The Impact of the Ubiquitin System in the Pathogenesis of Squamous Cell Carcinomas
Abstract
1. Introduction
1.1. Molecular Landscape of SCCs
1.2. Overview of the Ubiquitin System
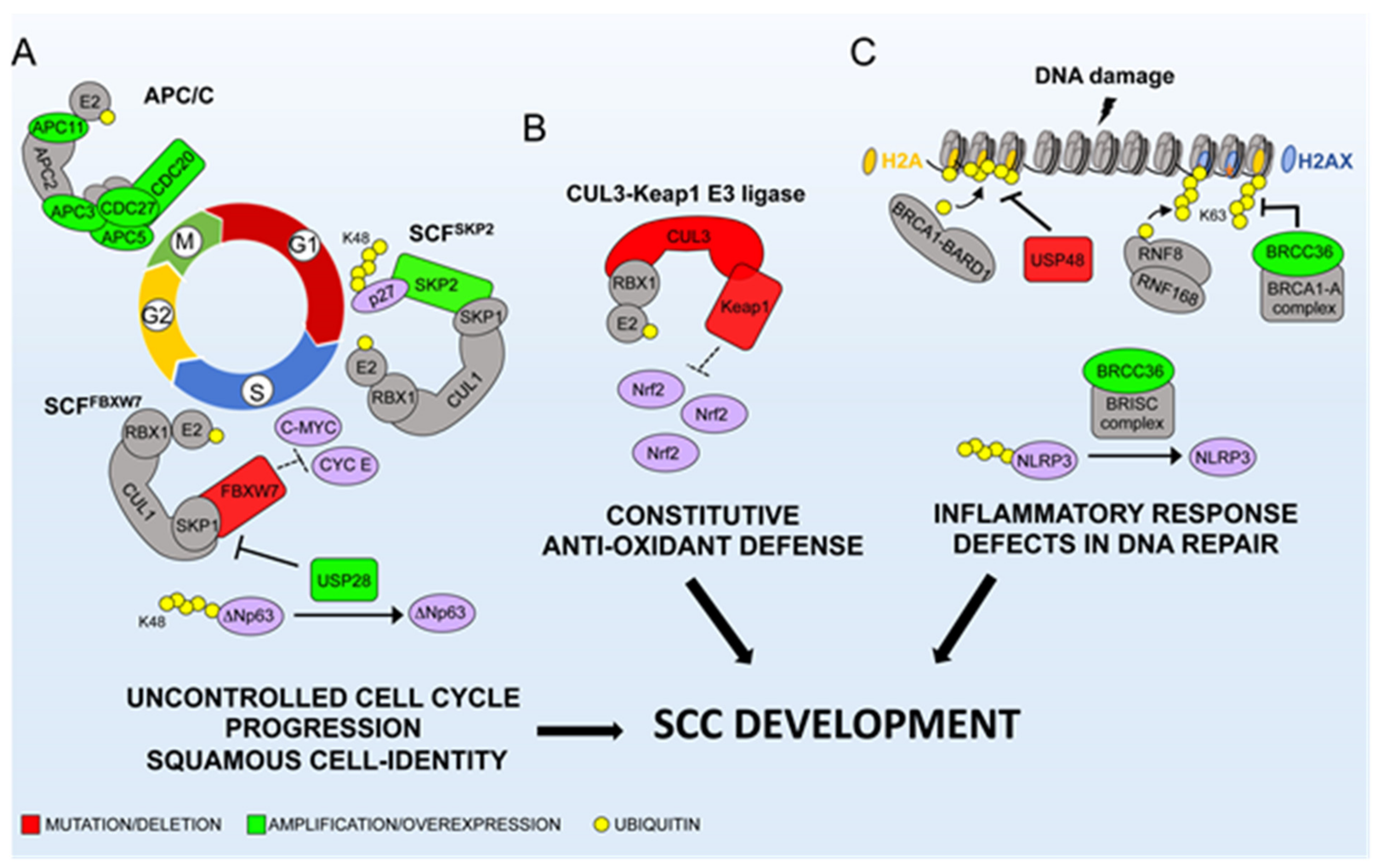
2. CRLs and SCC Etiology
2.1. SCFSKP2: A Canonical Oncogene in SCCs
2.2. Tumor Suppressor Activity of SCFFBXW7 in SCCs
2.3. Keap1/Cul3/Rbx1 E3-Ubiquitin Ligase
3. The APC/C Complex
4. MDM2 and E6AP Restrain p53 in SCCs
5. Additional E3s Involved in SCCs
6. DUBs
6.1. USP22
6.2. USP48 and BRCC3
6.3. USP28
6.4. USP9x
6.5. OTUD3 and USP7: a Two-Way Mode to Dampen PTEN in SCCs?
7. Concluding Remarks and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SCCs | squamous cell carcinomas |
| HNSCCs | squamous cell carcinomas of head and neck |
| LSCCs | lung squamous cell carcinomas |
| ESCCs | esophageal squamous cell carcinomas |
| CvSCCs | cervical squamous cell carcinomas |
| CSCCs | cutaneous squamous cell carcinomas |
| E3s | E3 ubiquitin ligases |
| DUBs | deubiquitinating enzymes |
| CDK | cyclin-dependent kinase |
| EGF-R | epidermal growth factor receptor |
| HPV | human papilloma viruses |
| PI3K | PI3kinase: phosphatidylinositol 3-kinase-Protein Kinase B |
| APC/C | anaphase-promoting complex/cyclosome |
| HDAC1 | histone deacetylases 1 |
| HDAC2 | histone deacetylases 2 |
| FGFR2 | Fibroblast Growth Factor Receptor 2 |
| CSN | copy number variations |
| VEGF | Vascular endothelial growth factor |
| DSB | DNA double-strand break |
| HR | homologous recombination |
| NHEJ | non-homologous end joining |
| BRCA1 | breast cancer type 1 susceptibility protein |
| BARD1 | BRCA1-associated RING domain protein 1 |
| TGFβ | transforming growth factor beta |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Dotto, G.P.; Rustgi, A.K. Squamous cell cancers: A unified perspective on biology and genetics. Cancer Cell 2016, 29, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.G.; Cohen, E.E. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Wuthrick, E.J.; Zhang, Q.; Machtay, M.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Fortin, A.; Silverman, C.L.; Raben, A.; Kim, H.E.; Horwitz, E.M.; et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Cai, X.; Yao, Z.; Huang, J. Progress in targeted therapeutic drugs for oral squamous cell carcinoma. Surg. Oncol. 2019, 31, 90–97. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Minion, L.E.; Tewari, K.S. Cervical cancer—state of the science: From angiogenesis blockade to checkpoint inhibition. Gynecol. Oncol. 2018, 148, 609–621. [Google Scholar] [CrossRef]
- Socinski, M.A.; Obasaju, C.; Gandara, D.; Hirsch, F.R.; Bonomi, P.; Bunn, P.A., Jr.; Kim, E.S.; Langer, C.J.; Natale, R.B.; Novello, S.; et al. Current and emergent therapy options for advanced squamous cell lung cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 165–183. [Google Scholar] [CrossRef]
- McCusker, M.G.; Orkoulas-Razis, D.; Mehra, R. Potential of pembrolizumab in metastatic or recurrent head and neck cancer: Evidence to date. OncoTargets Ther. 2020, 13, 3047–3059. [Google Scholar] [CrossRef]
- Campbell, J.D.; Yau, C.; Bowlby, R.; Liu, Y.; Brennan, K.; Fan, H.; Taylor, A.M.; Wang, C.; Walter, V.; Akbani, R.; et al. Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep. 2018, 23, 194–212. [Google Scholar] [CrossRef]
- Agrawal, N.; Jiao, Y.; Bettegowda, C.; Hutfless, S.M.; Wang, Y.; David, S.; Cheng, Y.; Twaddell, W.S.; Latt, N.L.; Shin, E.J.; et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2012, 2, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.B.; Chen, Z.L.; Li, J.G.; Hu, X.D.; Shi, X.J.; Sun, Z.M.; Zhang, F.; Zhao, Z.R.; Li, Z.T.; Liu, Z.Y.; et al. Genetic landscape of esophageal squamous cell carcinoma. Nat. Genet. 2014, 46, 1097–1102. [Google Scholar] [CrossRef]
- Kim, Y.; Hammerman, P.S.; Kim, J.; Yoon, J.A.; Lee, Y.; Sun, J.M.; Wilkerson, M.D.; Pedamallu, C.S.; Cibulskis, K.; Yoo, Y.K.; et al. Integrative and comparative genomic analysis of lung squamous cell carcinomas in east asian patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 121–128. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Baugh, E.H.; Ke, H.; Levine, A.J.; Bonneau, R.A.; Chan, C.S. Why are there hotspot mutations in the tp53 gene in human cancers? Cell Death Differ. 2018, 25, 154–160. [Google Scholar] [CrossRef]
- Kim, M.P.; Lozano, G. Mutant p53 partners in crime. Cell Death Differ. 2018, 25, 161–168. [Google Scholar] [CrossRef]
- Kaiser, A.M.; Attardi, L.D. Deconstructing networks of p53-mediated tumor suppression in vivo. Cell Death Differ. 2018, 25, 93–103. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Galbraith, M.D.; Andrysik, Z.; Espinosa, J.M. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2018, 25, 133–143. [Google Scholar] [CrossRef]
- Levine, A.J. Reviewing the future of the p53 field. Cell Death Differ. 2018, 25, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Duong, D.M.; Seyfried, N.T.; Cheng, D.; Xie, Y.; Robert, J.; Rush, J.; Hochstrasser, M.; Finley, D.; Peng, J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 2009, 137, 133–145. [Google Scholar] [CrossRef]
- Spit, M.; Rieser, E.; Walczak, H. Linear ubiquitination at a glance. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef]
- Oikawa, D.; Sato, Y.; Ito, H.; Tokunaga, F. Linear ubiquitin code: Its writer, erasers, decoders, inhibitors, and implications in disorders. Int. J. Mol. Sci. 2020, 21, 3381. [Google Scholar] [CrossRef]
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A.; Heller, H.; Haas, A.L.; Rose, I.A. Proposed role of atp in protein breakdown: Conjugation of protein with multiple chains of the polypeptide of atp-dependent proteolysis. Proc. Natl. Acad. Sci. USA 1980, 77, 1783–1786. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Ciechanover, A. The ubiquitin code in the ubiquitin-proteasome system and autophagy. Trends Biochem. Sci. 2017, 42, 873–886. [Google Scholar] [CrossRef]
- Varshavsky, A. N-degron and c-degron pathways of protein degradation. Proc. Natl. Acad. Sci. USA 2019, 116, 358–366. [Google Scholar] [CrossRef]
