What Are Young Women Living Conditions after Breast Cancer? Health-Related Quality of Life, Sexual and Fertility Issues, Professional Reinsertion
Abstract
1. Introduction
2. Materials and Methods
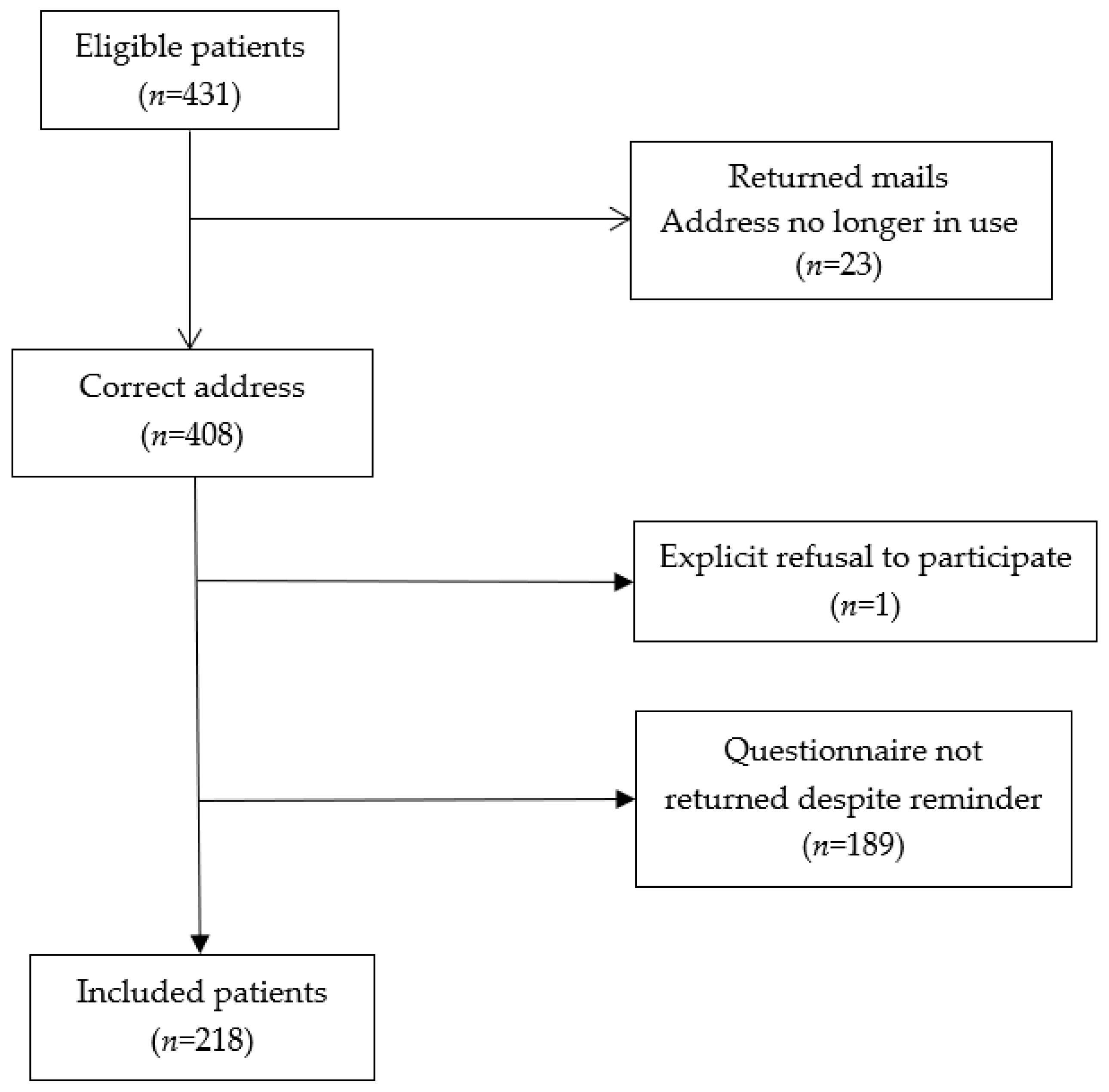
2.1. Patients
2.2. Outcomes, Measures, Study Variables
2.2.1. Outcome Variables
2.2.2. Predictor Variables
2.2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Participants
3.2. HRQoL, Sexual Function, Social Support, Anxiety and Depression Scores
3.2.1. HRQoL Scores
3.2.2. Sexual Function Scores
3.2.3. Social Support Scores
3.2.4. Anxiety and Depression Scores
3.3. Determinants of HRQoL
3.4. Fertility Data and Concerns
3.5. Professional Reinsertion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Defossez, G.; Le Guyader-Peyrou, S.; Uhry, Z.; Grosclaude, P.; Colonna, M.; Dantony, E.; Remontet, L.; Monnereau, A.; Woronof, A.-S.; Delafosse, P.; et al. Estimations Nationales de L’incidence et de la Mortalité par Cancer en France Métropolitaine Entre 1990 et 2018. Étude à Partir des Registres des Cancers du Réseau Francim. Résultats Préliminaires; Santé Publique France: Saint-Maurice, France, 2019; p. 161. [Google Scholar]
- Coleman, M.P.; Quaresma, M.; Berrino, F.; Lutz, J.M.; De Angelis, R.; Capocaccia, R.; John, L.; Koifman, S.; Storm, H.H.; Sant, M.; et al. Cancer survival in five continents: A worldwide population-based study (CONCORD). Lancet Oncol. 2008, 9730–9756. [Google Scholar] [CrossRef]
- Cowppli-Bony, A.; Uhry, Z.; Remontet, L.; Guizard, A.V.; Voirin, N.; Monnereau, A.; Bouvier, A.M.; Colonna, M.; Bossard, N.; Woronoff, A.S.; et al. Survie des Patients Atteints de Cancer en France, 1989-2013.–Etude à Partir des Registres des Cancers du Réseau Francim Partie 1–Tumeurs Solides; Institut de Veille Sanitaire: Saint-Maurice, France, 2016; pp. 184–191. [Google Scholar]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Nennecke, A.; Siseling, S.; Berrino, F.; et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Baumann, C.; Briançon, S.; Metz, V. Maladie chronique et qualité de vie: Enjeux, définition et mesure. Actualité Dossier Santé Publique 2010, 72, 19–21. [Google Scholar]
- Duijts, S.F.; Kieffer, J.M.; van Muijen, P.; van der Beek, A. Sustained employability and health-related quality of life in cancer survivors up to four years after diagnosis. Acta Oncol. 2017, 56, 174–182. [Google Scholar] [CrossRef]
- Meneses, K.; McNees, P.; Azuero, A.; Jukkala, A. Evaluation of the Fertility and Cancer Project (FCP) among young breast cancer survivors. Psychooncology 2010, 19, 1112–1115. [Google Scholar] [CrossRef]
- Auguste, A.; Cortet, M.; Dabakuyo-Yonli, T.S.; Launay, L.; Arnould, L.; Desmoulins, I.; Roignot, P.; Arveoux, P.; Bertaut, A.; Poillot, M.-L.; et al. Breast cancer subtype of French women is not influencedby socioeconomic status: A population based-study. PLoS ONE 2017, 2, e0170069. [Google Scholar] [CrossRef]
