Role of Tumor and Stroma-Derived IGF/IGFBPs in Pancreatic Cancer
Abstract
1. Introduction
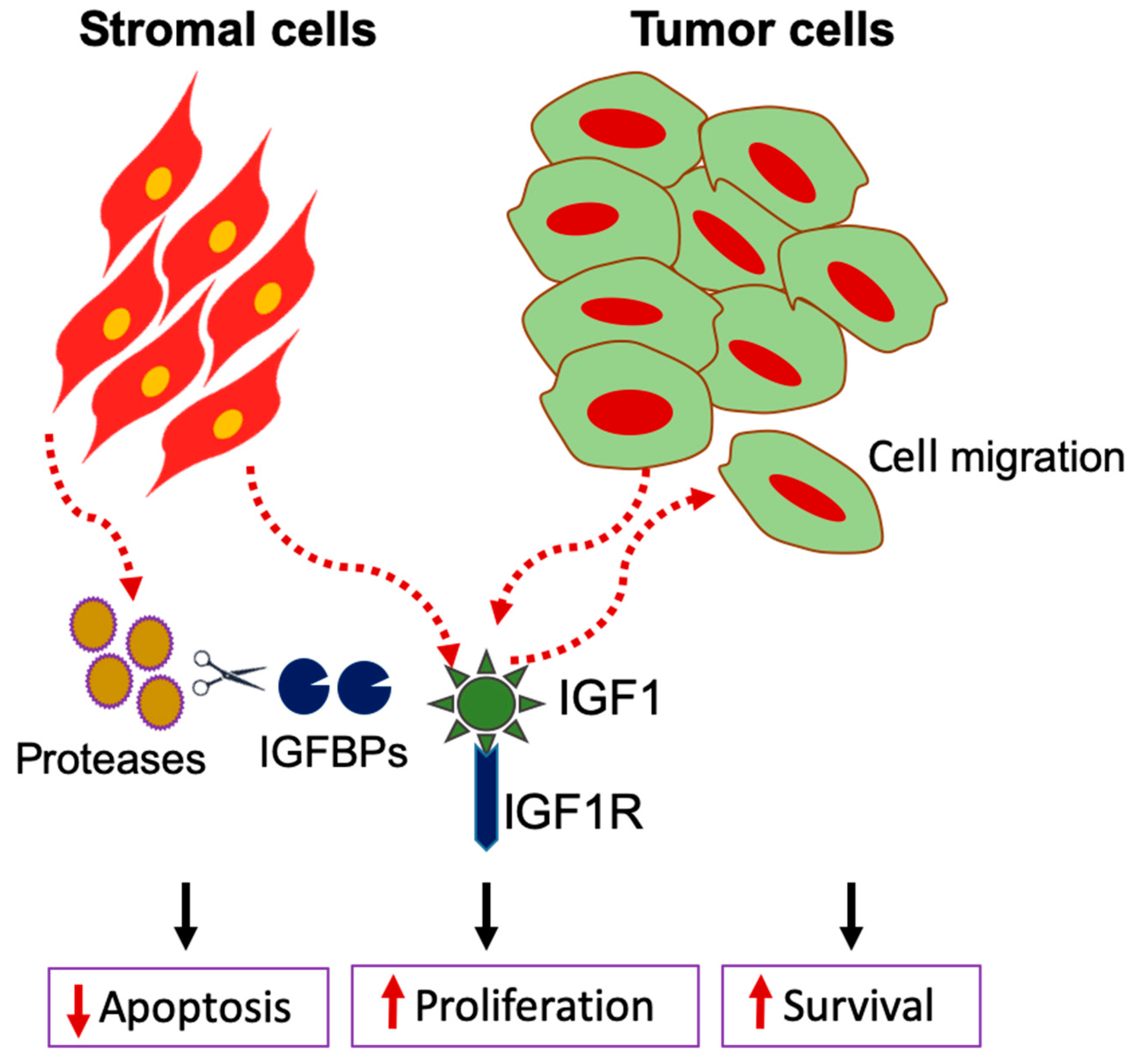
2. IGF Signaling in Tumor-Stromal Cell Transformation in Pancreas
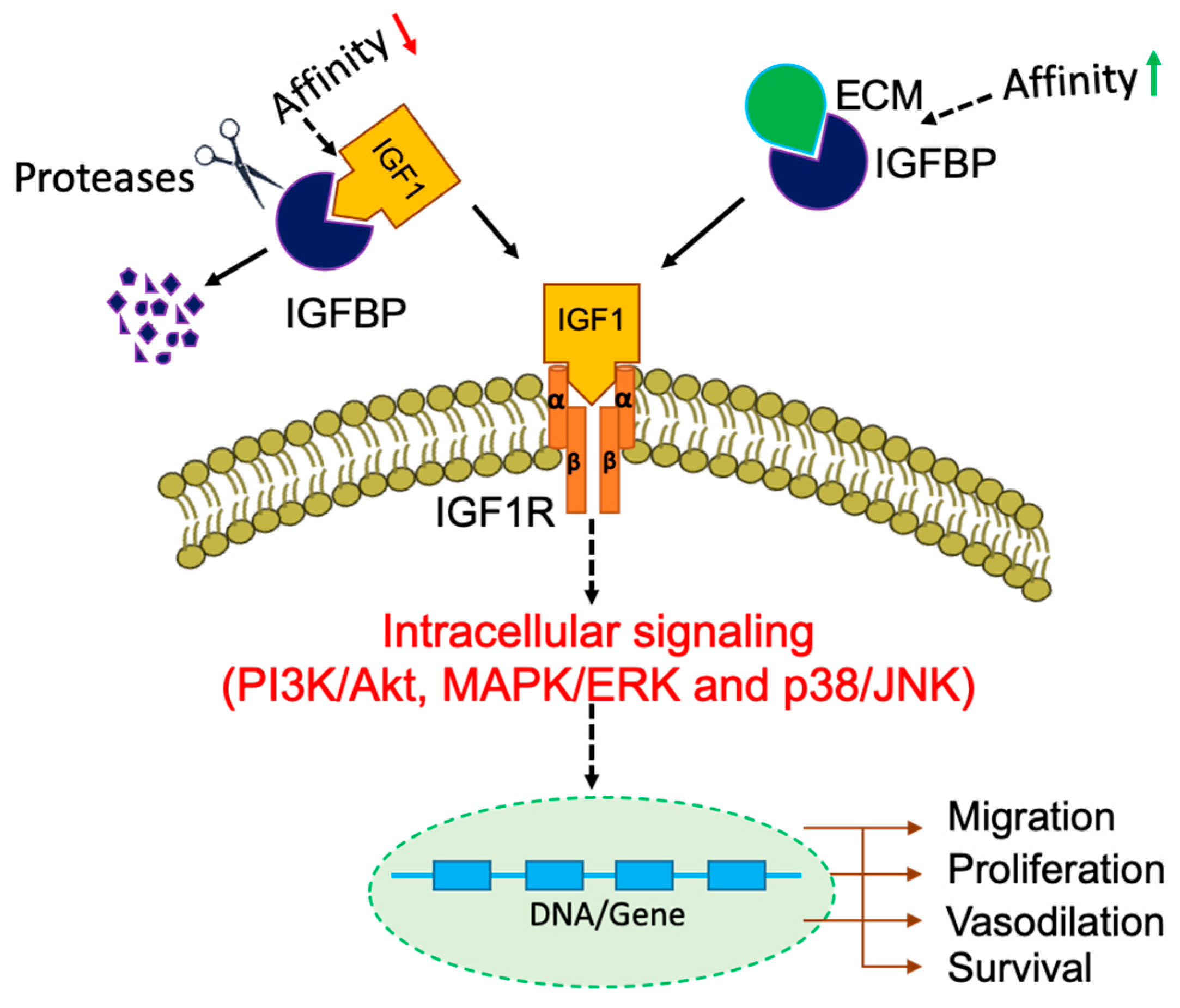
3. Insulin-Like Growth Factor Binding Proteins (IGFBPs)
4. IGF-Independent Actions of IGFBPs
5. IGFBPs and Extracellular Matrix
6. IGFBPs as Potential Cancer Biomarkers
7. The Therapeutic Relevance of IGFBPs
7.1. Knockout/Knockdown of IGFBPs as Targeted Therapy
7.2. Small Molecule Inhibitors of IGFBPs
8. Concluding Remarks and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| CAF | Cancer-associated fibroblasts |
| ECM | Extracellular matrix |
| TME | Tumor microenvironment |
| IGF | Insulin-like growth factor |
| IGFR | Insulin-like growth factor receptor |
| IGFBP | Insulin-like growth factor binding proteins |
| MMPs | Matrix metalloproteases |
| PC | Pancreatic cancer |
| PSC | Pancreatic stellate cells |
| TGF-β | Transforming growth factor-beta |
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Dusch, N.; Weiss, C.; Ströbel, P.; Kienle, P.; Post, S.; Niedergethmann, M. Factors Predicting Long-Term Survival Following Pancreatic Resection for Ductal Adenocarcinoma of the Pancreas: 40 Years of Experience. J. Gastrointest. Surg. 2013, 18, 674–681. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Zhu, H.; Wei, M.; Xu, J.; Hua, J.; Liang, C.; Meng, Q.; Zhang, Y.; Liu, J.; Zhang, B.; Yu, X.-J.; et al. PARP inhibitors in pancreatic cancer: Molecular mechanisms and clinical applications. Mol. Cancer 2020, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Liao, W.; Chan, T.; Chen, W.; Lee, C.; Shan, Y.; Huang, P.; Hou, Y.; Li, C.; Tsai, K.K. The differential distributions of ASPM isoforms and their roles in Wnt signaling, cell cycle progression, and pancreatic cancer prognosis. J. Pathol. 