- Bernassola, F.; Chillemi, G.; Melino, G. Hect-type e3 ubiquitin ligases in cancer. Trends Biochem. Sci. 2019, 44, 1057–1075. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Qi, J.; Ronai, Z.A. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat. Rev. Cancer 2018, 18, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M.; Peschiaroli, A.; Memmi, E.M.; Zhang, J.; Antonov, A.; Green, D.R.; Barlev, N.A.; Garabadgiu, A.V.; Zhou, P.; Melino, G.; et al. The cul4a-ddb1 e3 ubiquitin ligase complex represses p73 transcriptional activity. Oncogene 2013, 32, 4721–4726. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Busino, L. E3 ubiquitin ligases in b-cell malignancies. Cell. Immunol. 2019, 340, 103905. [Google Scholar] [CrossRef]
- Rotin, D.; Kumar, S. Physiological functions of the hect family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2009, 10, 398–409. [Google Scholar] [CrossRef]
- Petroski, M.D.; Deshaies, R.J. Function and regulation of cullin-ring ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2005, 6, 9–20. [Google Scholar] [CrossRef]
- Lydeard, J.R.; Schulman, B.A.; Harper, J.W. Building and remodelling cullin-ring e3 ubiquitin ligases. EMBO Rep. 2013, 14, 1050–1061. [Google Scholar] [CrossRef]
- Cope, G.A.; Deshaies, R.J. Targeted silencing of jab1/csn5 in human cells downregulates scf activity through reduction of f-box protein levels. BMC Biochem. 2006, 7, 1. [Google Scholar] [CrossRef]
- Duda, D.M.; Borg, L.A.; Scott, D.C.; Hunt, H.W.; Hammel, M.; Schulman, B.A. Structural insights into nedd8 activation of cullin-ring ligases: Conformational control of conjugation. Cell 2008, 134, 995–1006. [Google Scholar] [CrossRef]
- Zhou, C.; Wee, S.; Rhee, E.; Naumann, M.; Dubiel, W.; Wolf, D.A. Fission yeast cop9/signalosome suppresses cullin activity through recruitment of the deubiquitylating enzyme ubp12p. Mol. Cell 2003, 11, 927–938. [Google Scholar] [CrossRef]
- Rao, F.; Lin, H.; Su, Y. Cullin-ring ligase regulation by the cop9 signalosome: Structural mechanisms and new physiologic players. Adv. Exp. Med. Biol. 2020, 1217, 47–60. [Google Scholar] [PubMed]
- Mevissen, T.E.T.; Komander, D. Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 2017, 86, 159–192. [Google Scholar] [CrossRef] [PubMed]
- Clague, M.J.; Urbe, S.; Komander, D. Breaking the chains: Deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 2019, 20, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Beaudenon, S.; Huibregtse, J.M. Hpv e6, e6ap and cervical cancer. BMC Biochem. 2008, 9 (Suppl. S1–S4). [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Hsieh, S.C.; Chen, J.N.; Tsai, M.H.; Chang, C.C. Wwp1 gene is a potential molecular target of human oral cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chang, X.; Lian, S.; Zhu, W. Ww domain-containing e3 ubiquitin protein ligase 1 depletion evokes antitumor effect in cutaneous squamous cell carcinoma by inhibiting signal transducer and activator of transcription 3 signaling pathway. J. Int. Med Res. 2018, 46, 2898–2912. [Google Scholar] [CrossRef]
- Ge, Z.; Leighton, J.S.; Wang, Y.; Peng, X.; Chen, Z.; Chen, H.; Sun, Y.; Yao, F.; Li, J.; Zhang, H.; et al. Integrated genomic analysis of the ubiquitin pathway across cancer types. Cell Rep. 2018, 23, 213–226. [Google Scholar] [CrossRef]
- Sawada, R.; Maehara, R.; Oshikiri, T.; Nakamura, T.; Itoh, T.; Kodama, Y.; Kakeji, Y.; Zen, Y. Mdm2 copy number increase: A poor prognostic, molecular event in esophageal squamous cell carcinoma. Human Pathol. 2019, 89, 1–9. [Google Scholar] [CrossRef]
- Okamoto, H.; Fujishima, F.; Kamei, T.; Nakamura, Y.; Ozawa, Y.; Miyata, G.; Nakano, T.; Katsura, K.; Abe, S.; Taniyama, Y.; et al. Murine double minute 2 predicts response of advanced esophageal squamous cell carcinoma to definitive chemoradiotherapy. BMC Cancer 2015, 15, 208. [Google Scholar] [CrossRef]
- Xiao, F.K.; Guo, S.; Yang, F.; Zhao, L.S.; Wang, L.D. Mdm2 and its functional polymorphism snp309 contribute to the development of esophageal carcinoma. J. Gene Med. 2019, 21, e3086. [Google Scholar] [CrossRef]
- Xiao, Z.X.; Chen, J.; Levine, A.J.; Modjtahedi, N.; Xing, J.; Sellers, W.R.; Livingston, D.M. Interaction between the retinoblastoma protein and the oncoprotein mdm2. Nature 1995, 375, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dolloff, N.G.; El-Deiry, W.S. Erk and mdm2 prey on foxo3a. Nat. Cell Biol. 2008, 10, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Zong, C.S.; Xia, W.; Wei, Y.; Ali-Seyed, M.; Li, Z.; Broglio, K.; Berry, D.A.; Hung, M.C. Mdm2 promotes cell motility and invasiveness by regulating e-cadherin degradation. Mol. Cell. Biol. 2006, 26, 7269–7282. [Google Scholar] [CrossRef]
- Horn, E.J.; Albor, A.; Liu, Y.; El-Hizawi, S.; Vanderbeek, G.E.; Babcock, M.; Bowden, G.T.; Hennings, H.; Lozano, G.; Weinberg, W.C.; et al. Ring protein trim32 associated with skin carcinogenesis has anti-apoptotic and e3-ubiquitin ligase properties. Carcinogenesis 2004, 25, 157–167. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Wang, X.L.; Ly, P.; Belyi, V.; Xu-Monette, Z.Y.; Young, K.H.; Hu, W.; Feng, Z. E3 ubiquitin ligase trim32 negatively regulates tumor suppressor p53 to promote tumorigenesis. Cell Death Differ. 2014, 21, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, Z.; Chen, J.; Hu, X. Expression and the potential functions of trim32 in lung cancer tumorigenesis. J. Cell. Biochem. 2019, 120, 5232–5243. [Google Scholar] [CrossRef]
- Luo, Q.; Wu, X.; Nan, Y.; Chang, W.; Zhao, P.; Zhang, Y.; Su, D.; Liu, Z. Trim32/usp11 balances arid1a stability and the oncogenic/tumor-suppressive status of squamous cell carcinoma. Cell Rep. 2020, 30, 98–111. [Google Scholar] [CrossRef]
- Frescas, D.; Pagano, M. Deregulated proteolysis by the f-box proteins skp2 and beta-trcp: Tipping the scales of cancer. Nat. Rev. Cancer 2008, 8, 438–449. [Google Scholar] [CrossRef]
- Cai, Z.; Moten, A.; Peng, D.; Hsu, C.C.; Pan, B.S.; Manne, R.; Li, H.Y.; Lin, H.K. The skp2 pathway: A critical target for cancer therapy. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Kitajima, S.; Kudo, Y.; Ogawa, I.; Bashir, T.; Kitagawa, M.; Miyauchi, M.; Pagano, M.; Takata, T. Role of cks1 overexpression in oral squamous cell carcinomas: Cooperation with skp2 in promoting p27 degradation. Am. J. Pathol. 2004, 165, 2147–2155. [Google Scholar] [CrossRef]
- Wang, X.C.; Tian, L.L.; Tian, J.; Jiang, X.Y. Overexpression of skp2 promotes the radiation resistance of esophageal squamous cell carcinoma. Radiat. Res. 2012, 177, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Gstaiger, M.; Jordan, R.; Lim, M.; Catzavelos, C.; Mestan, J.; Slingerland, J.; Krek, W. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl. Acad. Sci. USA 2001, 98, 5043–5048. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Masuda, N.; Nakajima, M.; Fukai, Y.; Miyazaki, T.; Kato, H.; Kuwano, H. Inverse correlation between expression levels of p27 and the ubiquitin ligase subunit skp2 in early esophageal squamous cell carcinoma. Anticancer Res. 2004, 24, 777–783. [Google Scholar] [PubMed]