- Dialla, P.O.; Quipourt, V.; Gentil, J.; Marilier, S.; Poillot, M.L.; Roignot, P.; Dabakuyo-Yonli, T.S.; Arveux, P.; Guiu, S.; Darut-Jouve, A.; et al. In breast cancer, are treatments and survival the same whatever a patient’s age? A population-based study over the period 1998–2009. Geriatr. Gerontol. Int. 2015, 15, 617–626. [Google Scholar] [CrossRef]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Sullivan, M.; Prieto, L.; Leplege, A.; Kaasa, S.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 11, 1171–1178. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Turner-Bowker, D.M.; Gandek, B. How to Score Version 2 of the SF-12 Health Survey: With a Supplement Documenting Version 1; Quality Metric Inc.: Lincon, RI, USA, 2005. [Google Scholar]
- Lepine, J.P.; Godchau, M.; Brun, P. Anxiety and depression in patients. Lancet 1985, 2, 1425–1426. [Google Scholar] [CrossRef]
- Rascle, N.; Bruchon-Schweitzer, M.; Sarason, I.G. Short Form of Sarason’s Social Support Questionnaire: French Adaptation and Validation. Psychol. Rep. 2005, 97, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Sass, C.; Moulin, J.J.; Guéguen, R.; Abric, L.; Dauphinot, V.; Dupré, C.; La Rosa, E.; Magnier, P.; Lebbe, E.; Guenot, C.; et al. Le score Epices: Un score individuel de précarité. Construction du score et mesure des relations avec des données de santé, dans une population de 197389 personnes. Bull. Epidemiol. Hebdomadaire 2006, 14, 93–96. [Google Scholar]
- Rosen, R.; Brown, J.; Heiman, S.; Leiblum, C.; Meston, R.; Shabsigh, D.; Ferguson, R.; D’Agostino, R. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000, 26, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Wylomanski, S.; Bouquin, R.; Philippe, H.J.; Poulin, Y.; Hanf, M.; Dréno, B.; Quéreux, G.; Rouzier, R. Psychometric properties of the French Female Sexual Function Index (FSFI). Qual. Life Res. 2014, 23, 2079–2087. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Winchester, D.P.; Byrd, D.R.; Mayer, L.; Gress, D.M.; et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Mamguem Kamga, A.; Dumas, A.; Joly, F.; Billa, O.; Simon, J.; Poillot, M.L.; Dabakuyo-Yonli, T.S.; Jouve, A.D.; Fumoleau, P.; Countant, C.; et al. Long-Term Gynecological Cancer Survivors in Côte d’Or: Health-Related Quality of Life and Living Conditions. Oncologist 2019, 23, 1–11. [Google Scholar] [CrossRef]
- Chu, W.O.; Dialla, P.O.; Roignot, P.; Bone-Lepinoy, M.C.; Poillot, M.L.; Coutant, C.; Dabakuyo-Yonli, T.S.; Arveux, P. Determinants of quality of life among long-term breast cancer survivors. Qual. Life Res. 2016, 25, 1981–1990. [Google Scholar] [CrossRef]
