25, 2878–2886. [Google Scholar] [CrossRef]
- DiBrito, S.R.; Blair, A.B.; Prasath, V.; Habibi, M.; Harmon, J.W.; Duncan, M.D. Total Gastrectomy for CDH-1 Mutation Carriers: An Institutional Experience. J. Surg. Res. 2020, 247, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, T.; Buhl, K. Techniques of reconstruction after total gastrectomy for cancer. Br. J. Surg. 2004, 91, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Figueiredo, J.; Biffi, R.; Trentin, C.; Bonanni, B.; Feroce, I.; Serrano, D.; Cassano, E.; Annibale, B.; Melo, S.; et al. E-cadherin germline mutation carriers: Clinical management and genetic implications. Cancer Metastasis Rev. 2014, 33, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04206891 (accessed on 20 December 2019).
- Corso, G.; De Scalzi, A.; Feroce, I.; Veronesi, P.; Bonanni, B.; Galimberti, V. Clinical criteria revision for hereditary lobular breast cancer associated with E-cadherin germline mutations. Pers. Med. 2018, 15, 153–155. [Google Scholar] [CrossRef]
- Breast, T. WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2019. [Google Scholar]
- Guerini-Rocco, E.; Fusco, N. Premalignant and Pre-invasive Lesions of the Breast. In Methods in Molecular Biology; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 103–120. [Google Scholar]
- Marotti, J.D.; Schnitt, S.J. Genotype-Phenotype Correlations in Breast Cancer. Surg. Pathol. Clin. 2018, 11, 199–211. [Google Scholar] [CrossRef]
- Mirandola, S.; Pellini, F.; Granuzzo, E.; Lorenzi, M.; Accordini, B.; Ulgelmo, M.; Invento, A.; Lombardi, D.; Caldana, M.; Pollini, G.P. Multidisciplinary management of CDH1 germinal mutation and prophylactic management hereditary lobular breast cancer: A case report. Int. J. Surg. Case Rep. 2019, 58, 92–95. [Google Scholar] [CrossRef]
- Sickles, E.A. The subtle and atypical mammographic features of invasive lobular carcinoma. Radiology 1991, 178, 25–26. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Grady, D.; Barclay, J.; Sickles, E.A.; Ernster, V. Effect of Age, Breast Density, and Family History on the Sensitivity of First Screening Mammography. JAMA 1996, 276, 33. [Google Scholar] [CrossRef]
- Robertson, C.L. A private breast imaging practice: Medical audit of 25,788 screening and 1,077 diagnostic examinations. Radiology 1993, 187, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Hilleren, D.J.; Andersson, I.T.; Lindholm, K.; Linnell, F.S. Invasive lobular carcinoma: Mammographic findings in a 10-year experience. Radiology 1991, 178, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Krecke, K.N.; Gisvold, J.J. Invasive lobular carcinoma of the breast: Mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am. J. Roentgenol. 1993, 161, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, M.; Ollivier, L.; Asselain, B.; Meunier, M.; Laurent, M.; Vielh, P.; Neuenschwander, S. Mammographic features of 455 invasive lobular carcinomas. Radiology 1992, 185, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Paramagul, C.P.; A Helvie, M.; Adler, D.D. Invasive lobular carcinoma: Sonographic appearance and role of sonography in improving diagnostic sensitivity. Radiology 1995, 195, 231–234. [Google Scholar] [CrossRef]
- Selinko, V.L.; Middleton, L.P.; Dempsey, P.J. Role of sonography in diagnosing and staging invasive lobular carcinoma. J. Clin. Ultrasound 2004, 32, 323–332. [Google Scholar] [CrossRef]
- Mann, R.M.; Hoogeveen, Y.L.; Blickman, J.G.; Boetes, C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: A review of existing literature. Breast Cancer Res. Treat. 2007, 107, 1–14. [Google Scholar] [CrossRef]
- Mann, R.M.; Kuhl, C.K.; Kinkel, K.; Boetes, C. Breast MRI: Guidelines from the European Society of Breast Imaging. Eur. Radiol. 2008, 18, 1307–1318. [Google Scholar] [CrossRef]
- McGuire, K.P.; Mamounas, E.P. Management of Hereditary Breast Cancer: ASCO, ASTRO, and SSO Guideline. Ann. Surg. Oncol. 2020, 27, 1721–1723. [Google Scholar] [CrossRef]