- Kudo, Y.; Kitajima, S.; Ogawa, I.; Miyauchi, M.; Takata, T. Down-regulation of cdk inhibitor p27 in oral squamous cell carcinoma. Oral Oncol. 2005, 41, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Kitajima, S.; Sato, S.; Miyauchi, M.; Ogawa, I.; Takata, T. High expression of s-phase kinase-interacting protein 2, human f-box protein, correlates with poor prognosis in oral squamous cell carcinomas. Cancer Res. 2001, 61, 7044–7047. [Google Scholar]
- Qiu, L.; Lv, J.; Chen, Y.; Wang, J.; Wu, R. Expression of skp2 and p27(kip1) proteins in hypopharyngeal squamous cell carcinoma and its clinical significance. Oncol. Lett. 2015, 10, 3756–3760. [Google Scholar] [CrossRef]
- Liang, Y.; Hou, X.; Cui, Q.; Kang, T.B.; Fu, J.H.; Zhang, L.J.; Luo, R.Z.; He, J.H.; Zeng, Y.X.; Yang, H.X. Skp2 expression unfavorably impacts survival in resectable esophageal squamous cell carcinoma. J. Transl. Med. 2012, 10, 73. [Google Scholar] [CrossRef]
- Naganawa, Y.; Ishiguro, H.; Kuwabara, Y.; Kimura, M.; Mitsui, A.; Katada, T.; Tanaka, T.; Shiozaki, M.; Fujii, Y.; Takeyama, H. Decreased expression of fbxw7 is correlated with poor prognosis in patients with esophageal squamous cell carcinoma. Exp. Ther. Med. 2010, 1, 841–846. [Google Scholar] [CrossRef]
- Yokobori, T.; Mimori, K.; Iwatsuki, M.; Ishii, H.; Tanaka, F.; Sato, T.; Toh, H.; Sudo, T.; Iwaya, T.; Tanaka, Y.; et al. Copy number loss of fbxw7 is related to gene expression and poor prognosis in esophageal squamous cell carcinoma. Int. J. Oncol. 2012, 41, 253–259. [Google Scholar]
- Wang, L.; Ye, X.; Liu, Y.; Wei, W.; Wang, Z. Aberrant regulation of fbw7 in cancer. Oncotarget 2014, 5, 2000–2015. [Google Scholar] [CrossRef]
- Choi, M.; Kadara, H.; Zhang, J.; Parra, E.R.; Rodriguez-Canales, J.; Gaffney, S.G.; Zhao, Z.; Behrens, C.; Fujimoto, J.; Chow, C.; et al. Mutation profiles in early-stage lung squamous cell carcinoma with clinical follow-up and correlation with markers of immune function. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gombodorj, N.; Yokobori, T.; Tanaka, N.; Suzuki, S.; Kuriyama, K.; Kumakura, Y.; Yoshida, T.; Sakai, M.; Sohda, M.; Baatar, S.; et al. Correlation between high fbxw7 expression in pretreatment biopsy specimens and good response to chemoradiation therapy in patients with locally advanced esophageal cancer: A retrospective study. J. Surg. Oncol. 2018, 118, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.H.; Perez-Losada, J.; Wu, D.; Delrosario, R.; Tsunematsu, R.; Nakayama, K.I.; Brown, K.; Bryson, S.; Balmain, A. Fbxw7/cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 2004, 432, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Sailo, B.L.; Banik, K.; Girisa, S.; Bordoloi, D.; Fan, L.; Halim, C.E.; Wang, H.; Kumar, A.P.; Zheng, D.; Mao, X.; et al. Fbxw7 in cancer: What has been unraveled thus far? Cancers 2019, 11, 246. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Hosogane, M.; Okuyama, R.; Aoyama, S.; Onoyama, I.; Nakayama, K.I.; Nakayama, K. Opposing functions of fbxw7 in keratinocyte growth, differentiation and skin tumorigenesis mediated through negative regulation of c-myc and notch. Oncogene 2013, 32, 1921–1932. [Google Scholar] [CrossRef]
- Ma, J.Q.; Tuersun, H.; Jiao, S.J.; Zheng, J.H.; Xiao, J.B.; Hasim, A. Functional role of nrf2 in cervical carcinogenesis. PLoS ONE 2015, 10, e0133876. [Google Scholar] [CrossRef]
- Jeong, Y.; Hoang, N.T.; Lovejoy, A.; Stehr, H.; Newman, A.M.; Gentles, A.J.; Kong, W.; Truong, D.; Martin, S.; Chaudhuri, A.; et al. Role of keap1/nrf2 and tp53 mutations in lung squamous cell carcinoma development and radiation resistance. Cancer Discov. 2017, 7, 86–101. [Google Scholar] [CrossRef]
- Rolfs, F.; Huber, M.; Kuehne, A.; Kramer, S.; Haertel, E.; Muzumdar, S.; Wagner, J.; Tanner, Y.; Bohm, F.; Smola, S.; et al. Nrf2 activation promotes keratinocyte survival during early skin carcinogenesis via metabolic alterations. Cancer Res. 2015, 75, 4817–4829. [Google Scholar] [CrossRef]
- Namani, A.; Matiur Rahaman, M.; Chen, M.; Tang, X. Gene-expression signature regulated by the keap1-nrf2-cul3 axis is associated with a poor prognosis in head and neck squamous cell cancer. BMC Cancer 2018, 18, 46. [Google Scholar] [CrossRef]
- Kim, Y.R.; Oh, J.E.; Kim, M.S.; Kang, M.R.; Park, S.W.; Han, J.Y.; Eom, H.S.; Yoo, N.J.; Lee, S.H. Oncogenic nrf2 mutations in squamous cell carcinomas of oesophagus and skin. J. Pathol. 2010, 220, 446–451. [Google Scholar] [CrossRef]
- Jeong, Y.; Hellyer, J.A.; Stehr, H.; Hoang, N.T.; Niu, X.; Das, M.; Padda, S.K.; Ramchandran, K.; Neal, J.W.; Wakelee, H.; et al. Role of keap1/nfe2l2 mutations in the chemotherapeutic response of patients with non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Mondal, G.; Sengupta, S.; Panda, C.K.; Gollin, S.M.; Saunders, W.S.; Roychoudhury, S. Overexpression of cdc20 leads to impairment of the spindle assembly checkpoint and aneuploidization in oral cancer. Carcinogenesis 2007, 28, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Wang, J.; Bao, L.; Wang, L.; Huo, J.; Wang, X. Global analysis of chromosome 1 genes among patients with lung adenocarcinoma, squamous carcinoma, large-cell carcinoma, small-cell carcinoma, or non-cancer. Cancer Metastasis Rev. 2015, 34, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, J.W.; Lee, J.H.; Kim, Y.S. Mad2 and cdc20 are upregulated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the uterine cervix. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2014, 33, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hu, T.; Liang, Y.; Li, P.; Chen, X.; Zhang, J.; Ma, Y.; Hao, Q.; Wang, J.; Zhang, P.; et al. Neddylation inhibition activates the extrinsic apoptosis pathway through atf4-chop-dr5 axis in human esophageal cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4145–4157. [Google Scholar] [CrossRef]
- McHugh, A.; Fernandes, K.; Chinner, N.; Ibrahim, A.F.M.; Garg, A.K.; Boag, G.; Hepburn, L.A.; Proby, C.M.; Leigh, I.M.; Saville, M.K. The identification of potential therapeutic targets for cutaneous squamous cell carcinoma. J. Investig. Dermatol. 2019, 140, 1154–1165. [Google Scholar] [CrossRef]