- Dialla, P.O.; Chu, W.O.; Roignot, P.; Bone-Lepinoy, M.C.; Poillot, M.L.; Coutant, C.; Dabakuyo-Yonli, T.S.; Arveux, P. Impact of age-related socio-economic and clinical determinants of quality of life among long-term breast cancer survivors. Maturitas 2015, 81, 362–370. [Google Scholar] [CrossRef]
- Short, H.; Al Sayah, F.; Ohinmaa, A.; Lahtinen, M.; Johnson, J.A. The relationship of neighbourhood-level material and social deprivation with health-related quality of life. Qual Life Res. 2018, 27, 3265–3274. [Google Scholar] [CrossRef]
- Johansson, R.; Carlbring, P.; Heedman, A.; Paxling, B.; Andersson, G. Depression, anxiety and their comorbidity in the Swedish general population: Point prevalence and the effect on health-related quality of life. PeerJ 2013, 1, 1–18. [Google Scholar] [CrossRef]
- Helgeson, V.S. Social support and quality of life. Qual. Life Res. 2003, 12 (Suppl. 1), 25–31. [Google Scholar] [CrossRef] [PubMed]
- Schultz, P.N.; Klein, M.J.; Beck, M.L.; Stava, C.; Sellin, R.V. Breast cancer: Relationship between menopausal symptoms, physiologic health effects of cancer treatment and physical constraints on quality of life in long-term survivors. J. Clin. Nurs. 2005, 14, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Cuenca, A.I.; Martin-Espinosa, N.M.; Sampietro-Crespo, A.; Rodríguez-Borrego, M.A.; Carmona-Torres, J.M. Sexual dysfunction in Spanish women with breast cancer. PLoS ONE 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Abril-Requena, A.; García-Torres, F.; Alós, F.J. Sexual dysfunction and phobic anxiety in breast cancer survivors. Psycho Oncology 2019, 195–197. [Google Scholar] [CrossRef]
- Dizon, D.S. Quality of Life after Breast Cancer: Survivorship and Sexuality. Breast J. 2009, 15, 500–504. [Google Scholar] [CrossRef]
- Raggio, G.A.; Butryn, M.L.; Arigo, D.; Mikorski, R.; Palmer, S.C. Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol. Health 2014, 29, 632–650. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Mertens, A.C.; Spencer, J.B.; Manatunga, A.K.; Howards, P.P. Menses resumption after cancer treatment-induced amenorrhea occurs early or not at all. Fertil. Steril. 2016, 105, 765–772. [Google Scholar] [CrossRef]
- Chin, H.B.; Howards, P.P.; Kramer, M.R.; Mertens, A.C.; Spencer, J.B. Which Female Cancer Patients Fail to Receive Fertility Counseling Before Treatment in the State of Georgia. Fertil. Steril. 2016, 106, 1763–1771. [Google Scholar] [CrossRef]
- Jukkala, A.M.; Azuero, A.; McNees, P.; Bates, G.W.; Meneses, K. Self-assessed knowledge of treatment and fertility preservation in young women with breast cancer. Fertil. Steril. 2010, 94, 2396–2398. [Google Scholar] [CrossRef]