- Jakub, J.; Peled, A.W.; Gray, R.J.; Greenup, R.A.; Kiluk, J.V.; Sacchini, V.; McLaughlin, S.A.; Tchou, J.C.; Vierkant, R.A.; Degnim, A.C.; et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population with BRCA Mutations: A Multi-institutional Study. JAMA Surg. 2018, 153, 123–129. [Google Scholar] [CrossRef]
- Valachis, A.; Nearchou, A.D.; Lind, P. Surgical management of breast cancer in BRCA-mutation carriers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2014, 144, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Tsangaris, T.N.; Kouprina, N.; Wu, L.S.-F.; Balch, C.M.; Vang, R.; Argani, P. The superficial margin of the skin-sparing mastectomy for breast carcinoma: Factors predicting involvement and efficacy of additional margin sampling. Ann. Surg. Oncol. 2008, 15, 1330–1340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muller, T.; Baratte, A.; Bruant-Rodier, C.; Bodin, F.; Mathelin, C. Oncological safety of nipple-sparing prophylactic mastectomy: A review of the literature on 3716 cases. Ann. Chir. Plast. Esthet. 2017, 63, e6–e13. [Google Scholar] [CrossRef]
- Weber, W.P.; Haug, M.; Kurzeder, C.; Bjelic-Radisic, V.; Koller, R.; Reitsamer, R.; Fitzal, F.; Biazus, J.; Brenelli, F.; Urban, C.; et al. Oncoplastic Breast Consortium consensus conference on nipple-sparing mastectomy. Breast Cancer Res. Treat. 2018, 172, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Headon, H.L.; Kasem, A.; Mokbel, K. The Oncological Safety of Nipple-Sparing Mastectomy: A Systematic Review of the Literature with a Pooled Analysis of 12,358 Procedures. Arch. Plast. Surg. 2016, 43, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Morigi, C.; Bagnardi, V.; Corso, G.; Vicini, E.; Fontana, S.K.R.; Naninato, P.; Ratini, S.; Magnoni, F.; Toesca, A.; et al. Oncological Outcomes of Nipple-Sparing Mastectomy: A Single-Center Experience of 1989 Patients. Ann. Surg. Oncol. 2018, 25, 3849–3857. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Vicini, E.; Corso, G.; Morigi, C.; Fontana, S.; Sacchini, V.; Veronesi, P. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast 2017, 34 (Suppl. S1), S82–S84. [Google Scholar] [CrossRef]
- Van Verschuer, V.M.; Mureau, M.A.; Gopie, J.P.; Vos, E.L.; Verhoef, C.; Menke-Pluijmers, M.B.; Koppert, L.B. Patient satisfaction and nipple-areola sensitivity after bilateral prophylactic mastectomy and immediate implant breast reconstruction in a high breast cancer risk population: Nipple-sparing mastectomy versus skin-sparing mastectomy. Ann. Plast. Surg. 2016, 77, 145–152. [Google Scholar] [CrossRef]
- Razdan, S.N.; Patel, V.; Jewell, S.; McCarthy, C.M. Quality of life among patients after bilateral prophylactic mastectomy: A systematic review of patient-reported outcomes. Qual. Life Res. 2015, 25, 1409–1421. [Google Scholar] [CrossRef]
- Franceschini, G.; Masetti, R. What The Surgeons Should Know About The Bilateral Prophylactic Mastectomy in BRCA Mutation Carriers. Eur. J. Breast Health 2019, 15, 135–136. [Google Scholar] [CrossRef]
- Corso, G.; De Lorenzi, F.; Vicini, E.; Pagani, G.; Veronesi, P.; Sargenti, M.; Magnoni, F.; Naninato, P.; Maisonneuve, P.; Sangalli, C.; et al. Nipple-sparing mastectomy with different approaches: Surgical incisions, complications, and cosmetic results. Preliminary results of 100 consecutive patients at a single center. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Toesca, A.; Invento, A.; Massari, G.; Girardi, A.; Peradze, N.; Lissidini, G.; Sangalli, C.; Maisonneuve, P.; Manconi, A.; Gottardi, A.; et al. Update on the Feasibility and Progress on Robotic Breast Surgery. Ann. Surg. Oncol. 2019, 26, 3046–3051. [Google Scholar] [CrossRef] [PubMed]
- European Institute of Oncology. Robotic Nipple-Sparing Mastectomy vs. Conventional Open Technique; ClinicalTrials.gov identifier: NCT 03440398. Available online: www.clinicaltrials.gov/ct2/show/NCT03440398 (accessed on 22 February 2018).
