Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis
Abstract
1. Introduction
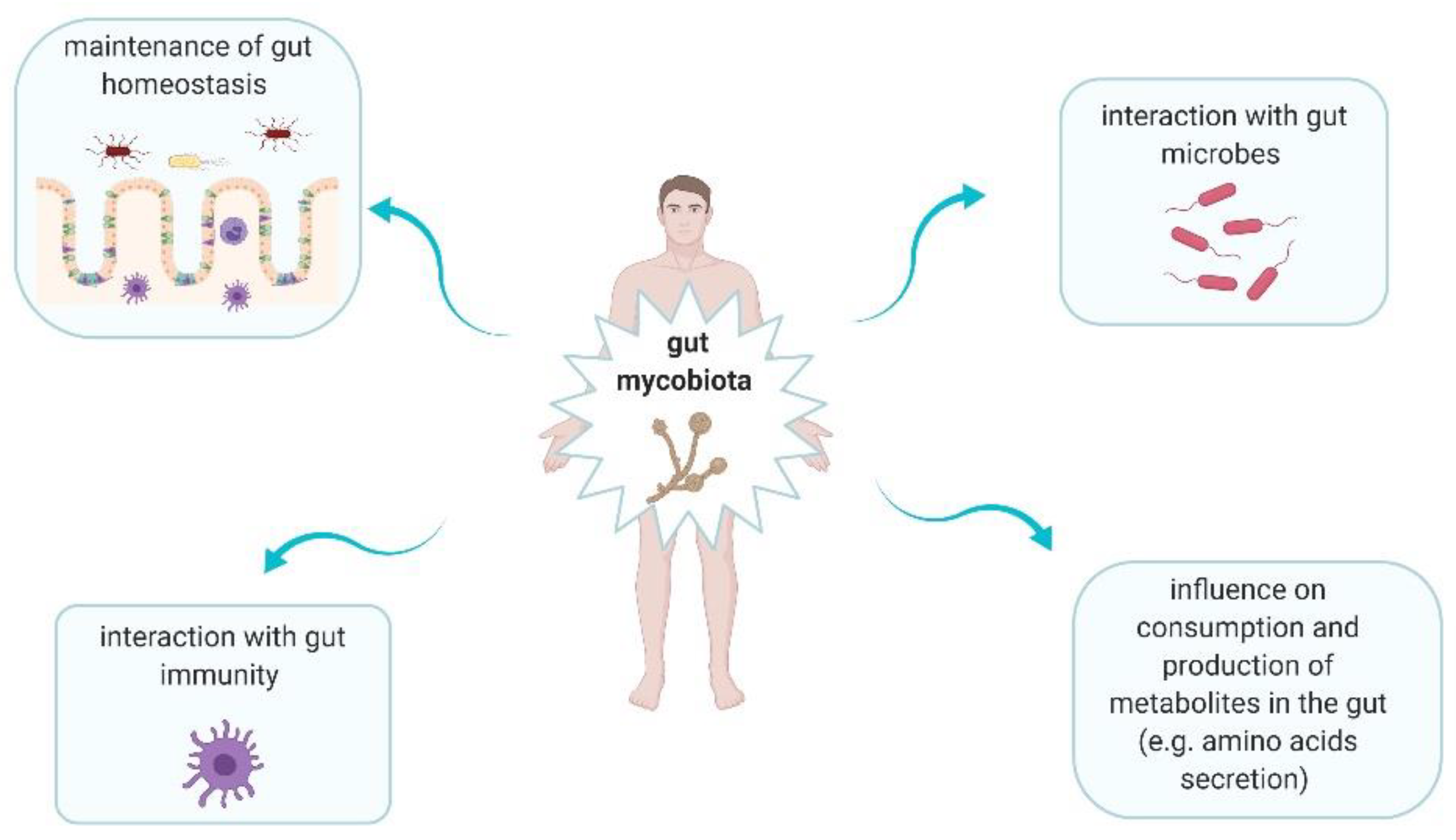
2. Gut Mycobiota in Healthy Gut
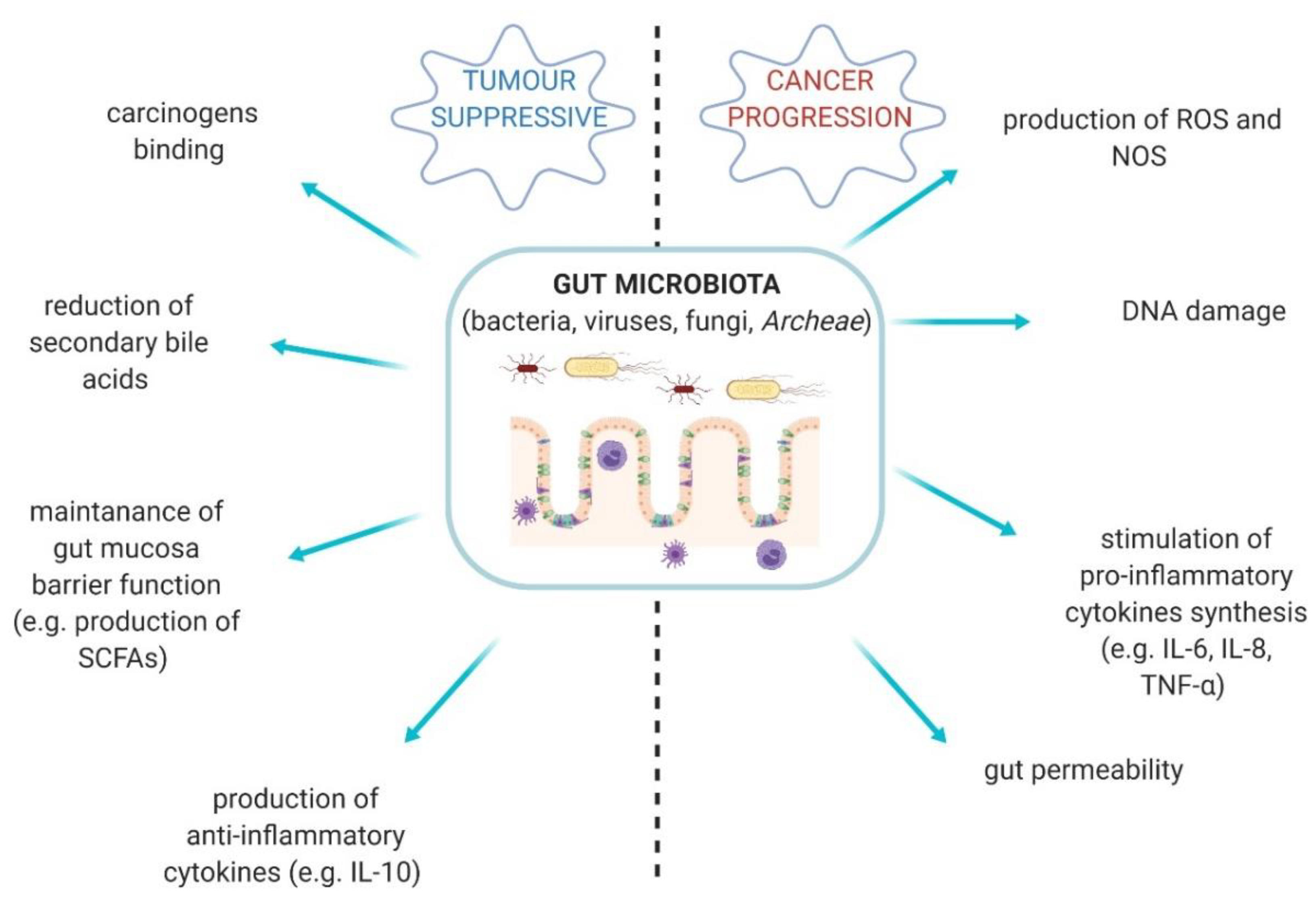
3. The Link between Fungal Microbiota Dysbiosis and Carcinogenesis
3.1. Colorectal Cancer
3.2. Oral Cancers
3.3. Pancreatic Cancer
4. Fungal Probiotics in Oncology
4.1. S. boulardii—Characteristics and Properties
4.2. S. boulardii in Oncohematological Patients
4.3. S. boulardii in Immunosuppressed/Critically Ill Patients
4.4. S. boulardii—Prevention of Cancer Development
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahnic, A.; Rupnik, M. Different host factors are associated with patterns in bacterial and fungal gut microbiota in Slovenian healthy cohort. PLoS ONE 2018, 12, e0209209. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. Cancer J. Clin. 2017, 4, 326–344. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 1, 38. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Ruszkowski, J.; Skonieczna-Żydecka, K.; Jędrzejczak, J.; Folwarski, M.; Makarewicz, W. Gastrointestinal cancers: The role of microbiota in carcinogenesis and the role of probiotics and microbiota in the anti-cancer therapy efficiency. Cent. Eur. J. Immunol. (accepted).