- Thewes, B.; Meiser, B.; Taylor, A.; Phillips, K.A.; Pendlebury, S.; Capp, A.; Dalley, D.; Goldstein, D.; Baber, R.; Friedlander, M.L. Fertility-and menopause-related information needs of younger women with a diagnosis of early breast cancer. J. Clin. Oncol. 2005, 23, 5155–5165. [Google Scholar] [CrossRef]
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The impact of cancer on subsequent chance of pregnancy: A population-based analysis. Hum. Reprod. 2018, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Takahashi, M.; Sairenchi, T.; Muto, T. The impact of breast cancer on employment among Japanese women. J. Occup. Health 2014, 56, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.S.; Overgaard, C.; Bøggild, H.; Garne, J.P.; Lund, T.; Overvad, K.; Fonager, K. The long-term financial consequences of breast cancer: A Danish registry-based cohort study. BMC Public Health 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Cho, J.; Shin, D.W.; Park, B.W.; Ahn, S.H.; Noh, D.Y.; Yun, Y.H.; Lee, E.S.; Nam, S.J. Impact of breast cancer diagnosis and treatment on work-related life and factors affecting them. Breast Cancer Res. Treat. 2009, 116, 609–616. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Scherer, S.; Wiskemann, J.; Steindorf, K. Return to work after breast cancer: The role of treatment-related side effects and potential impact on quality of life. Eur. J. Cancer. Care. 2019, 29. [Google Scholar] [CrossRef]
- Abrahams, H.J.; Gielissen, M.F.; Schmits, I.C.; Verhagen, C.A.; Rovers, M.M.; Knoop, H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Ann. Oncol. 2016, 27, 965–974. [Google Scholar] [CrossRef]
- Jones, J.M.; Olson, K.; Catton, P.; Catton, C.N.; Fleshner, N.E.; Krzyzanowska, M.K.; McCready, D.R.; Wong, R.K.S.; Jiang, H.; Howel, D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J. Cancer Surviv. 2016, 10, 51–61. [Google Scholar] [CrossRef]
| Characteristics | No. of Patients | Median (Min–Max) | % |
|---|---|---|---|
| Age at diagnosis, years | |||
| ≤35 | 27 | 12.4 | |
| >35 | 191 | 87.6 | |
| Age at time of survey, years | |||
| ≤45 | 60 | 27.5 | |
| >45 | 158 | 72.5 | |
| BMI at time of survey | |||
| ≤25 | 134 | 62.0 | |
| >25 | 82 | 38.0 | |
| Missing data | 2 | ||
| Marital status at time of survey | |||
| Married/living maritally | 132 | 60.6 | |
| Single/divorced/widowed | 86 | 39.4 | |
| Missing data | 0 | ||
| Educational level | |||
| Less than high school diploma | 57 | 26.5 | |
| High school diploma or higher | 158 | 73.5 | |
| Missing data | 3 | ||
| Employment at time of survey | |||
| Employed | 189 | 87.5 | |
| Unemployed | 27 | 12.5 | |
| Missing data | 2 | ||
| Time since diagnosis, months | |||
| Mean | 87.7 | ||
| Median (min-max) | 218 | 86 (36–155) | |
| SD | 34.2 | ||
| Time since diagnosis, months | |||
