Soluble PD-L1 in NSCLC Patients Treated with Checkpoint Inhibitors and Its Correlation with Metabolic Parameters
Abstract
1. Introduction
2. Results
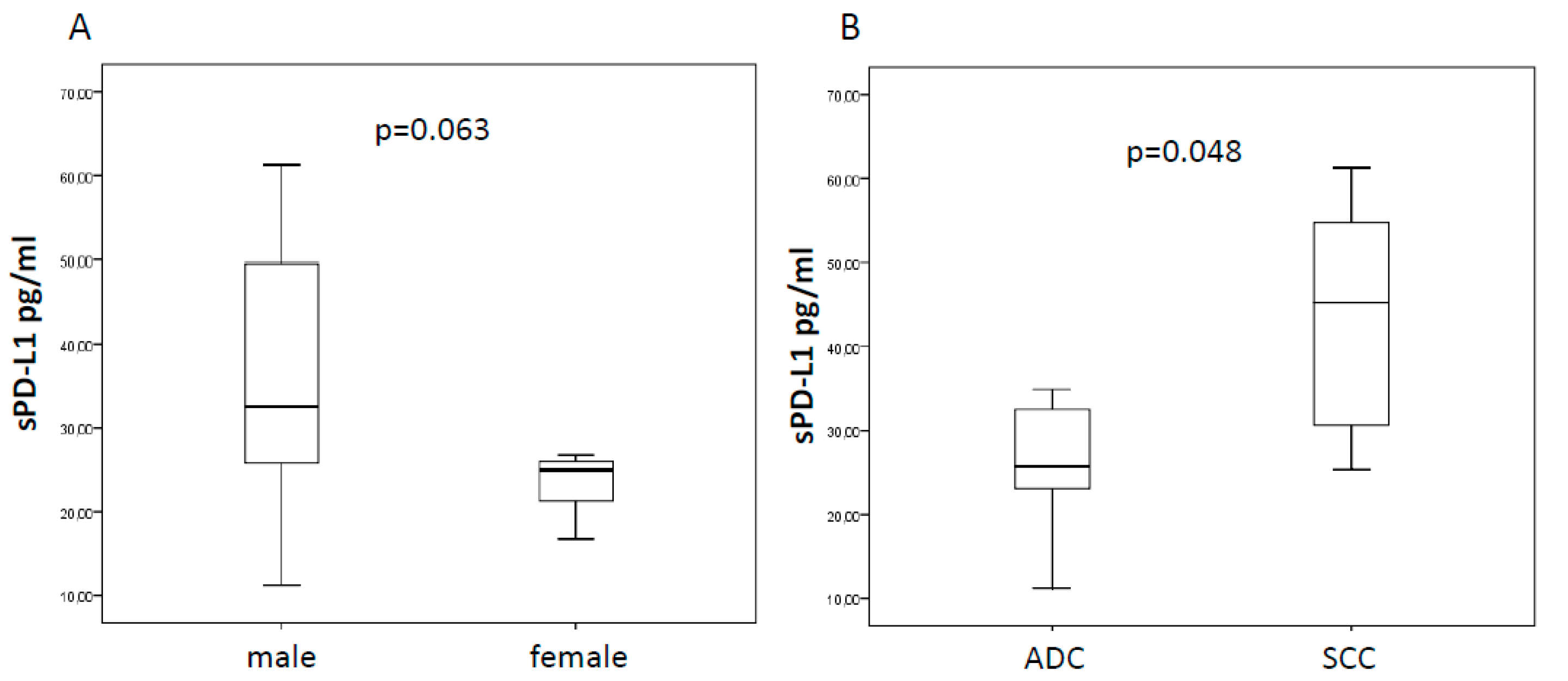
2.1. Baseline sPD-L1 and Patients’ Characteristics
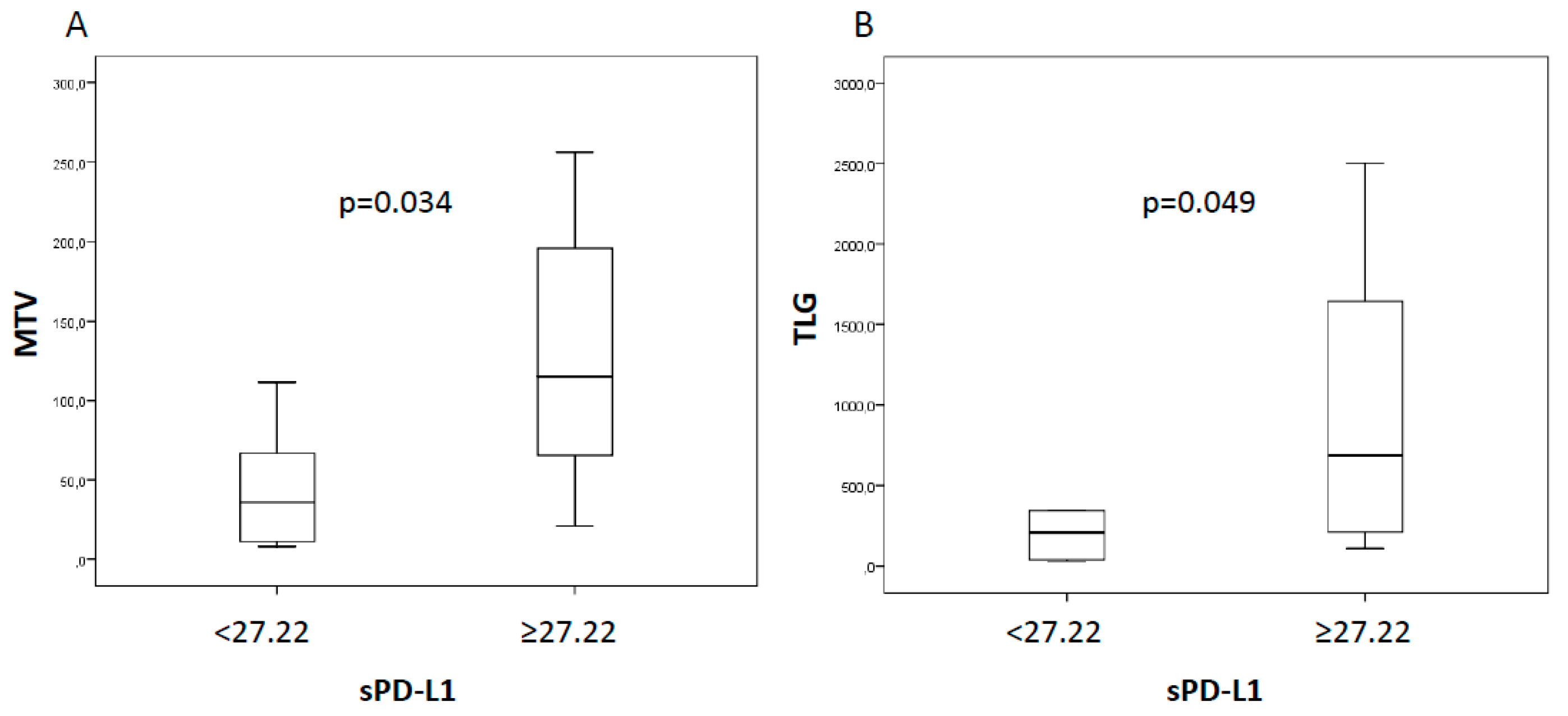
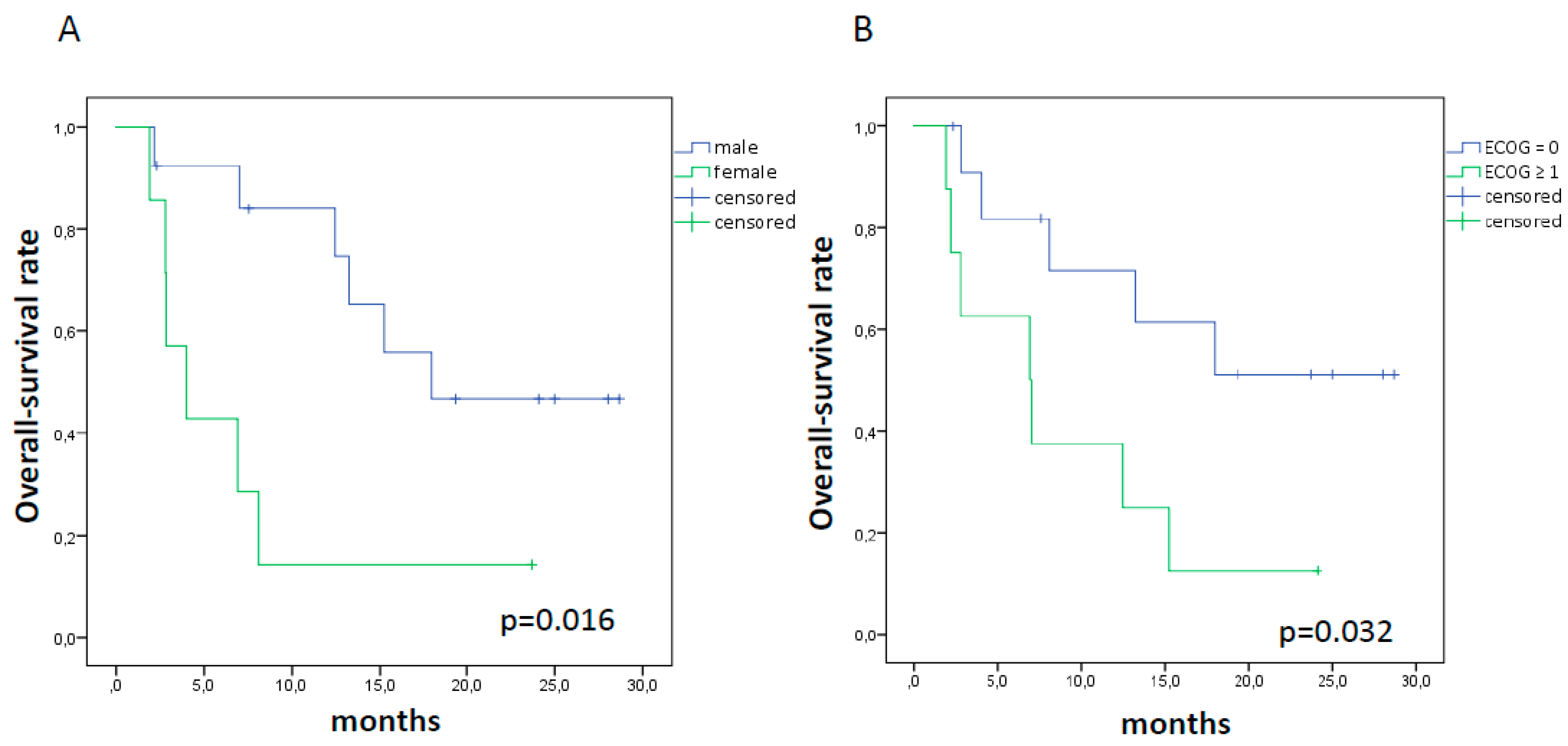
2.2. sPD-L1, Tumor Response, and Survival
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. Clinical Endpoints
4.3. Quantification of Circulating PD-L1
4.4. Imaging Protocol and Tumor Delineation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. 2016. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety. activity. and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Huang, Z.; Teng, F.; Xing, L.; Yu, J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat. Rev. 2015, 41, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Buder-Bakhaya, K.; Hassel, J.C. Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment-A Review From the Melanoma Perspective and Beyond. Front. Immunol. 2018, 9, 1474. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Bu, Z.; Liu, X.; Zhang, L.; Li, Z.; Wu, A.; Wu, X.; Cheng, X.; Xing, X.; Du, H.; et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin. J. Cancer Res. 2014, 26, 104e111. [Google Scholar]
- Zhang, J.; Gao, J.; Li, Y.; Nie, J.; Dai, L.; Hu, W.; Chen, X.; Han, J.; Ma, X.; Tian, G.; et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac. Cancer. 2015, 6, 534e538. [Google Scholar] [CrossRef]
- Zhou, J.; Mahoney, K.M.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.H.; et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol. Res. 2017, 5, 480e492. [Google Scholar] [CrossRef]