- Ilonzo, N.; Tsang, A.; Tsantes, S.; Estabrook, A.; Ma, A.M.T. Breast reconstruction after mastectomy: A ten-year analysis of trends and immediate postoperative outcomes. Breast 2017, 32, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, P.; Ballardini, B.; De Lorenzi, F.; Magnoni, F.; Lissidini, G.; Caldarella, P.; Galimberti, V. Immediate breast reconstruction after mastectomy. Breast 2011, 20 (Suppl. S3), S104–S107. [Google Scholar] [CrossRef]
- Kamali, P.; Zettervall, S.L.; Wu, W.; Ibrahim, A.M.S.; Medin, C.; Rakhorst, H.; Schermerhorn, M.; Lee, B.T.; Lin, S.J. Differences in the Reporting of Racial and Socioeconomic Disparities among Three Large National Databases for Breast Reconstruction. Plast. Reconstr. Surg. 2017, 139, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.D.; Salibian, A.A.; Karp, N.S.; Choi, M. Implant-Based Breast Reconstruction: Hot Topics, Controversies, and New Directions. Plast. Reconstr. Surg. 2019, 143, 404e–416e. [Google Scholar] [CrossRef]
- Sbitany, H.; Piper, M.; Lentz, R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast. Reconstr. Surg. 2017, 140, 432–443. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Yu, N.; Huang, J.; Long, X. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Meta-analysis. Ann. Plast. Surg. 2020. [Google Scholar] [CrossRef]
- Sigalove, S.; Maxwell, G.P.; Sigalove, N.M.; Storm-Dickerson, T.L.; Pope, N.; Rice, J.; Gabriel, A. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast. Reconstr. Surg. 2017, 139, 287–294. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Hardwick, R.; Huntsman, D.; Carneiro, F.; Guilford, P.; Blair, V.; Chung, D.C.; Norton, J.; Ragunath, K.; Van Krieken, J.H.; et al. Hereditary diffuse gastric cancer: Updated consensus guidelines for clinical management and directions for future research. J. Med. Genet. 2010, 47, 436–444. [Google Scholar] [CrossRef]
- Pharoah, P.D.; Guilford, P.; Caldas, C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001, 121, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Seruca, R.; Carneiro, F. Genetics, Pathology, and Clinics of Familial Gastric Cancer. Int. J. Surg. Pathol. 2006, 14, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Senz, J.; Kaurah, P.; Pinheiro, H.; Sanges, R.; Haegert, A.; Corso, G.; Schouten, J.; Fitzgerald, R.; Vogelsang, H.; et al. Germline CDH1 deletions in hereditary diffuse gastric cancer families. Hum. Mol. Genet. 2009, 18, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Plon, S.E.; Eccles, D.M.; Easton, U.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V.; et al. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 29, 1282–1291. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Lee, K.; Krempely, K.; Roberts, M.E.; Anderson, M.J.; Carneiro, F.; Chao, E.; Dixon, K.; Figueiredo, J.; Ghosh, R.; Huntsman, D.; et al. Specifications of the ACMG/AMP variant curation guidelines for the analysis of germline CDH1 sequence variants. Hum. Mutat. 2018, 39, 1553–1568. [Google Scholar] [CrossRef]
- Kurian, A.W.; Hare, E.E.; Mills, M.A.; Kingham, K.E.; McPherson, L.; Whittemore, A.S.; McGuire, V.; Ladabaum, U.; Kobayashi, Y.; Lincoln, S.E.; et al. Clinical Evaluation of a Multiple-Gene Sequencing Panel for Hereditary Cancer Risk Assessment. J. Clin. Oncol. 2014, 32, 2001–2009. [Google Scholar] [CrossRef]