| ≤86 | 110 | 50.5 | |
| >86 | 108 | 49.5 | |
| AJCC stage | |||
| 1 | 93 | 42.7 | |
| 2/3 | 125 | 57.3 | |
| Missing data | 0 | ||
| Tumor grade | |||
| I | 41 | 19.6 | |
| II | 92 | 44.0 | |
| III | 76 | 36.4 | |
| Missing data | 9 | ||
| Hormone Receptor status | |||
| Positive | 166 | 76.5 | |
| Negative | 51 | 23.5 | |
| Missing data | 1 | ||
| HER2 status | |||
| Positive | 47 | 21.8 | |
| Negative | 169 | 78.2 | |
| Missing data | 2 | ||
| Triple negative status | |||
| Yes | 37 | 17.1 | |
| No | 179 | 82.9 | |
| Missing data | 2 | ||
| Menopausal status at time of survey | |||
| Menopausal | 112 | 53.6 | |
| Non-menopausal | 97 | 46.4 | |
| Missing data | 9 | ||
| Charlson comorbidity Index | |||
| =0 | 195 | 90.3 | |
| ≥1 | 21 | 9.7 | |
| Missing data | 2 | ||
| Surgery | 100 | ||
| Yes | 216 | ||
| No | 0 | ||
| Missing data | 2 | ||
| Chemotherapy | |||
| Yes | 168 | 77.4 | |
| No | 49 | 22.6 | |
| Missing data | 1 | ||
| Radiotherapy | |||
| Yes | 186 | 85.7 | |
| No | 31 | 14.3 | |
| Missing data | 1 | ||
| Endocrine therapy | |||
| Yes | 156 | 71.9 | |
| No | 61 | 28.1 | |
| Missing data | 1 | ||
| EPICES deprivation score a | 213 | ||
| Mean | 17.3 | ||
| Median [min-max] | 13.6 (0–75.7) | ||
| SD | 17.2 | ||
| Missing data | 5 | ||
| EPICES deprivation score a | |||
| EPICES ≤ 30 | 174 | 81.7 | |
| EPICES > 30 | 39 | 18.3 | |
| Missing data | 5 |
| Dimensions | No. of Patients | Mean (SD) | Median (Min-Max) |
|---|---|---|---|
| Health-Related Quality of Life: SF12 a | |||
| General health | 217 | 67.3 (19.1) | 60 (25–100) |
| Physical functioning | 217 | 80.8 (27.1) | 100 (0–100) |
| Role physical | 216 | 68.3 (28.2) | 75 (0–100) |
| Role emotional | 217 | 67.8 (26.6) | 75 (0–100) |
| Bodily pain | 217 | 75.0 (27.3) | 75 (0–100) |
| Mental health | 217 | 59.9 (19.6) | 62.5 (0–100) |
| Vitality | 217 | 50.7 (24.6) | 50 (0–100) |
| Social functioning | 215 | 68.5 (25.8) | 75 (0–100) |
| Sexual function b | |||
| Desire | 215 | 3 (1.3) | 3 (1.2–6) |
| Arousal | 213 | 3.2 (1.9) | 3.6 (0–6) |
| Pain | 211 | 3.6 (2.4) | 4.4 (0–6) |
| Satisfaction | 194 | 4.2 (1.7) | 4.4 (0.8–6) |
| Lubrication | 215 | 3.5 (2.3) | 4.2 (0–6) |
| Orgasm | 211 | 3.4 (2.2) | 4 (0–6) |
| Global Score | 188 | 22.1 (10.5) | 25.1 (2–36) |
| Sexual dysfunction (%) | |||
| Yes | 104 (55.3) | ||
| No | 84 (44.7) | ||
| Missing data | 30 | ||
| Social support c | |||
| Social support availability | 212 | 20.5 (11.2) | 19 (0–54) |
| Social support satisfaction | 196 | 28.8 (7.9) | 31 (6–36) |
| Social support availability (%) | |||
| <19 | 102 (48.1) | ||
| ≥19 | 110 (51.9) | ||
| Missing data | 6 | ||
| Social support satisfaction (%) | |||
| <31 | 97 (49.5) | ||
| ≥31 | 99 (50.5) | ||
| Missing data | 22 | ||
| HADS d | |||