- Vanderdys, V.; Allak, A.; Guessous, F.; Benamar, M.; Read, P.W.; Jameson, M.J.; Abbas, T. The neddylation inhibitor pevonedistat (mln4924) suppresses and radiosensitizes head and neck squamous carcinoma cells and tumors. Mol. Cancer Ther. 2018, 17, 368–380. [Google Scholar] [CrossRef]
- Uddin, S.; Bhat, A.A.; Krishnankutty, R.; Mir, F.; Kulinski, M.; Mohammad, R.M. Involvement of f-box proteins in progression and development of human malignancies. Semin. Cancer Biol. 2016, 36, 18–32. [Google Scholar] [CrossRef]
- Masumoto, K.; Kitagawa, M. E3 ubiquitin ligases as molecular targets in human oral cancers. Curr. Cancer Drug Targets 2016, 16, 130–135. [Google Scholar] [CrossRef]
- Nakayama, K.; Nagahama, H.; Minamishima, Y.A.; Miyake, S.; Ishida, N.; Hatakeyama, S.; Kitagawa, M.; Iemura, S.; Natsume, T.; Nakayama, K.I. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 2004, 6, 661–672. [Google Scholar] [CrossRef]
- Carrano, A.C.; Eytan, E.; Hershko, A.; Pagano, M. Skp2 is required for ubiquitin-mediated degradation of the cdk inhibitor p27. Nat. Cell Biol. 1999, 1, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ganoth, D.; Bornstein, G.; Ko, T.K.; Larsen, B.; Tyers, M.; Pagano, M.; Hershko, A. The cell-cycle regulatory protein cks1 is required for scf(skp2)-mediated ubiquitinylation of p27. Nat. Cell Biol. 2001, 3, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Pagano, M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. Cancer Biol. 2003, 13, 41–47. [Google Scholar] [CrossRef]
- Loda, M.; Cukor, B.; Tam, S.W.; Lavin, P.; Fiorentino, M.; Draetta, G.F.; Jessup, J.M.; Pagano, M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 1997, 3, 231–234. [Google Scholar] [CrossRef]
- Lin, L.H.; Chang, K.W.; Cheng, H.W.; Liu, C.J. Smad4 somatic mutations in head and neck carcinoma are associated with tumor progression. Front. Oncol. 2019, 9, 1379. [Google Scholar] [CrossRef]
- Martin, D.; Abba, M.C.; Molinolo, A.A.; Vitale-Cross, L.; Wang, Z.; Zaida, M.; Delic, N.C.; Samuels, Y.; Lyons, J.G.; Gutkind, J.S. The head and neck cancer cell oncogenome: A platform for the development of precision molecular therapies. Oncotarget 2014, 5, 8906–8923. [Google Scholar] [CrossRef]
- Clement, E.; Inuzuka, H.; Nihira, N.T.; Wei, W.; Toker, A. Skp2-dependent reactivation of akt drives resistance to pi3k inhibitors. Sci. Signal. 2018, 11, eaao3810. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The pi3k-akt network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. Akt/pkb signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Lin, H.K.; Wang, G.; Chen, Z.; Teruya-Feldstein, J.; Liu, Y.; Chan, C.H.; Yang, W.L.; Erdjument-Bromage, H.; Nakayama, K.I.; Nimer, S.; et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of skp2 by akt/pkb. Nat. Cell Biol. 2009, 11, 420–432. [Google Scholar] [CrossRef]
- Nogueira, V.; Sundararajan, D.; Kwan, J.M.; Peng, X.D.; Sarvepalli, N.; Sonenberg, N.; Hay, N. Akt-dependent skp2 mrna translation is required for exiting contact inhibition, oncogenesis, and adipogenesis. EMBO J. 2012, 31, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.; Saur, D.; Hamacher, R.; Schmid, R.M.; Schneider, G. Phosphoinositide-3-kinase signaling controls s-phase kinase-associated protein 2 transcription via e2f1 in pancreatic ductal adenocarcinoma cells. Cancer Res. 2007, 67, 4149–4156. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of yap/taz in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Furth, N.; Aylon, Y.; Oren, M. P53 shades of hippo. Cell Death Differ. 2018, 25, 81–92. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhu, Y.; Yuan, C.; Wang, D.; Zhang, W.; Qi, B.; Qiu, J.; Song, X.; Ye, J.; et al. The hippo transducer taz promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 2015, 9, 1091–1105. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. Yap/taz at the roots of cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Liu, Q.; Li, Y.; Sun, X.; Hong, L.; Ji, S.; Liu, C.; Geng, J.; Zhang, W.; et al. Hippo signaling suppresses cell ploidy and tumorigenesis through skp2. Cancer Cell 2017, 31, 669–684. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Maglic, D.; Dill, M.T.; Mojumdar, K.; Ng, P.K.; Jeong, K.J.; Tsang, Y.H.; Moreno, D.; Bhavana, V.H.; et al. Comprehensive molecular characterization of the hippo signaling pathway in cancer. Cell Rep. 2018, 25, 1304–1317. [Google Scholar] [CrossRef]
- Saladi, S.V.; Ross, K.; Karaayvaz, M.; Tata, P.R.; Mou, H.; Rajagopal, J.; Ramaswamy, S.; Ellisen, L.W. Actl6a is co-amplified with p63 in squamous cell carcinoma to drive yap activation, regenerative proliferation, and poor prognosis. Cancer Cell 2017, 31, 35–49. [Google Scholar] [CrossRef]
- Tanaka, N.; Zhao, M.; Tang, L.; Patel, A.A.; Xi, Q.; Van, H.T.; Takahashi, H.; Osman, A.A.; Zhang, J.; Wang, J.; et al. Gain-of-function mutant p53 promotes the oncogenic potential of head and neck squamous cell carcinoma cells by targeting the transcription factors foxo3a and foxm1. Oncogene 2018, 37, 1279–1292. [Google Scholar] [CrossRef]
- Kurashige, J.; Watanabe, M.; Iwatsuki, M.; Kinoshita, K.; Saito, S.; Hiyoshi, Y.; Kamohara, H.; Baba, Y.; Mimori, K.; Baba, H. Overexpression of microrna-223 regulates the ubiquitin ligase fbxw7 in oesophageal squamous cell carcinoma. Br. J. Cancer 2012, 106, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Z.; Wang, K.P.; Song, H.J.; Xia, J.H.; Jiang, Y.; Wang, Y.L. Mir-27a-3p promotes esophageal cancer cell proliferation via f-box and wd repeat domain-containing 7 (fbxw7) suppression. Int. J. Clin. Exp. Med. 2015, 8, 15556–15562. [Google Scholar] [PubMed]
- Zhao, J.; Hu, C.; Chi, J.; Li, J.; Peng, C.; Yun, X.; Li, D.; Yu, Y.; Li, Y.; Gao, M.; et al. Mir-24 promotes the proliferation, migration and invasion in human tongue squamous cell carcinoma by targeting fbxw7. Oncol. Rep. 2016, 36, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- De Blasio, C.; Zonfrilli, A.; Franchitto, M.; Mariano, G.; Cialfi, S.; Verma, N.; Checquolo, S.; Bellavia, D.; Palermo, R.; Benelli, D.; et al. Plk1 targets notch1 during DNA damage and mitotic progression. J. Biol. Chem. 2019, 294, 17941–17950. [Google Scholar] [CrossRef]
- Cialfi, S.; Palermo, R.; Manca, S.; De Blasio, C.; Vargas Romero, P.; Checquolo, S.; Bellavia, D.; Uccelletti, D.; Saliola, M.; D’Alessandro, A.; et al. Loss of notch1-dependent p21(waf1/cip1) expression influences the notch1 outcome in tumorigenesis. Cell Cycle 2014, 13, 2046–2055. [Google Scholar] [CrossRef]