- Kurian, A.W.; Ward, K.C.; Hamilton, A.S.; Deapen, D.M.; Abrahamse, P.; Bondarenko, I.; Li, Y.; Hawley, S.T.; Morrow, M.; Jagsi, R.; et al. Uptake, Results, and Outcomes of Germline Multiple-Gene Sequencing After Diagnosis of Breast Cancer. JAMA Oncol. 2018, 4, 1066. [Google Scholar] [CrossRef]
- Hall, M.J.; Forman, A.D.; Pilarski, R.; Wiesner, G.; Giri, V.N. Gene panel testing for inherited cancer risk. Natl. Compr. Canc. Netw. 2014, 12, 1339–1346. [Google Scholar] [CrossRef]
- Lowstuter, K.; Espenschied, C.R.; Sturgeon, D.; Ricker, C.; Karam, R.; LaDuca, H.; Culver, J.O.; Dolinsky, J.S.; Chao, E.; Sturgeon, J.; et al. Unexpected CDH1 Mutations Identified on Multigene Panels Pose Clinical Management Challenges. JCO Precis. Oncol. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Cicero, G.; De Luca, R.; Dorangricchia, P.; Coco, G.L.; Guarnaccia, C.; Fanale, D.; Calò, V.; Russo, A. Risk Perception and Psychological Distress in Genetic Counselling for Hereditary Breast and/or Ovarian Cancer. J. Genet. Couns. 2017, 26, 999–1007. [Google Scholar] [CrossRef]
- Ringwald, J.; Wochnowski, C.; Bosse, K.; Giel, K.E.; Schaeffeler, N.; Zipfel, S.; Teufel, M. Psychological Distress, Anxiety, and Depression of Cancer-Affected BRCA1/2 Mutation Carriers: A Systematic Review. J. Genet. Couns. 2016, 25, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Esteban, I.; Vilaró, M.; Adrover, E.; Angulo, A.; Carrasco, E.; Gadea, N.; Sanchez, A.; Ocana, T.; Llort, G.; Jover, R.; et al. Psychological impact of multigene cancer panel testing in patients with a clinical suspicion of hereditary cancer across Spain. Psycho-Oncology 2018, 27, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Meiser, B.; Quinn, V.F.; Mitchell, G.; Tucker, K.; Watts, K.J.; Rahman, B.; Peate, M.; Saunders, C.; Geelhoed, E.; Gleeson, M.; et al. Psychological outcomes and surgical decisions after genetic testing in women newly diagnosed with breast cancer with and without a family history. Eur. J. Hum. Genet. 2018, 26, 972–983. [Google Scholar] [CrossRef]
- Bechara, A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004, 55, 30–40. [Google Scholar] [CrossRef]
- Martin, L.N.; Delgado, M.R. The influence of emotion regulation on decision-making under risk. J. Cogn. Neurosci. 2011, 23, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, P.; Lucchiari, C.; Santoro, L.; Sangalli, C.; Luini, A.; Pravettoni, G. PTSD symptoms as a consequence of breast cancer diagnosis: Clinical implications. SpringerPlus 2014, 3, 392. [Google Scholar] [CrossRef]
- Renzi, C.; Riva, S.; Masiero, M.; Pravettoni, G.; Information, P.E.K.F.C. The choice dilemma in chronic hematological conditions: Why choosing is not only a medical issue? A psycho-cognitive perspective. Crit. Rev. Oncol. 2016, 99, 134–140. [Google Scholar] [CrossRef]
- Fioretti, C.; Mazzocco, K.; Pravettoni, G. Psychological Support in Breast Cancer Patients: A Personalized Approach. In Methods in Molecular Biology; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 841–847. [Google Scholar] [CrossRef]
- Kondylakis, H.; Kazantzaki, E.; Koumakis, L.; Genitsaridi, I.; Marias, K.; Gorini, A.; Mazzocco, K.; Pravettoni, G.; Burke, D.; McVie, G.; et al. Development of interactive empowerment services in support of personalised medicine. Ecancermedicalscience 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Gorini, A.; Pravettoni, G. P5 medicine: A plus for a personalized approach to oncology. Nat. Rev. Clin. Oncol. 2011, 8, 444. [Google Scholar] [CrossRef]