| Anxiety | 216 | 8.7 (3.8) | 8 (2–20) |
| Depression | 217 | 4.7 (3.6) | 4 (0–16) |
| Anxiety | |||
| <11 | 153 (70.8) | ||
| ≥11 | 63 (29.1) | ||
| Missing data | 2 | ||
| Depression | |||
| <11 | 198 (91.2) | ||
| Dimensions | No. of Patients | Mean (SD) | Median (Min-Max) |
| ≥11 | 19 (8.8) | ||
| Missing data | 1 |
| Scales Scores of the SF-12 and Variables | Estimate | Standard Error | p-Value |
|---|---|---|---|
| General health a | |||
| Depression | 0.0006 | ||
| <11/≥11 | 17.34 | 4.99 | |
| Social support satisfaction | 0.0042 | ||
| ≥31/<31 | 7.30 | 2.52 | |
| Menopausal status at time of survey | 0.0051 | ||
| No/Yes | 7.19 | 2.53 | |
| Physical functioning b | |||
| Depression | 0.0028 | ||
| <11/≥11 | 20.34 | 6.70 | |
| Scales scores of the SF-12 and variables | Estimate | Standard error | p-value |
| Charlson’s comorbidity score | 0.0055 | ||
| =0/≥1 | 6.03 | 4.02 | |
| Role physical c | |||
| Depression | 0.0008 | ||
| <11/≥11 | 24.75 | 7.23 | |
| Role emotional d | |||
| Anxiety | <0.0001 | ||
| <11/≥11 | 20.03 | 3.63 | |
| Depression | <0.0001 | ||
| <11/≥11 | 32.62 | 6.26 | |
| Bodily pain e | |||
| Anxiety | 0.0015 | ||
| <11/≥11 | 13.40 | 4.15 | |
| EPICES deprivation score | 0.0037 | ||
| ≤30/>30 | 13.44 | 6.96 | |
| Mental health f | |||
| Anxiety | <0.0001 | ||
| <11/≥11 | 19.52 | 2.58 | |
| Depression | <0.0001 | ||
| <11/≥11 | 18.57 | 4.04 | |
| EPICES deprivation score | 0.0013 | ||
| ≤30/>30 | 9.40 | 2.87 | |
| Vitality g | |||
| Depression | <0.0001 | ||
| <11/≥11 | 29.22 | 5.82 | |
| Social functioning h | |||
| Anxiety | <0.0001 | ||
| <11/≥11 | 15.90 | 3.74 | |
| Depression | <0.0001 | ||
| <11/≥11 | 26.67 | 6.25 | |
| Social support satisfaction | 0.0005 | ||
| ≥31/<31 | 11.31 | 3.21 |
| Variables | No. of Patients | % |
|---|---|---|
| Contraception at time of diagnosis | ||
| No | 44 | 20.9 |
| Yes | 167 | 79.2 |
| Missing data | 7 | |
| Menstrual cycle before treatment | ||
| Non-existent | 49 | 22.8 |
| Regular | 140 | 65.1 |
| Irregular | 26 | 12.1 |
| Missing data | 3 | |
| Menstrual cycle after treatment | ||
| Non-existent | 121 | 57.1 |
| Regular | 36 | 17.0 |
| Irregular | 55 | 25.9 |
| Missing data | 6 | |
| Children at the time of diagnosis | ||
| No | 32 | 14.8 |
| Yes | 184 | 85.2 |
| Missing data | 2 | |
| Pregnancy project at time of diagnosis | ||
| No | 194 | 90.2 |
| Yes | 21 | 9.8 |
| Missing data | 3 | |
| Gave up a pregnancy project after treatment | ||
| Yes | 66 | 33.2 |
| No | 133 | 66.8 |
| Missing data | 19 | |
| Planned pregnancy project after treatment | ||
| Yes | 15 | 7.3 |
| No | 190 | 92.7 |
| Missing data | 13 | |
| Children at time of diagnosis among women who had pregnancy project at time of diagnosis (n = 21) | ||
| No | 7 | 33.3 |
| Yes | 14 | 66.7 |