- Kaźmierczak-Siedlecka, K.; Fic, M.; Folwarski, M.; Makarewicz, W. Diet & Microbes: Gut health for the brain and body. In Proceedings of the 6th Nutrition Winter School 2020, Levi, Finland, 27–31 January 2020. [Google Scholar]
- Vyshenska, D.; Lam, K.C.; Shulzhenko, N.; Morgun, A. Interplay between viruses and bacterial microbiota in cancer development. Semin. Immunol. 2017, 32, 14–24. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Beebe-Dimmer, J. The cancer burden attributable to biologic agents. Ann. Epidemiol. 2015, 3, 183–187. [Google Scholar] [CrossRef]
- Coker, O.O.; Nakatsu, G.; Dai, R.Z.; Wu, W.K.K.; Wong, S.H.; Ng, S.C.; Leung chan, F.K.; Jao Yiu Sung, J.; Yu, J. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 2019, 4, 654–662. [Google Scholar] [CrossRef]
- Klimesova, K.; Jiraskova Zakostelska, Z.; Tlaskalova-Hogenova, H. Oral Bacterial and Fungal Microbiome Impacts Colorectal Carcinogenesis. Front. Microbiol. 2018, 9, 774. [Google Scholar] [CrossRef]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal Fungi in Health and Disease. Cell Host Microbe 2017, 2, 156–165. [Google Scholar] [CrossRef]
- Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf?fbclid=IwAR24wyni_jp6BAwwQ0Xj7-1E2aSFSjHPq4BrG-KSMcF2Vuee5o-wJMuaalc (accessed on 15 May 2020).
- Mukherjee, P.K.; Sendid, B.; Hoarau, G.; Colombel, J.F.; Poulain, D.; Ghannoum, M.A. Mycobiota in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2015, 2, 77–87. [Google Scholar] [CrossRef]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE 2013, 6, e66019. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Leonardi, I. Fungal Dysbiosis: Immunity and Interactions at Mucosal Barriers. Nat. Rev. Immunol. 2017, 10, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 1, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Murzyn, A.; Krasowska, A.; Stefanowicz, P.; Dziadkowiec, D.; Łukaszewicz, M. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS ONE 2010, 8, e12050. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 1, 120. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Di Paola, M.; Stefanini, I.; Albanese, D.; Rizzetto, L.; Lionetti, P.; Calabrò, A.; Jousson, O.; Donati, C.; Cavalieri, D.; et al. Age and Gender Affect the Composition of Fungal Population of the Human Gastrointestinal Tract. Front. Microbiol. 2016, 7, 1227. [Google Scholar] [CrossRef]
- Kapitan, M.; Niemiec, M.J.; Steimle, A.; Frick, J.S.; Jacobsen, I.D. Fungi as Part of the Microbiota and Interactions with Intestinal Bacteria. Curr. Top. Microbiol. Immunol. 2019, 422, 265–301. [Google Scholar]
- Paterson, M.J.; Oh, S.; Underhill, D.M. Host-microbe Interactions: Commensal Fungi in the Gut. Curr. Opin. Microbiol. 2017, 40, 131–137. [Google Scholar] [CrossRef]
- Richard, M.L.; Sokol, H. The Gut Mycobiota: Insights Into Analysis, Environmental Interactions and Role in Gastrointestinal Diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 6, 331–345. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Wu, W.; Chen, D.Z.; Xiao, M.; Huang, X. The opportunistic human fungal pathogen Candida albicans promotes the growth and proliferation of commensal Escherichia coli through an iron-responsive pathway. Microbiol. Res. 2018, 207, 232–239. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Hogan, D.A.; Mylonakis, E. Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 2010, 8, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Chiaro, T.R.; Soto, R.; Stephens, W.Z.; Kubinak, J.L.; Petersen, C.; Gogokhia, L. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci. Transl. Med. 2017, 380, eaaf9044. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, J.; An, Y.; Li, P.; Xiong, W.; Li, J.; Yan, D.; Wang, M.Y.; Zhong, G.S. Chitooligosaccharides Prevents the Development of Colitis-Associated Colorectal Cancer by Modulating the Intestinal Microbiota and Mycobiota. Front. Microbiol. 