- Lopci, E.; Haanen, J. Cancer management in the era of immunotherapy: “much more than what strikes the eyes”. Q. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Galon, J.; Lanzi, A. Immunoscore and its introduction in clinical practice. Q. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.C.; Recondo, G.; Rassy, E.; Mezquita, L. The LIPI score and inflammatory biomarkers for selection of patients with solid tumors treated with checkpoint inhibitors. Q. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, S.; Passiglia, F.; D’Incecco, A.; Gallo, M.; De Luca, A.; Rossi, E.; D’Incà, F.; Minuti, G.; Landi, L.; Bennati, C.; et al. Circulating programmed death ligand-1 (cPD-L1) in non-small-cell lung cancer (NSCLC). Oncotarget 2018, 9, 17554–17563. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Carbone, F.G.; Rossi, S.; Monterisi, S.; Federico, D.; Toschi, L.; Lopci, E. Circulating Tumor Cells and Metabolic Parameters in NSCLC Patients Treated with Checkpoint Inhibitors. Cancers 2020, 12, 487. [Google Scholar] [CrossRef]
- Seban, R.D.; Mezquita, L.; Berenbaum, A.; Dercle, L.; Botticella, A.; Le Pechoux, C.; Caramella, C.; Deutsch, E.; Grimaldi, S.; Adam, J.; et al. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1147–1157. [Google Scholar] [CrossRef]
- Grizzi, F.; Castello, A.; Qehajaj, D.; Toschi, L.; Rossi, S.; Pistillo, D.; Paleari, V.; Veronesi, G.; Novellis, P.; Monterisi, S.; et al. Independent expression of circulating and tissue levels of PD-L1: Correlation of clusters with tumor metabolism and outcome in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2019, 68, 1537–1545. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Ulrich, B.C.; Guibert, N. Immunotherapy efficacy and gender: Discovery in precision medicine. Transl. Lung Cancer Res. 2018, 7, S211–S213. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y.; Hosomi, Y.; Nakahara, Y.; Watanabe, K.; Sagawa, Y.; Homma, S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer 2017, 104, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zheng, J.; Zhu, J.; Xie, C.; Zhang, X.; Han, X.; Song, B.; Ma, Y.; Liu, J. PD-L1 gene polymorphism and high level of plasma soluble PD-L1 protein may be associated with small cell lung cancer. Int. J. Biol. Markers. 2015, 30, e364–e368. [Google Scholar] [CrossRef]
- Abu Hejleh, T.; Furqan, M.; Ballas, Z.; Clamon, G. The clinical significance of soluble PD-1 and PD-L1 in lung cancer. Crit. Rev. Oncol. Hematol. 2019, 143, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Dronca, R.S.; Mansfield, A.S.; Liu, X.; Harrington, S.; Enninga, E.A.; Kottschade, L.A.; Koo, C.W.; McWilliams, R.R.; Block, M.S.; Nevala, W.K. Bim and soluble PD-L1 (sPD-L1) as predictive biomarkers of response to anti-1 therapy in patients with melanoma and lung carcinoma. J. Clin. Oncol. 2017, 35, S11534. [Google Scholar] [CrossRef]
- Costantini, A.; Julie, C.; Dumenil, C.; Hélias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.F.; et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology 2018, 7, e1452581. [Google Scholar] [CrossRef]