- Glatzer, M.; Panje, C.M.; Sirén, C.; Cihoric, N.; Putora, P.M. Decision Making Criteria in Oncology. Oncology 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, R.; Garland, S.N.; Vaska, M.; Carlson, L.E. Who benefits from psychosocial interventions in oncology? A systematic review of psychological moderators of treatment outcome. J. Behav. Med. 2012, 35, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Petrocchi, S.; Iannello, P.; Lecciso, F.; Levante, A.; Antonietti, A.; Schulz, P. Interpersonal trust in the context of doctor-patient relationship: Dyadic analysis with one-with-many design. Soc. Sci. Med. 2019, 235, 112391. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, A.; Munthe, C.; Törner, M.; Forsander, G. The Counseling, Self-Care, Adherence Approach to Person-Centered Care and Shared Decision Making: Moral Psychology, Executive Autonomy, and Ethics in Multi-Dimensional Care Decisions. Health Commun. 2016, 31, 964–973. [Google Scholar] [CrossRef]
- Jones, P.S.; Meleis, A.I. Health is empowerment. ANS Adv. Nurs. Sci. 1993, 15, 1–14. [Google Scholar] [CrossRef]
- Mandelblatt, J.; Kreling, B.; Figeuriedo, M.; Feng, S. What Is the Impact of Shared Decision Making on Treatment and Outcomes for Older Women with Breast Cancer? J. Clin. Oncol. 2006, 24, 4908–4913. [Google Scholar] [CrossRef]
- Shay, L.A.; Lafata, J.E. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med. Decis. Mak. 2014, 35, 114–131. [Google Scholar] [CrossRef]
- Majithia, A.R.; Tsuda, B.; Agostini, M.; Gnanapradeepan, K.; Rice, R.; Peloso, G.; Patel, K.A.; Zhang, X.; Broekema, M.F.; Patterson, N.; et al. Prospective functional classification of all possible missense variants in PPARG. Nat. Genet. 2016, 48, 1570–1575. [Google Scholar] [CrossRef]
- Findlay, G.M.; Daza, R.M.; Martin, B.; Zhang, M.D.; Leith, A.P.; Gasperini, M.; Janizek, J.D.; Huang, X.; Starita, L.M.; Shendure, J. Accurate classification of BRCA1 variants with saturation genome editing. Nature 2018, 562, 217–222. [Google Scholar] [CrossRef]
- Raraigh, K.S.; Han, S.T.; Davis-Marcisak, E.F.; Evans, T.A.; Pellicore, M.; McCague, A.F.; Joynt, A.T.; Lu, Z.; Atalar, M.; Sharma, N.; et al. Functional Assays Are Essential for Interpretation of Missense Variants Associated with Variable Expressivity. Am. J. Hum. Genet. 2018, 102, 1062–1077. [Google Scholar] [CrossRef]
- Suriano, G.; Seixas, S.; Rocha, J.; Seruca, R. A model to infer the pathogenic significance of CDH1 germline missense variants. J. Mol. Med. 2006, 84, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.C.; Caldas, C. Clinical implications of E-cadherin associated hereditary diffuse gastric cancer. Gut 2004, 53, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.; Seruca, R. Germline Missense Mutants in Hereditary Diffuse Gastric Cancer. In Spotlight on Familial and Hereditary Gastric Cancer; Springer Science and Business Media LLC: Berlin, Germany, 2013; pp. 77–86. [Google Scholar] [CrossRef]