- Compagnone, M.; Gatti, V.; Presutti, D.; Ruberti, G.; Fierro, C.; Markert, E.K.; Vousden, K.H.; Zhou, H.; Mauriello, A.; Anemone, L.; et al. Deltanp63-mediated regulation of hyaluronic acid metabolism and signaling supports hnscc tumorigenesis. Proc. Natl. Acad. Sci. USA 2017, 114, 13254–13259. [Google Scholar] [CrossRef] [PubMed]
- Gatti, V.; Fierro, C.; Annicchiarico-Petruzzelli, M.; Melino, G.; Peschiaroli, A. Deltanp63 in squamous cell carcinoma: Defining the oncogenic routes affecting epigenetic landscape and tumour microenvironment. Mol. Oncol. 2019, 13, 981–1001. [Google Scholar] [CrossRef]
- Gatti, V.; Bongiorno-Borbone, L.; Fierro, C.; Annicchiarico-Petruzzelli, M.; Melino, G.; Peschiaroli, A. P63 at the crossroads between stemness and metastasis in breast cancer. Int. J. Mol. Sci. 2019, 20, 2683. [Google Scholar] [CrossRef]
- Gatti, V.; Fierro, C.; Compagnone, M.; Giangrazi, F.; Markert, E.K.; Bongiorno-Borbone, L.; Melino, G.; Peschiaroli, A. Deltanp63 regulates the expression of hyaluronic acid-related genes in breast cancer cells. Oncogenesis 2018, 7, 65. [Google Scholar] [CrossRef]
- Regina, C.; Compagnone, M.; Peschiaroli, A.; Lena, A.; Annicchiarico-Petruzzelli, M.; Piro, M.C.; Melino, G.; Candi, E. Setdb1, a novel interactor of deltanp63, is involved in breast tumorigenesis. Oncotarget 2016, 7, 28836–28848. [Google Scholar] [CrossRef]
- Napoli, M.; Venkatanarayan, A.; Raulji, P.; Meyers, B.A.; Norton, W.; Mangala, L.S.; Sood, A.K.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Vin, H.; et al. Deltanp63/dgcr8-dependent micrornas mediate therapeutic efficacy of hdac inhibitors in cancer. Cancer Cell 2016, 29, 874–888. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, M.; Hu, B.; Windle, B.; Ge, X.; Li, G.; Sun, Y. Sorting nexin 5 controls head and neck squamous cell carcinoma progression by modulating fbw7. J. Cancer 2019, 10, 2942–2952. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Torres-Lockhart, K.; Forster, N.; Ramakrishnan, S.; Greninger, P.; Garnett, M.J.; McDermott, U.; Rothenberg, S.M.; Benes, C.H.; Ellisen, L.W. Mcl-1 and fbw7 control a dominant survival pathway underlying hdac and bcl-2 inhibitor synergy in squamous cell carcinoma. Cancer Discov. 2013, 3, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Pentimalli, F. Bcl2: A 30-year tale of life, death and much more to come. Cell Death Differ. 2018, 25, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Letai, A. Why do bcl-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The keap1-nrf2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the keap1-nrf2 pathway. Mol. Cell. Biol. 2020, 40. [Google Scholar] [CrossRef]
- Menegon, S.; Columbano, A.; Giordano, S. The dual roles of nrf2 in cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef]
- Ramos-Gomez, M.; Kwak, M.K.; Dolan, P.M.; Itoh, K.; Yamamoto, M.; Talalay, P.; Kensler, T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 3410–3415. [Google Scholar] [CrossRef]
- Xu, C.; Huang, M.T.; Shen, G.; Yuan, X.; Lin, W.; Khor, T.O.; Conney, A.H.; Kong, A.N. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in c57bl/6 mice by sulforaphane is mediated by nuclear factor e2-related factor 2. Cancer Res. 2006, 66, 8293–8296. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced nrf2 transcription promotes ros detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Jonsson, M.; Lauss, M.; Brunnstrom, H.; Jonsson, P.; Borg, A.; Jonsson, G.; Ringner, M.; Planck, M.; Staaf, J. Genome-wide DNA methylation analysis of lung carcinoma reveals one neuroendocrine and four adenocarcinoma epitypes associated with patient outcome. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 6127–6140. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. Keap1 recruits neh2 through binding to etge and dlg motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 2006, 26, 2887–2900. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Zhang, W.; Gross, A.M.; Von Dollen, J.; Johnson, J.R.; Franks-Skiba, K.E.; Swaney, D.L.; Johnson, T.L.; Jang, G.M.; Shah, P.S.; et al. Multiple routes to oncogenesis are promoted by the human papillomavirus-host protein network. Cancer Discov. 2018, 8, 1474–1489. [Google Scholar] [CrossRef] [PubMed]
- Reshmi, S.C.; Gollin, S.M. Chromosomal instability in oral cancer cells. J. Dent. Res. 2005, 84, 107–117. [Google Scholar] [CrossRef]
- Reing, J.E.; Gollin, S.M.; Saunders, W.S. The occurrence of chromosome segregational defects is an intrinsic and heritable property of oral squamous cell carcinoma cell lines. Cancer Genet. Cytogenet. 2004, 150, 57–61. [Google Scholar] [CrossRef]
- Thirthagiri, E.; Robinson, C.M.; Huntley, S.; Davies, M.; Yap, L.F.; Prime, S.S.; Paterson, I.C. Spindle assembly checkpoint and centrosome abnormalities in oral cancer. Cancer Lett. 2007, 258, 276–285. [Google Scholar] [CrossRef]
- Gisselsson, D.; Jonson, T.; Yu, C.; Martins, C.; Mandahl, N.; Wiegant, J.; Jin, Y.; Mertens, F.; Jin, C. Centrosomal abnormalities, multipolar mitoses, and chromosomal instability in head and neck tumours with dysfunctional telomeres. Br. J. Cancer 2002, 87, 202–207. [Google Scholar] [CrossRef]
- Biswas, N.K.; Das, C.; Das, S.; Maitra, A.; Nair, S.; Gupta, T.; D’Cruz, A.K.; Sarin, R.; Majumder, P.P. Lymph node metastasis in oral cancer is strongly associated with chromosomal instability and DNA repair defects. Int. J. Cancer 2019, 145, 2568–2579. [Google Scholar] [CrossRef]
- Schrock, M.S.; Stromberg, B.R.; Scarberry, L.; Summers, M.K. Apc/c ubiquitin ligase: Functions and mechanisms in tumorigenesis. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kapanidou, M.; Curtis, N.L.; Bolanos-Garcia, V.M. Cdc20: At the crossroads between chromosome segregation and mitotic exit. Trends Biochem. Sci. 2017, 42, 193–205. [Google Scholar] [CrossRef]
- Moura, I.M.; Delgado, M.L.; Silva, P.M.; Lopes, C.A.; do Amaral, J.B.; Monteiro, L.S.; Bousbaa, H. High cdc20 expression is associated with poor prognosis in oral squamous cell carcinoma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2014, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, P. The function of apc/ccdh1 in cell cycle and beyond. Cell Div. 2009, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Higuera, I.; Manchado, E.; Dubus, P.; Canamero, M.; Mendez, J.; Moreno, S.; Malumbres, M. Genomic stability and tumour suppression by the apc/c cofactor cdh1. Nat. Cell Biol. 2008, 10, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Rokudai, S.; Li, Y.; Otaka, Y.; Fujieda, M.; Owens, D.M.; Christiano, A.M.; Nishiyama, M.; Prives, C. Stxbp4 regulates apc/c-mediated p63 turnover and drives squamous cell carcinogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E4806–E4814. [Google Scholar] [CrossRef]