2019, 10, 2101. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Sherafat, S.; Alebouyeh, M.; Moghim, S.; Ahmadi Amoli, H.; Ghasemian-Safaei, H. Role of gut microbiota in the pathogenesis of colorectal cancer; a review article. Gastroenterol. Hepatol. Bed Bench 2018, 2, 101–109. [Google Scholar]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of Gut Microbiota in the Development and Treatment of Colorectal Cancer. Digestion 2019, 1, 72–78. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, J.; Wu, Q.; Chen, J.; Liu, J.; Wang, L.; Chen, C.W.; Xu, J.M.; Zhang, H.P.; Shi, C.F.; et al. The role of microbiota in the development of colorectal cancer. Int. J. Cancer 2019, 8, 2032–2041. [Google Scholar] [CrossRef]
- Gagnière, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.-A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 2, 501–518. [Google Scholar]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Fic, M.; van de Wetering, T.; Folwarski, M.; Makareiwcz, W. Therapeutic methods of gut microbiota modification in colorectal cancer management–fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes 2020. (accepted). [Google Scholar] [CrossRef]
- Kim, S.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 4, 357–368. [Google Scholar] [CrossRef]
- Caiqin, Q.; Wei, W.; Huie, P.; Rong, H.; Wei, L. Preparation and properties of reduced chitooligomers. Carbohydr. Polym. 2008, 4, 701–706. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 6, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Nurhayati, Y.; Abdul Manaf, A.; Osman, H.; Che Abdullah, A.B.; Huat Tang, J.Y. Effect of Chitosan Oligosaccharides on the Growth of Bifidobacterium Species. Mal. J. Appl. Sci. 2016, 1, 13–23. [Google Scholar]
- Pan, X.; Chen, F.; Wu, T.; Tang, H.; Zhao, Z. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J. Zhejiang Univ. Sci. B 2009, 4, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Habib, K.; Maggiotto, F.; Pignede, G.; Vandekerckove, P.; Maes, E.; Dubuquoy, L.; Fontaine, T.; Guerardel, Y.; Poulain, D. Modulation of Intestinal Inflammation by Yeasts and Cell Wall Extracts: Strain Dependence and Unexpected Anti-Inflammatory Role of Glucan Fractions. PLoS ONE 2012, 7, e40648. [Google Scholar] [CrossRef]
- Gao, R.; Kong, C.; Li, H.; Huang, L.; Qu, X.; Qin, N.; Qin, H. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 12, 2457–2468. [Google Scholar] [CrossRef]
- Luan, C.; Xie, L.; Yang, X.; Miao, H.; Lv, N.; Zhang, R.; Xiao, X.; Hu, Y.F.; Liu, Y.L.; Wu, N.; et al. Dysbiosis of Fungal Microbiota in the Intestinal Mucosa of Patients with Colorectal Adenomas. Sci. Rep. 2015, 5, 7980. [Google Scholar] [CrossRef]
- Arzmi, M.H.; Dashper, S.; McCullough, M. Polymicrobial interactions of Candida albicans and its role in oral carcinogenesis. J. Oral Pathol. Med. 2019, 7, 546–551. [Google Scholar] [CrossRef]
- Pushalkar, S.; Mane, S.P.; Ji, X.; Li, Y.; Evans, C.; Crasta, O.R.; Morse, D.; Meagher, R.; Singh, A.; Saxena, D. Microbial diversity in saliva of oral squamous cell carcinoma. Immunol. Med. Microbiol. 2011, 3, 269–277. [Google Scholar] [CrossRef]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016, 8, 805–814. [Google Scholar] [CrossRef]
- Stornetta, A.; Guidolin, V.; Balbo, S. Alcohol-Derived Acetaldehyde Exposure in the Oral Cavity. Cancers 2018, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, A.; Nawaz, A.; Mäkitie, A.; Meurman, J.H. Role of Non-Albicans Candida and Candida Albicans in Oral Squamous Cell Cancer Patients. J. Oral Maxillofac. Surg. 2018, 12, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Wang, H.; Retuerto, M.; Zhang, H.; Burkey, B.; Ghannoum, M.A.; Eng, C. Bacteriome and mycobiome associations in oral tongue cancer. Oncotarget 2017, 57, 97273–97289. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2016, 2, 181–193. [Google Scholar]