| Spontaneous pregnancy before diagnosis (n = 14) | ||
| No | 1 | 7.1 |
| Yes | 13 | 92.9 |
| Pregnancy with MAP before diagnosis (n = 14) | ||
| 0 | 7 | 77.8 |
| 1 | 2 | 22.2 |
| Missing data | 5 | |
| Information received about impact of treatment on fertility and ovarian function | ||
| Yes | 70 | 33.8 |
| No | 68 | 32.9 |
| I forgot | 69 | 33.3 |
| Missing data | 11 | |
| Fertility preservation-related information | ||
| Yes | 56 | 27.2 |
| No | 150 | 72.8 |
| Missing data | 12 | |
| Would have liked to be informed if fertility preservation-related information not given (n = 150) | ||
| Yes | 55 | 38.7 |
| No | 87 | 61.3 |
| Missing data | 8 | |
| MAP Consultation (n = 21) | ||
| No | 16 | 84.2 |
| Yes | 3 | 15.8 |
| Missing data | 2 | |
| Fertility preservation proposed if MAP consultation a (n = 3) | ||
| No | 0 | |
| Yes | 3 | 100 |
| Agreed to proposed fertility preservation (n = 3) | ||
| No | 1 | 66.7 |
| Yes | 2 | 33.3 |
| Spontaneous pregnancy after treatment (n = 21) | ||
| 0 | 13 | 86.7 |
| 1 | 2 | 13.3 |
| Missing data | 6 | |
| Pregnancy with MAP after treatment (n = 21) | ||
| 0 | 13 | 92.9 |
| 1 | 1 | 7.1 |
| Missing data | 7 | |
| Adoption (n = 21) | ||
| Yes | 1 | 5.3 |
| No | 18 | 94.7 |
| Missing data | 2 | |
| Abortion since cancer (n = 21) | ||
| Yes | 0 | |
| No | 20 | 100 |
| Missing data | 1 | |
| Spontaneous miscarriages (n = 21) | ||
| Yes | 1 | 5.0 |
| No | 19 | 95.0 |
| Missing data | 1 |
| Variables | No. of Patients | % |
|---|---|---|
| Income since cancer diagnosis | ||
| Increased | 43 | 19.8 |
| Unchanged | 114 | 52.5 |
| Decreased | 60 | 27.7 |
| Missing data | 1 | |
| Bank loan in progress | ||
| No | 64 | 29.4 |
| Yes | 154 | 70.6 |
| Difficulties repaying bank loans in progress | ||
| No | 139 | 64.9 |
| Yes | 28 | 13.1 |
| Not concerned | 47 | 22.0 |
| Missing data | 4 | |
| Asked for a loan since treatment | ||
| No | 94 | 45.2 |
| Yes | 114 | 54.8 |
| Missing data | 10 | |
| Answer to a home loan request (n = 61) a | ||
| Agreement without conditions | 10 | 17.5 |
| Difficulties with insurance (refusal, exclusions and/or increased premium) | 47 | 82.5 |
| Missing data | 4 | |
| Employment at time of diagnosis | ||
| Employed | 183 | 84.3 |
| Unemployed | 34 | 15.7 |
| Missing data | 1 | |
| Arduous working conditions at time of diagnosis | ||
| Yes | 91 | 46.2 |
| No | 106 | 53.8 |
| Missing data | 21 | |
| Decreased ability to work after the end of treatment | ||
| Yes | 160 | 73.4 |
| No | 53 | 24.3 |
| Not concerned | 5 | 2.3 |
| Reasons for decreased ability to work after the end of treatment | ||
| Fatigue | 135 | 84.4 |
| Pain | 69 | 43.1 |
| Limitation of some of my movements | 69 | 43.1 |
| Limitation of my cognitive abilities | 80 | 50.0 |
| Emotional problems | 58 | 36.3 |