- Otaka, Y.; Rokudai, S.; Kaira, K.; Fujieda, M.; Horikoshi, I.; Iwakawa-Kawabata, R.; Yoshiyama, S.; Yokobori, T.; Ohtaki, Y.; Shimizu, K.; et al. Stxbp4 drives tumor growth and is associated with poor prognosis through pdgf receptor signaling in lung squamous cell carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3442–3452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peart, M.J.; Prives, C. Stxbp4 regulates deltanp63 stability by suppression of rack1-dependent degradation. Mol. Cell. Biol. 2009, 29, 3953–3963. [Google Scholar] [CrossRef]
- Karni-Schmidt, O.; Lokshin, M.; Prives, C. The roles of mdm2 and mdmx in cancer. Ann. Rev. Pathol. 2016, 11, 617–644. [Google Scholar] [CrossRef]
- Wu, D.; Prives, C. Relevance of the p53-mdm2 axis to aging. Cell Death Differ. 2018, 25, 169–179. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Yarbrough, W.G. Arf promotes mdm2 degradation and stabilizes p53: Arf-ink4a locus deletion impairs both the rb and p53 tumor suppression pathways. Cell 1998, 92, 725–734. [Google Scholar] [CrossRef]
- Pectasides, E.; Rampias, T.; Sasaki, C.; Perisanidis, C.; Kouloulias, V.; Burtness, B.; Zaramboukas, T.; Rimm, D.; Fountzilas, G.; Psyrri, A. Markers of epithelial to mesenchymal transition in association with survival in head and neck squamous cell carcinoma (hnscc). PLoS ONE 2014, 9, e94273. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.F.; Zullig, L.; Soltermann, A.; Roessle, M.; Graf, N.; Haerle, S.K.; Studer, G.; Jochum, W.; Moch, H.; Stoeckli, S.J. Down regulation of e-cadherin (ecad)—a predictor for occult metastatic disease in sentinel node biopsy of early squamous cell carcinomas of the oral cavity and oropharynx. BMC Cancer 2011, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, S.; Batra, M.; Abidullah, M.; Bhuvinder, S.; Katragadda, P. Role of e-cadherin in progression of oral squamous cell carcinoma: A retrospective immunohistochemical study. J. Contemp. Dent. Pract. 2018, 19, 1105–1110. [Google Scholar]
- Nardi, C.E.; Dedivitis, R.A.; Camillo de Almeida, R.; de Matos, L.L.; Cernea, C.R. The role of e-cadherin and beta-catenin in laryngeal cancer. Oncotarget 2018, 9, 30199–30209. [Google Scholar] [CrossRef][Green Version]
- Reymond, A.; Meroni, G.; Fantozzi, A.; Merla, G.; Cairo, S.; Luzi, L.; Riganelli, D.; Zanaria, E.; Messali, S.; Cainarca, S.; et al. The tripartite motif family identifies cell compartments. EMBO J. 2001, 20, 2140–2151. [Google Scholar] [CrossRef]
- Lai, K.P.; Chen, J.; Tse, W.K.F. Role of deubiquitinases in human cancers: Potential targeted therapy. Int. J. Mol. Sci. 2020, 21, 2548. [Google Scholar] [CrossRef]
- Jeusset, L.M.; McManus, K.J. Ubiquitin specific peptidase 22 regulates histone h2b mono-ubiquitination and exhibits both oncogenic and tumor suppressor roles in cancer. Cancers 2017, 9, 167. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, Y. Usp22 nuclear expression is significantly associated with progression and unfavorable clinical outcome in human esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2012, 138, 1291–1297. [Google Scholar] [CrossRef]
- Liu, T.; Liu, J.; Chen, Q.; Jin, S.; Mi, S.; Shao, W.; Kudo, Y.; Zeng, S.; Qi, G. Expression of usp22 and the chromosomal passenger complex is an indicator of malignant progression in oral squamous cell carcinoma. Oncol. Lett. 2019, 17, 2040–2046. [Google Scholar] [CrossRef]
- Dou, Y.; Lin, J.; Shu, H.; Jiang, N. Role of ubiquitin-specific peptidase 22 in carcinogenesis of human pharyngeal squamous cell carcinoma. Mol. Med. Rep. 2014, 10, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Densham, R.M.; Garvin, A.J.; Stone, H.R.; Strachan, J.; Baldock, R.A.; Daza-Martin, M.; Fletcher, A.; Blair-Reid, S.; Beesley, J.; Johal, B.; et al. Human brca1-bard1 ubiquitin ligase activity counteracts chromatin barriers to DNA resection. Nat. Struct. Mol. Boil. 2016, 23, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Xu, B.; Qu, C.; Tao, Y.; Chen, C.; Hua, W.; Feng, G.; Chang, H.; Liu, Z.; Li, G.; et al. Brcc3 acts as a prognostic marker in nasopharyngeal carcinoma patients treated with radiotherapy and mediates radiation resistance in vitro. Radiat. Oncol. 2015, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Zhang, L.; Yang, Z.; Chen, X.; Luo, J.; Zhou, Z.; Mei, X.; Yu, X.; Shao, Z.; et al. Targeting deubiquitinase usp28 for cancer therapy. Cell Death Dis. 2018, 9, 186. [Google Scholar] [CrossRef]
- Prieto-Garcia, C.; Hartmann, O.; Reissland, M.; Braun, F.; Fischer, T.; Walz, S.; Schulein-Volk, C.; Eilers, U.; Ade, C.P.; Calzado, M.A.; et al. Maintaining protein stability of np63 via usp28 is required by squamous cancer cells. EMBO Mol. Med. 2020, 12, e11101. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Liu, W.; He, X.; Cui, L.; Chen, X.; Yang, M.; Liu, H.; Wei, W.; Liu, S.; et al. Usp9x expression correlates with tumor progression and poor prognosis in esophageal squamous cell carcinoma. Diagn. Pathol. 2013, 8, 177. [Google Scholar] [CrossRef]
- Wei, B.; Xu, L.; Hui, H.; Sun, Y.; Wu, J. Usp9x mrna expression predicts clinical outcome for esophageal squamous cell carcinoma treated with cisplatin-based therapy. Clin. Res. Hepatol. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Nanayakkara, D.M.; Nguyen, M.N.; Wood, S.A. Deubiquitylating enzyme, usp9x, regulates proliferation of cells of head and neck cancer lines. Cell Prolif. 2016, 49, 494–502. [Google Scholar] [CrossRef]
- India Project Team of the International Cancer Genome Consortium. Mutational landscape of gingivo-buccal oral squamous cell carcinoma reveals new recurrently-mutated genes and molecular subgroups. Nat. Commun. 2013, 4, 2873. [Google Scholar] [CrossRef]
- Fadlullah, M.Z.; Chiang, I.K.; Dionne, K.R.; Yee, P.S.; Gan, C.P.; Sam, K.K.; Tiong, K.H.; Ng, A.K.; Martin, D.; Lim, K.P.; et al. Genetically-defined novel oral squamous cell carcinoma cell lines for the development of molecular therapies. Oncotarget 2016, 7, 27802–27818. [Google Scholar] [CrossRef]