- Melo, S.; Figueiredo, J.; Fernandes, M.S.; Gonçalves, M.; De Sá, E.M.; Sanches, J.M.; Seruca, R. Predicting the Functional Impact of CDH1 Missense Mutations in Hereditary Diffuse Gastric Cancer. Int. J. Mol. Sci. 2017, 18, 2687. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Pinheiro, H.; Figueiredo, J.; Seruca, R.; Carneiro, F. Familial gastric cancer: Genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015, 16, e60–e70. [Google Scholar] [CrossRef]
- Correia, J.S.; Figueiredo, J.; Lopes, R.; Stricher, F.; Oliveira, C.; Serrano, L.; Seruca, R. E-Cadherin Destabilization Accounts for the Pathogenicity of Missense Mutations in Hereditary Diffuse Gastric Cancer. PLoS ONE 2012, 7, e33783. [Google Scholar] [CrossRef]
- Suriano, G.; Oliveira, C.; Ferreira, P.; Machado, J.C.; Bordin, M.C.; De Wever, O.; Bruyneel, E.A.; Moguilevsky, N.; Grehan, N.; Porter, T.R.; et al. Identification of CDH1 germline missense mutations associated with functional inactivation of the E-cadherin protein in young gastric cancer probands. Hum. Mol. Genet. 2003, 12, 575–582. [Google Scholar] [CrossRef]
- Figueiredo, J.; Söderberg, O.; Correia, J.S.; Grannas, K.; Suriano, G.; Seruca, R. The importance of E-cadherin binding partners to evaluate the pathogenicity of E-cadherin missense mutations associated to HDGC. Eur. J. Hum. Genet. 2012, 21, 301–309. [Google Scholar] [CrossRef]
- Sanches, J.M.; Figueiredo, J.; Fonseca, M.; Durães, C.; Melo, S.; Esménio, S.; Seruca, R. Quantification of mutant E-cadherin using bioimaging analysis of in situ fluorescence microscopy. A new approach to CDH1 missense variants. Eur. J. Hum. Genet. 2014, 23, 1072–1079. [Google Scholar] [CrossRef]
- Mestre, T.; Figueiredo, J.; Ribeiro, A.S.; Paredes, J.; Seruca, R.; Sanches, J.M. Quantification of topological features in cell meshes to explore E-cadherin dysfunction. Sci. Rep. 2016, 6, 25101. [Google Scholar] [CrossRef]
- Pinho, S.S.; Seruca, R.; Gärtner, F.; Yamaguchi, Y.; Gu, J.; Taniguchi, N.; Reis, C.A. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell. Mol. Life Sci. 2010, 68, 1011–1020. [Google Scholar] [CrossRef]
- Pena-Couso, L.; Perea, J.; Melo, S.; Mercadillo, F.; Figueiredo, J.; Sanches, J.M.; Sanchez-Ruiz, A.; Robles, L.; Oliveira, C.; Urioste, M. Clinical and functional characterization of the CDH1 germline variant c.1679C>G in three unrelated families with hereditary diffuse gastric cancer. Eur. J. Hum. Genet. 2018, 26, 1348–1353. [Google Scholar] [CrossRef]
- Correia, J.S.; Figueiredo, J.; Oliveira, C.; Van Hengel, J.; Seruca, R.; Van Roy, F.; Suriano, G. Endoplasmic reticulum quality control: A new mechanism of E-cadherin regulation and its implication in cancer. Hum. Mol. Genet. 2008, 17, 3566–3576. [Google Scholar] [CrossRef]
- Suriano, G.; Oliveira, M.J.; Huntsman, D.; Mateus, A.R.; Ferreira, P.; Casares, F.; Oliveira, C.; Carneiro, F.; Machado, J.C.; Mareel, M.; et al. E-cadherin germline missense mutations and cell phenotype: Evidence for the independence of cell invasion on the motile capabilities of the cells. Hum. Mol. Genet. 2003, 12, 3007–3016. [Google Scholar] [CrossRef]
- Boterberg, T.; E Bracke, M.; A Bruyneel, E.; Mareel, M.; Brooks, S.A.; Schumacher, U. Cell Aggregation Assays. Methods Mol. Med. 2001, 58, 33–45. [Google Scholar] [PubMed]