- Tiako Meyo, M.; Jouinot, A.; Giroux-Leprieur, E.; Fabre, E.; Wislez, M.; Alifano, M.; Leroy, K.; Boudou-Rouquette, P.; Tlemsani, C.; Khoudour, N.; et al. Predictive Value of Soluble PD-1. PD-L1. VEGFA. CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study. Cancers 2020, 12, 473. [Google Scholar] [CrossRef]
- Ugurel, S.; Schadendorf, D.; Horny, K.; Sucker, A.; Schramm, S.; Utikal, J.; Pföhler, C.; Herbst, R.; Schilling, B.; Blank, C.; et al. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann. Oncol. 2020, 31, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Ito, K.; Teng, R.; Schöder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. 18F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef]
| Patient | Gender (M/F) | Age | ICI | sPD-L1 | PFS Months | OS Months | ||
|---|---|---|---|---|---|---|---|---|
| Pre- | Post- | % | ||||||
| 1 | F | 76 | pembrolizumab | 24.98 | 61.45 | 146.00 | 4.0 | 8.1 |
| 2 | M | 86 | nivolumab | 58.54 | 46.30 | −20.91 | 6.5 | 15.2 |
| 3 | M | 79 | nivolumab | 25.86 | 39.69 | 53.48 | 12.1 | 13.2 |
| 4 | F | 77 | pembrolizumab | 56.52 | 49.56 | −12.31 | 1.5 | 2.8 |
| 5 | F | 55 | nivolumab | 23.04 | 31.41 | 36.33 | 1.7 | 2.8 |
| 6 | M | 65 | pembrolizumab | 27.71 | 47.53 | 71.53 | 28.0 | 28.0 |
| 7 | M | 79 | nivolumab | 49.64 | 41.89 | −15.61 | 5.8 | 7.0 |
| 8 | M | 70 | nivolumab | 24.18 | 19.60 | −18.94 | 5.0 | 12.4 |
| 9 | M | 80 | nivolumab | 54.75 | 77.84 | 42.17 | 4.9 | 7.5 |
| 10 | F | 78 | nivolumab | 16.69 | 45.41 | 172.08 | 23.7 | 23.7 |
| 11 | M | 70 | nivolumab + ipilimumab | 30.61 | 54.40 | 77.72 | 23.2 | 24.1 |
| 12 | M | 52 | nivolumab | 34.93 | 51.49 | 47.41 | 1.6 | 2.2 |