| Other symptoms | 10 | 6.3 |
| Decreased ability to work at the time of survey | ||
| Yes | 128 | 59.0 |
| No | 85 | 39.2 |
| Not concerned | 4 | 1.8 |
| Missing data | 1 | |
| Reasons for decreased ability to work at the time of the survey | ||
| Fatigue | 96 | 75.0 |
| Pain | 53 | 41.4 |
| Limitation of some of my movements | 51 | 39.8 |
| Limitation of my cognitive abilities | 54 | 42.2 |
| Emotional problems | 39 | 30.5 |
| Other symptoms | 4 | 3.1 |
| Impact of cancer on work | ||
| Positive | 45 | 21.2 |
| Negative | 82 | 38.7 |
| None | 85 | 40.1 |
| Missing | 6 | |
| Perceived discrimination | ||
| Yes | 61 | 28.4 |
| No | 123 | 57.2 |
| Not applicable | 31 | 14.4 |
| Missing data | 3 | |
| Events since the end of treatment | ||
| Retirement, stopped working | 1 | 0.5 |
| Made redundant | 22 | 10.8 |
| Bankruptcy, sale or cessation of an independent activity | 6 | 3.0 |
| Resignation | 11 | 5.4 |
| Different position, transfer (within the same company) | 36 | 17.7 |
| None | 127 | 62.6 |
| Missing data | 15 | |
| Role of cancer or its after-effects in the aforementioned events (n = 76) b | ||
| No | 30 | 39.5 |
| Yes | 46 | 60.5 |
| Missing data |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assogba, E.L.F.; Mamguem Kamga, A.; Costaz, H.; Jankowski, C.; Dumas, A.; Roignot, P.; Jolimoy, G.; Coutant, C.; Arveux, P.; Dabakuyo-Yonli, T.S. What Are Young Women Living Conditions after Breast Cancer? Health-Related Quality of Life, Sexual and Fertility Issues, Professional Reinsertion. Cancers 2020, 12, 1564. https://doi.org/10.3390/cancers12061564
Assogba ELF, Mamguem Kamga A, Costaz H, Jankowski C, Dumas A, Roignot P, Jolimoy G, Coutant C, Arveux P, Dabakuyo-Yonli TS. What Are Young Women Living Conditions after Breast Cancer? Health-Related Quality of Life, Sexual and Fertility Issues, Professional Reinsertion. Cancers. 2020; 12(6):1564. https://doi.org/10.3390/cancers12061564
Chicago/Turabian StyleAssogba, Emerline L. F., Ariane Mamguem Kamga, Helène Costaz, Clémentine Jankowski, Agnès Dumas, Patrick Roignot, Geneviève Jolimoy, Charles Coutant, Patrick Arveux, and Tienhan Sandrine Dabakuyo-Yonli. 2020. "What Are Young Women Living Conditions after Breast Cancer? Health-Related Quality of Life, Sexual and Fertility Issues, Professional Reinsertion" Cancers 12, no. 6: 1564. https://doi.org/10.3390/cancers12061564
APA StyleAssogba, E. L. F., Mamguem Kamga, A., Costaz, H., Jankowski, C., Dumas, A., Roignot, P., Jolimoy, G., Coutant, C., Arveux, P., & Dabakuyo-Yonli, T. S. (2020). What Are Young Women Living Conditions after Breast Cancer? Health-Related Quality of Life, Sexual and Fertility Issues, Professional Reinsertion. Cancers, 12(6), 1564. https://doi.org/10.3390/cancers12061564