- Suriano, G.; Mulholland, D.; De Wever, O.; Ferreira, P.; Mateus, A.R.; Bruyneel, E.; Nelson, C.C.; Mareel, M.M.; Yokota, J.; Huntsman, D.; et al. The intracellular E-cadherin germline mutation V832 M lacks the ability to mediate cell–cell adhesion and to suppress invasion. Oncogene 2003, 22, 5716–5719. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Kleinman, H.K.; McGarvey, M.L.; Liotta, L.A.; Robey, P.G.; Tryggvason, K.; Martin, G.R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 1982, 21, 6188–6193. [Google Scholar] [CrossRef]
- Mateus, A.R.; Correia, J.S.; Figueiredo, J.; Heindl, S.; Alves, C.C.; Suriano, G.; Luber, B.; Seruca, R. E-cadherin mutations and cell motility: A genotype–phenotype correlation. Exp. Cell Res. 2009, 315, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.R.; Seruca, R.; Machado, J.C.; Keller, G.; Oliveira, M.J.; Suriano, G.; Luber, B. EGFR regulates RhoA-GTP dependent cell motility in E-cadherin mutant cells. Hum. Mol. Genet. 2007, 16, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.W.; Conchello, J.A. Fluorescence microscopy. Nat. Methods 2005, 2, 910–919. [Google Scholar] [CrossRef]
- Muzzey, D.; Van Oudenaarden, A. Quantitative time-lapse fluorescence microscopy in single cells. Annu. Rev. Cell Dev. Biol. 2009, 25, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Sandison, D.R.; Williams, R.M.; Wells, K.S.; Strickler, J.; Webb, W.W. Quantitative Fluorescence Confocal Laser Scanning Microscopy (CLSM). In Handbook of Biological Confocal Microscopy; Springer Science and Business Media LLC: Berlin, Germany, 1995; pp. 39–53. [Google Scholar]
- Nakano, A. Spinning-disk Confocal Microscopy—A Cutting-Edge Tool for Imaging of Membrane Traffic. Cell Struct. Funct. 2002, 27, 349–355. [Google Scholar] [CrossRef]
- Waters, J.C. Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol. 2009, 185, 1135–1148. [Google Scholar] [CrossRef]
- A Hamilton, N. Quantification and its Applications in Fluorescent Microscopy Imaging. Traffic 2009, 10, 951–961. [Google Scholar] [CrossRef]
- Figueiredo, J.; Rodrigues, I.; Ribeiro, J.; Fernandes, M.S.; Melo, S.; Sousa, B.; Paredes, J.; Oliveira, C.; Sanches, J.M. Geometric compensation applied to image analysis of cell populations with morphological variability: A new role for a classical concept. Sci. Rep. 2018, 8, 10266. [Google Scholar] [CrossRef]
- Fonseca, L.M.G.; Manjunath, B.S. Registration techniques for multisensor remotely sensed imagery. Photogramm. Eng. Remote Sens. 1996, 62, 1049–1056. [Google Scholar]
- Zitová, B.; Flusser, J. Image registration methods: A survey. Image Vis. Comput. 2003, 21, 977–1000. [Google Scholar] [CrossRef]
- Sanches, J.M.; Marques, J. Joint image registration and volume reconstruction for 3D ultrasound. Pattern Recognit. Lett. 2003, 24, 791–800. [Google Scholar] [CrossRef]
- Li, S.; Wakefield, J.; Noble, J.A. Automated Segmentation and Alignment of Mitotic Nuclei for Kymograph Visualisation. In Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 March–2 April 2011; pp. 622–625. [Google Scholar] [CrossRef]
- Figueiredo, J.; Melo, S.; Gamet, K.; Godwin, T.; Seixas, S.; Sanches, J.M.; Guilford, P.; Oliveira, C. E-cadherin signal sequence disruption: A novel mechanism underlying hereditary cancer. Mol. Cancer 2018, 17, 112. [Google Scholar] [CrossRef]