| 13 | F | 77 | nivolumab | 25.33 | 28.85 | 13.90 | 6.0 | 6.9 |
| 14 | M | 73 | pembrolizumab | 61.27 | 27.18 | −55.64 | 28.7 | 28.7 |
| 15 | M | 51 | nivolumab | 40.92 | 50.44 | 23.26 | 2.3 | 2.3 |
| 16 | M | 78 | nivolumab | 32.55 | 42.33 | 30.05 | 5.6 | 18.0 |
| 17 | M | 53 | pembrolizumab | 11.23 | 26.56 | 136.51 | 1.8 | 19.4 |
| 18 | F | 80 | nivolumab | 26.74 | 21.89 | −18.14 | 1.8 | 4.0 |
| 19 | F | 80 | pembrolizumab | 19.51 | 64.71 | 231.68 | 0.1 | 1.9 |
| 20 | M | 75 | pembrolizumab | 25.50 | 42.33 | 66.00 | 25.0 | 25.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castello, A.; Rossi, S.; Toschi, L.; Mansi, L.; Lopci, E. Soluble PD-L1 in NSCLC Patients Treated with Checkpoint Inhibitors and Its Correlation with Metabolic Parameters. Cancers 2020, 12, 1373. https://doi.org/10.3390/cancers12061373
Castello A, Rossi S, Toschi L, Mansi L, Lopci E. Soluble PD-L1 in NSCLC Patients Treated with Checkpoint Inhibitors and Its Correlation with Metabolic Parameters. Cancers. 2020; 12(6):1373. https://doi.org/10.3390/cancers12061373
Chicago/Turabian StyleCastello, Angelo, Sabrina Rossi, Luca Toschi, Luigi Mansi, and Egesta Lopci. 2020. "Soluble PD-L1 in NSCLC Patients Treated with Checkpoint Inhibitors and Its Correlation with Metabolic Parameters" Cancers 12, no. 6: 1373. https://doi.org/10.3390/cancers12061373
APA StyleCastello, A., Rossi, S., Toschi, L., Mansi, L., & Lopci, E. (2020). Soluble PD-L1 in NSCLC Patients Treated with Checkpoint Inhibitors and Its Correlation with Metabolic Parameters. Cancers, 12(6), 1373. https://doi.org/10.3390/cancers12061373