- Dongari-Bagtzoglou, A.; Kashleva, H. Candida albicans triggers interleukin-8 secretion by oral epithelial cells. Microb. Pathog. 2003, 4, 169–177. [Google Scholar] [CrossRef]
- Simoes, P.K.; Olson, S.H.; Saldia, A.; Kurtz, R.C. Epidemiology of pancreatic adenocarcinoma. Chin. Clin. Oncol. 2017, 3, 24. [Google Scholar] [CrossRef]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinform. 2018, 1, 33–49. [Google Scholar] [CrossRef]
- Wei, M.Y.; Shi, S.; Liang, C.; Meng, Q.C.; Hua, J.; Zhang, Y.Y.; Liu, J.; Zhang, B.; Xu, J.; Yu, X.-J. The microbiota and microbiome in pancreatic cancer: More influential than expected. Mol. Cancer 2019, 18, 97. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.L.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 7777, 264–267. [Google Scholar] [CrossRef]
- Turner, M.W. The role of mannose-binding lectin in health and disease. Mol. Immunol. 2003, 7, 423–429. [Google Scholar] [CrossRef]
- Eissa, M.A.L.; Lerner, L.; Abdelfatah, E.; Shankar, N.; Canner, J.K.; Hasan, N.M.; Yaghoobi, V.; Huang, B.; Kerner, Z.; Takaesu, F.; et al. Promoter methylation of ADAMTS1 and BNC1 as potential biomarkers for early detection of pancreatic cancer in blood. Clin. Epigenetics 2019, 1, 59. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 1, 141. [Google Scholar] [CrossRef] [PubMed]
- Mendez, R.; Kesh, K.; Arora, N.; Di Martino, L.; McAllister, F.; Merchant, N.; Banerjee, S. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis 2019, bgz116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xu, J.W.; Cheng, Y.G.; Gao, J.Y.; Hu, S.Y.; Wang, L.; Zhan, H.-X. Early detection of pancreatic cancer: Where are we now and where are we going? Int. J. Cancer 2017, 2, 231–241. [Google Scholar] [CrossRef]
- Selander, C.; Engblom, C.; Nilsson, G.; Scheynius, A.; Andersson, C.L. TLR2/MyD88-dependent and -independent activation of mast cell IgE responses by the skin commensal yeast Malassezia sympodialis. J. Immunol. 2009, 7, 4208–4216. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Shen, S.; Hou, Y.; Chen, Y.; Wang, T. The Mycobiota of the Human Body: A Spark Can Start a Prairie Fire. Gut Microbes 2020, 9, 1–25. [Google Scholar] [CrossRef]
- Conche, C.; Greten, F.R. Fungi Enter the Stage of Colon Carcinogenesis. Immunity 2018, 3, 384–386. [Google Scholar] [CrossRef]
- Montoya, A.M.; González, G.M.; Martinez-Castilla, A.M.; Aguilar, S.A.; Franco-Molina, M.A.; Coronado-Cerda, E. Cytokines Profile in Immunocompetent Mice During Trichosporon Asahii Infection. Med. Mycol. 2018, 1, 103–109. [Google Scholar] [CrossRef]
- Edwards-Ingram, L.; Gitsham, P.; Burton, N.; Warhurst, G.; Clarke, I.; Hoyle, D.; Oliver, S.G.; Stateva, L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 8, 2458–2467. [Google Scholar] [CrossRef]
- Czerucka, D.; Rampal, P. Diversity of Saccharomyces boulardii CNCM I-745 mechanisms of action against intestinal infections. World J. Gastroenterol. 2019, 18, 2188–2203. [Google Scholar] [CrossRef]
- Neut, C.; Mahieux, S.; Dubreuil, L.J. Antibiotic Susceptibility of Probiotic Strains: Is It Reasonable to Combine Probiotics with Antibiotics? Med. Mal. Infect. 2017, 7, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Moré, M.I.; Swidsinski, A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis–a review. Clin. Exp. Gastroenterol. 2015, 8, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.P.; Zheng, Y.; Wu, T.; He, Q.; Teng, G.G.; Wang, H.H. Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chin. Med. J. 2019, 16, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Fic, M.; Ruszkowski, J.; Folwarski, M.; Makarewicz, W. Saccharomyces boulardii (CNCM I-745): A non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr. Microbiol. (under review).