- Ribeiro, A.; Carvalho, F.; Figueiredo, J.; Mestre, T.; Monteiro, J.; Guedes, A.F.; Fonseca, M.; Sanches, J.M.; Seruca, R.; Santos, N.C.; et al. Atomic force microscopy and graph analysis to study the P-cadherin/SFK mechanotransduction signalling in breast cancer cells. Nanoscale 2016, 8, 19390–19401. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Long, J.M.; Brandt, A.C.; Brower, J.; Symecko, H.; Salo-Mullen, E.E.; Christian, S.N.; Harstad, T.; Couch, F.J.; Garber, J.E.; et al. Patients’ Medical and Psychosocial Experiences After Detection of a CDH1 Variant with Multigene Panel Testing. JCO Precis. Oncol. 2019, 1–14. [Google Scholar] [CrossRef]
- Marrelli, D.; Pedrazzani, C.; Corso, G.; Neri, A.; Di Martino, M.; Pinto, E.; Roviello, F. Different Pathological Features and Prognosis in Gastric Cancer Patients Coming From High-Risk and Low-Risk Areas of Italy. Ann. Surg. 2009, 250, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Palli, D.; Russo, A.G.; DeCarli, A. Dietary patterns, nutrient intake and gastric cancer in a high-risk area of Italy. Cancer Causes Control 2001, 12, 163–172. [Google Scholar] [CrossRef] [PubMed]
- A González, C.; Jakszyn, P.; Pera, G.; Agudo, A.; Bingham, S.; Palli, D.; Ferrari, P.; Boeing, H.; Del Giudice, G.; Plebani, M.; et al. Meat Intake and Risk of Stomach and Esophageal Adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2006, 98, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Marrelli, D.; Pascale, V.; Vindigni, C.; Roviello, F. Frequency of CDH1 germline mutations in gastric carcinoma coming from high- and low-risk areas: Metanalysis and systematic review of the literature. BMC Cancer 2012, 12, 8. [Google Scholar] [CrossRef]
| Markers | DGC | LBC |
|---|---|---|
| Cytokeratin 7 | +/- | + |
| Cytocheratin 20 | -/+ | - |
| GATA3 | - | + |
| CDX2 | +/- | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corso, G.; Montagna, G.; Figueiredo, J.; La Vecchia, C.; Fumagalli Romario, U.; Fernandes, M.S.; Seixas, S.; Roviello, F.; Trovato, C.; Guerini-Rocco, E.; et al. Hereditary Gastric and Breast Cancer Syndromes Related to CDH1 Germline Mutation: A Multidisciplinary Clinical Review. Cancers 2020, 12, 1598. https://doi.org/10.3390/cancers12061598
Corso G, Montagna G, Figueiredo J, La Vecchia C, Fumagalli Romario U, Fernandes MS, Seixas S, Roviello F, Trovato C, Guerini-Rocco E, et al. Hereditary Gastric and Breast Cancer Syndromes Related to CDH1 Germline Mutation: A Multidisciplinary Clinical Review. Cancers. 2020; 12(6):1598. https://doi.org/10.3390/cancers12061598
Chicago/Turabian StyleCorso, Giovanni, Giacomo Montagna, Joana Figueiredo, Carlo La Vecchia, Uberto Fumagalli Romario, Maria Sofia Fernandes, Susana Seixas, Franco Roviello, Cristina Trovato, Elena Guerini-Rocco, and et al. 2020. "Hereditary Gastric and Breast Cancer Syndromes Related to CDH1 Germline Mutation: A Multidisciplinary Clinical Review" Cancers 12, no. 6: 1598. https://doi.org/10.3390/cancers12061598
APA StyleCorso, G., Montagna, G., Figueiredo, J., La Vecchia, C., Fumagalli Romario, U., Fernandes, M. S., Seixas, S., Roviello, F., Trovato, C., Guerini-Rocco, E., Fusco, N., Pravettoni, G., Petrocchi, S., Rotili, A., Massari, G., Magnoni, F., De Lorenzi, F., Bottoni, M., Galimberti, V., ... Bonanni, B. (2020). Hereditary Gastric and Breast Cancer Syndromes Related to CDH1 Germline Mutation: A Multidisciplinary Clinical Review. Cancers, 12(6), 1598. https://doi.org/10.3390/cancers12061598