- Perez-Mancera, P.A.; Rust, A.G.; van der Weyden, L.; Kristiansen, G.; Li, A.; Sarver, A.L.; Silverstein, K.A.; Grutzmann, R.; Aust, D.; Rummele, P.; et al. The deubiquitinase usp9x suppresses pancreatic ductal adenocarcinoma. Nature 2012, 486, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, J.; Sha, B.; Li, M.; Wang, L.; Zhang, Y.; Liu, X.; Dong, Z.; Liu, Z.; Li, P.; et al. Targeting the overexpressed usp7 inhibits esophageal squamous cell carcinoma cell growth by inducing noxa-mediated apoptosis. Mol. Carcinog. 2019, 58, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Chen, D.S.; Li, H.; Liu, W.W.; Han, G.Y.; Han, Y.F. Clinical significance of usp7 and ezh2 in predicting prognosis of laryngeal squamous cell carcinoma and their possible functional mechanism. Int. J. Clin. Exp. Pathol. 2019, 12, 2184–2194. [Google Scholar] [PubMed]
- Zhao, G.Y.; Lin, Z.W.; Lu, C.L.; Gu, J.; Yuan, Y.F.; Xu, F.K.; Liu, R.H.; Ge, D.; Ding, J.Y. Usp7 overexpression predicts a poor prognosis in lung squamous cell carcinoma and large cell carcinoma. Tumor Boil. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 1721–1729. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Varthi, M.; Sykes, S.M.; Phillips, C.; Warzecha, C.; Zhu, W.; Wyce, A.; Thorne, A.W.; Berger, S.L.; McMahon, S.B. The putative cancer stem cell marker usp22 is a subunit of the human saga complex required for activated transcription and cell-cycle progression. Mol. Cell 2008, 29, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Koutelou, E.; Hirsch, C.L.; Dent, S.Y. Multiple faces of the saga complex. Curr. Opin. Cell Boil. 2010, 22, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; Clifton-Bligh, R.; Marsh, D.J. Histone h2b monoubiquitination: Roles to play in human malignancy. Endocr. Relat. Cancer 2015, 22, T19–T33. [Google Scholar] [CrossRef] [PubMed]
- Ao, N.; Liu, Y.; Feng, H.; Bian, X.; Li, Z.; Gu, B.; Zhao, X.; Liu, Y. Ubiquitin-specific peptidase usp22 negatively regulates the stat signaling pathway by deubiquitinating sirt1. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 1863–1875. [Google Scholar] [CrossRef]
- Joukov, V.; Groen, A.C.; Prokhorova, T.; Gerson, R.; White, E.; Rodriguez, A.; Walter, J.C.; Livingston, D.M. The brca1/bard1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell 2006, 127, 539–552. [Google Scholar] [CrossRef]
- Uckelmann, M.; Densham, R.M.; Baas, R.; Winterwerp, H.H.K.; Fish, A.; Sixma, T.K.; Morris, J.R. Usp48 restrains resection by site-specific cleavage of the brca1 ubiquitin mark from h2a. Nat. Commun. 2018, 9, 229. [Google Scholar] [CrossRef]
- Tarsounas, M.; Sung, P. The antitumorigenic roles of brca1-bard1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 2020, 21, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.; Mallery, D.L.; Larkin, C.; Huang, J.T.; Hiom, K. Brca1 is a histone-h2a-specific ubiquitin ligase. Cell Rep. 2014, 8, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Greenberg, R.A. Deciphering the brca1 tumor suppressor network. J. Biol. Chem. 2015, 290, 17724–17732. [Google Scholar] [CrossRef] [PubMed]
- Ochs, F.; Somyajit, K.; Altmeyer, M.; Rask, M.B.; Lukas, J.; Lukas, C. 53bp1 fosters fidelity of homology-directed DNA repair. Nat. Struct. Mol. Boil. 2016, 23, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Kastan, M.B. Repair versus checkpoint functions of brca1 are differentially regulated by site of chromatin binding. Cancer Res. 2015, 75, 2699–2707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kakarougkas, A.; Ismail, A.; Katsuki, Y.; Freire, R.; Shibata, A.; Jeggo, P.A. Co-operation of brca1 and poh1 relieves the barriers posed by 53bp1 and rap80 to resection. Nucleic Acids Res. 2013, 41, 10298–10311. [Google Scholar] [CrossRef]
- Hu, X.; Kim, J.A.; Castillo, A.; Huang, M.; Liu, J.; Wang, B. Nba1/merit40 and bre interaction is required for the integrity of two distinct deubiquitinating enzyme brcc36-containing complexes. J. Biol. Chem. 2011, 286, 11734–11745. [Google Scholar] [CrossRef]
- Uckelmann, M.; Sixma, T.K. Histone ubiquitination in the DNA damage response. DNA Repair 2017, 56, 92–101. [Google Scholar] [CrossRef]
- Dong, Y.; Hakimi, M.A.; Chen, X.; Kumaraswamy, E.; Cooch, N.S.; Godwin, A.K.; Shiekhattar, R. Regulation of brcc, a holoenzyme complex containing brca1 and brca2, by a signalosome-like subunit and its role in DNA repair. Mol. Cell 2003, 12, 1087–1099. [Google Scholar] [CrossRef]
- Rabl, J.; Bunker, R.D.; Schenk, A.D.; Cavadini, S.; Gill, M.E.; Abdulrahman, W.; Andres-Pons, A.; Luijsterburg, M.S.; Ibrahim, A.F.M.; Branigan, E.; et al. Structural basis of brcc36 function in DNA repair and immune regulation. Mol. Cell 2019, 75, 483–497.e9. [Google Scholar] [CrossRef]
- Py, B.F.; Kim, M.S.; Vakifahmetoglu-Norberg, H.; Yuan, J. Deubiquitination of nlrp3 by brcc3 critically regulates inflammasome activity. Mol. Cell 2013, 49, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Chen, L.; Li, Y.C.; Wu, L.; Yu, G.T.; Zhang, W.F.; Sun, Z.J. Nlrp3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. CR 2017, 36, 116. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Du, H.D.; Tang, D.; Zhang, D.; Zhou, J.; Zhai, C.W.; Yuan, C.C.; Hsueh, C.Y.; Li, S.J.; Heng, Y.; et al. Correlation between the nlrp3 inflammasome and the prognosis of patients with lscc. Front. Oncol. 2019, 9, 588. [Google Scholar] [CrossRef]
- Yu, S.; Yin, J.J.; Miao, J.X.; Li, S.G.; Huang, C.Z.; Huang, N.; Fan, T.L.; Li, X.N.; Wang, Y.H.; Han, S.N.; et al. Activation of nlrp3 inflammasome promotes the proliferation and migration of esophageal squamous cell carcinoma. Oncol. Rep. 2020, 43, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zaugg, K.; Mak, T.W.; Elledge, S.J. A role for the deubiquitinating enzyme usp28 in control of the DNA-damage response. Cell 2006, 126, 529–542. [Google Scholar] [CrossRef]
- Bassermann, F.; Frescas, D.; Guardavaccaro, D.; Busino, L.; Peschiaroli, A.; Pagano, M. The cdc14b-cdh1-plk1 axis controls the g2 DNA-damage-response checkpoint. Cell 2008, 134, 256–267. [Google Scholar] [CrossRef]
- Benevolo, M.; Musio, A.; Vocaturo, A.; Dona, M.G.; Rollo, F.; Terrenato, I.; Carosi, M.; Pescarmona, E.; Vocaturo, G.; Mottolese, M. Claspin as a biomarker of human papillomavirus-related high grade lesions of uterine cervix. J. Transl. Med. 2012, 10, 132. [Google Scholar] [CrossRef]