- Sulik-Tyszka, B.; Snarski, E.; Niedźwiedzka, M.; Augustyniak, M.; Myhre, T.N.; Kacprzyk, A.; Swoboda-Kopeć, E.; Roszkowska, M.; Dwilewicz-Trojaczek, J.; Jędrzejczak, W.W.; et al. Experience with Saccharomyces boulardii Probiotic in Oncohaematological Patients. Probiotics Antimicro. Proteins 2018, 2, 350–355. [Google Scholar] [CrossRef]
- Burkhardt, O.; Köhnlein, T.; Pletz, M.; Welte, T. Saccharomyces boulardii induced sepsis: Successful therapy with voriconazole after treatment failure with fluconazole. Scand. J. Infect. Dis. 2005, 1, 69–72. [Google Scholar] [CrossRef]
- Lolis, N.; Veldekis, D.; Moraitou, H.; Kanavaki, S.; Velegraki, A.; Triandafyllidis, C.; Tasioudis, C.; Pefanis, A.; Pneumatikos, I. Saccharomyces boulardii fungaemia in an intensive care unit patient treated with caspofungin. Crit. Care Lond. Engl. 2008, 2, 414. [Google Scholar]
- Cesaro, S.; Chinello, P.; Rossi, L.; Zanesco, L. Saccharomyces cerevisiae fungemia in a neutropenic patient treated with Saccharomyces boulardii. Support Care Cancer 2000, 6, 504–505. [Google Scholar] [CrossRef]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.-A.H.; Boeckh, M.J. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol. Blood Marrow Transplant. 2009, 10, 1143–1238. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Piekarska, A.; Lubieniecka-Archutowska, E.; Bicz, M.; Folwarski, M.; Makarewicz, W. Nutritional status in patients after hematopoietic cell transplantation. Acta Haematol. Pol. 2019, 1, 1–9. [Google Scholar]
- Muñoz, P.; Bouza, E.; Cuenca-Estrella, M.; Eiros, J.M.; Pérez, M.J.; Sánchez-Somolinos, M.; Rincón, C.; Hortal, J.; Peláez, T. Saccharomyces cerevisiae Fungemia: An Emerging Infectious Disease. Clin. Infect. Dis. 2005, 11, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Anaissie, E.; Bodey, G.P.; Kantarjian, H.; Ro, J.; Vartivarian, S.E.; Hopfer, R.; Hoy, J.; Rolston, K. New spectrum of fungal infections in patients with cancer. Rev. Infect. Dis. 1989, 3, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Aucott, J.N.; Fayen, J.; Grossnicklas, H.; Morrissey, A.; Lederman, M.M.; Salata, R.A. Invasive infection with Saccharomyces cerevisiae: Report of three cases and review. Rev. Infect. Dis. 1990, 3, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Appel-da-Silva, M.C.; Narvaez, G.A.; Perez, L.R.R.; Drehmer, L.; Lewgoy, J. Saccharomyces cerevisiae var. boulardii fungemia following probiotic treatment. Med. Mycol. Case Rep. 2017, 18, 15–17. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Zhang, X.; Yang, H.; Qian, J.; Li, J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019, 1, 246. [Google Scholar] [CrossRef]
- Fortin, O.; Aguilar-Uscanga, B.R.; Vu, K.D.; Salmieri, S.; Lacroix, M. Effect of Saccharomyces Boulardii Cell Wall Extracts on Colon Cancer Prevention in Male F344 Rats Treated with 1,2-Dimethylhydrazine. Nutr. Cancer 2018, 4, 632–642. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szajda, S.D.; Konarzewska-Duchnowska, E.; Zalewska-Szajda, B.; Gałązkowski, R.; Sawko, A.; Nammous, H.; Buko, V.; Szulc, A.; Zwierz, K.; et al. Serum β-glucuronidase as a potential colon cancer marker: A preliminary study. Postepy Hig. Med. Dosw. 2015, 69, 436–439. [Google Scholar] [CrossRef]
| Fungal Genus | Mechanisms in Cancer Development | References |
|---|---|---|
| Candida |
| [42,46,57,58] |
| Malassezia |
| [39,51,59] |
| Trichosporon |
| [38,60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak-Siedlecka, K.; Dvořák, A.; Folwarski, M.; Daca, A.; Przewłócka, K.; Makarewicz, W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers 2020, 12, 1326. https://doi.org/10.3390/cancers12051326
Kaźmierczak-Siedlecka K, Dvořák A, Folwarski M, Daca A, Przewłócka K, Makarewicz W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers. 2020; 12(5):1326. https://doi.org/10.3390/cancers12051326
Chicago/Turabian StyleKaźmierczak-Siedlecka, Karolina, Aleš Dvořák, Marcin Folwarski, Agnieszka Daca, Katarzyna Przewłócka, and Wojciech Makarewicz. 2020. "Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis" Cancers 12, no. 5: 1326. https://doi.org/10.3390/cancers12051326
APA StyleKaźmierczak-Siedlecka, K., Dvořák, A., Folwarski, M., Daca, A., Przewłócka, K., & Makarewicz, W. (2020). Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers, 12(5), 1326. https://doi.org/10.3390/cancers12051326