- Murtaza, M.; Jolly, L.A.; Gecz, J.; Wood, S.A. La fam fatale: Usp9x in development and disease. Cell. Mol. Life Sci. CMLS 2015, 72, 2075–2089. [Google Scholar] [CrossRef]
- Dupont, S.; Mamidi, A.; Cordenonsi, M.; Montagner, M.; Zacchigna, L.; Adorno, M.; Martello, G.; Stinchfield, M.J.; Soligo, S.; Morsut, L.; et al. Fam/usp9x, a deubiquitinating enzyme essential for tgfbeta signaling, controls smad4 monoubiquitination. Cell 2009, 136, 123–135. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The bcl-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. Bcl-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fu, L.; Sui, Y.; Zhang, L. The function and regulation of otu deubiquitinases. Front. Med. 2019, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lv, Y.; Li, H.; Gao, H.; Song, S.; Zhang, Y.; Xing, G.; Kong, X.; Wang, L.; Li, Y.; et al. Deubiquitylase otud3 regulates pten stability and suppresses tumorigenesis. Nat. Cell Biol. 2015, 17, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Carracedo, A.; Egia, A.; Lo-Coco, F.; Teruya-Feldstein, J.; Pandolfi, P.P. The deubiquitinylation and localization of pten are regulated by a hausp-pml network. Nature 2008, 455, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, D.; Shiloh, A.; Luo, J.; Nikolaev, A.Y.; Qin, J.; Gu, W. Deubiquitination of p53 by hausp is an important pathway for p53 stabilization. Nature 2002, 416, 648–653. [Google Scholar] [CrossRef]
- Sheng, Y.; Saridakis, V.; Sarkari, F.; Duan, S.; Wu, T.; Arrowsmith, C.H.; Frappier, L. Molecular recognition of p53 and mdm2 by usp7/hausp. Nat. Struct. Mol. Boil. 2006, 13, 285–291. [Google Scholar] [CrossRef]
- Chan, C.H.; Morrow, J.K.; Li, C.F.; Gao, Y.; Jin, G.; Moten, A.; Stagg, L.J.; Ladbury, J.E.; Cai, Z.; Xu, D.; et al. Pharmacological inactivation of skp2 scf ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell 2013, 154, 556–568. [Google Scholar] [CrossRef]
- Liao, R.G.; Watanabe, H.; Meyerson, M.; Hammerman, P.S. Targeted therapy for squamous cell lung cancer. Lung Cancer Manag. 2012, 1, 293–300. [Google Scholar] [CrossRef]
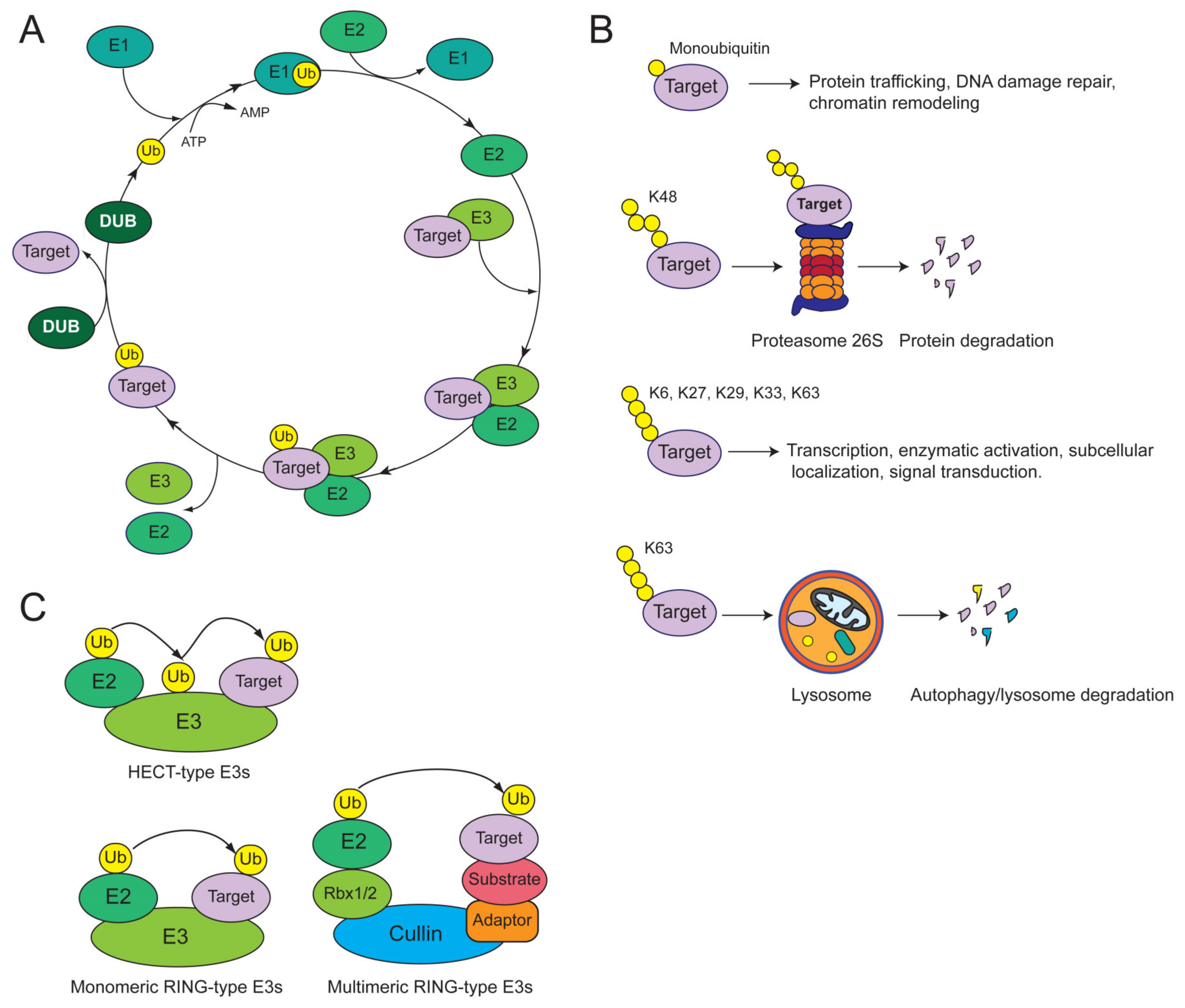
| E3 Ligase | Function | Cancer-Associated Substrates | Alterations in SSC Cells | Clinical Correlations | References |
|---|---|---|---|---|---|
| E6-AP | HECT-type E3 | p53, PML | In HPV-positive SCCs, E6-AP associates with E6 and acquires substrate specificity towards p53 (oncogene) | It contributes to HPV-driven carcinogenesis | [44] |
| WWP1 | HECT-type E3 | p53, ΔNp63, p27Kip1, Notch, Smad2, Smad4 | Overexpression in HNSCC patients (oncogene) | In CSCC patients, high expression is associated with lymph node metastasis. | [45,46] |
| MDM2 | RING-type E3 | p53, Rb, p21, FoxO3A, E-cadherin | Amplification, overexpression, hyperactivation as a result of p14/ARF loss (oncogene) | Correlation with SCC progression, adverse prognosis and chemoresistance | [47,48,49,50,51,52,53] |
| TRIM32 | RING-type E3 | ARID1A | Overexpression in HNSCC (oncogene) | N.D. | [54,55,56,57] |
| SKP2 | F-box protein, substrate receptor of the CRL1 E3 (SCF complex) | p27, AKT, Smad4, Tob, c-Myc, cdk9, Mll1, Rassf1, Mkp1 | Overexpression (oncogene) | Correlation with SCC progression, lymph node metastasis and radioresistance | [58,59,60,61,62,63,64,65,66,67] |
| FBXW7 | F-box protein, substrate receptor of the CRL1 E3 (SCF complex) | c-Myc, Notch1, Cyclin E, MCL-1, ΔNp63 | Missense and nonsense mutations, gene deletion, promoter hypermethylation, miRNA (tumor suppressor) | Correlation with adverse prognosis of ESCC patients and reactivity to chemoradiation therapy | [11,12,13,14,15,68,69,70,71,72,73,74,75] |
| KEAP1 | Substrate receptor of the CRL3 E3 | Nrf2 | Mutations, promoter hypermethylation, inactivation by HPV (oncogene) | Correlation with adverse prognosis in HNSCC. In LSCC mutations are associated with poor response to adjuvant therapy, and poorer overall survival. | [11,12,13,14,15,47,71,76,77,78,79,80,81] |
| CUL3 | Cullin subunit of the CRL3 E3 | Nrf2 | Mutations (oncogene) | Correlation with adverse prognosis in HNSCC and lung SCC patients | [47] |
| CDC20 | Substrate adaptor of the APC/C E3 complex (early mitosis) | Cyclin A, Cyclin B1, Securin | Amplification, overexpression (oncogene) | An APC/C signature is predictive of poor prognosis in HNSCC patients | [47,82,83,84] |
| DUB | Class | Cancer-Associated Substrates | Alterations in SSC Cells | Clinical Correlations | References |
|---|---|---|---|---|---|
| USP22 | USP | H2B, SIRT1, FBP1 | Overexpression (oncogene) | Positive correlation with SCC progression and lymph node metastasis, poor prognosis | [158,159,160,161] |
| USP48 | USP | H2A | Deletion (tumor suppressor) | Genomic instability | [47] |
| BRCC36 (BRCA1or BRISC complex) | JAMM | H2A, γH2AX, NLRP3, ATF, | Overexpression (oncogene) | Radioresistance, poor prognosis | [47,162,163] |
| USP28 | USP | ΔNp63, Claspin, 53BP1, FBW7 | Overexpression (oncogene) | Poor prognosis | [164,165] |
| USP9x | USP | β-catenin, MCL1, ASK-1, DLK, SMAD4, SMURF1 | Deletion, mutation (tumor suppressor) in oral cancer. In ESCC high expression | In ESCC high expression correlates with lymph node metastasis and poorer survival rate | [166,167,168,169,170,171] |
| OTUD3 | OTU | PTEN | Deletion (tumor suppressor) | N.D. | [47] |
| USP7 | USP | p53, MDM2, PTEN | Overexpression (oncogene) | In LSCC correlation with a more advanced tumor stage, lymph node metastasis and shorter overall survival | [172,173,174] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, V.; Bernassola, F.; Talora, C.; Melino, G.; Peschiaroli, A. The Impact of the Ubiquitin System in the Pathogenesis of Squamous Cell Carcinomas. Cancers 2020, 12, 1595. https://doi.org/10.3390/cancers12061595
Gatti V, Bernassola F, Talora C, Melino G, Peschiaroli A. The Impact of the Ubiquitin System in the Pathogenesis of Squamous Cell Carcinomas. Cancers. 2020; 12(6):1595. https://doi.org/10.3390/cancers12061595
Chicago/Turabian StyleGatti, Veronica, Francesca Bernassola, Claudio Talora, Gerry Melino, and Angelo Peschiaroli. 2020. "The Impact of the Ubiquitin System in the Pathogenesis of Squamous Cell Carcinomas" Cancers 12, no. 6: 1595. https://doi.org/10.3390/cancers12061595
APA StyleGatti, V., Bernassola, F., Talora, C., Melino, G., & Peschiaroli, A. (2020). The Impact of the Ubiquitin System in the Pathogenesis of Squamous Cell Carcinomas. Cancers, 12(6), 1595. https://doi.org/10.3390/cancers12061595