2019, 249, 498–508. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardiere, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. New Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Yoshida, K.; Iwashita, T.; Uemura, S.; Maruta, A.; Okuno, M.; Ando, N.; Iwata, K.; Kawaguchi, J.; Mukai, T.; Shimizu, M. A multicenter prospective phase II study of first-line modified FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget 2017, 8, 111346–111355. [Google Scholar] [CrossRef]
- Pandol, S.; Edderkaoui, M.; Gukovsky, I.; Lugea, A.; Gukovskaya, A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2009, 7, S44–S47. [Google Scholar] [CrossRef]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef]
- Korc, M. Pancreatic cancer–associated stroma production. Am. J. Surg. 2007, 194, S84–S86. [Google Scholar] [CrossRef]
- Chu, G.C.; Kimmelman, A.C.; Hezel, A.F.; DePinho, R.A. Stromal biology of pancreatic cancer. J. Cell. Biochem. 2007, 101, 887–907. [Google Scholar] [CrossRef]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Freitas, J.P.; Hussain, S.M.; Glazer, E.S. TGF-β Inhibitors in Metastatic Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Cancer 2019, 50, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Puzzoni, M.; Ziranu, P.; Pretta, A.; Impera, V.; Mariani, S.; Liscia, N.; Soro, P.; Musio, F.; Persano, M.; et al. New therapeutic targets in pancreatic cancer. Cancer Treat. Rev. 2019, 81, 101926. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Sapalidis, K.; Kotidis, E.; Mixalopoulos, N.; Zarogoulidis, P.; Tsavlis, D.; Baka, S.; Man, Y.-G.; Kanellos, J. Pancreatic cancer from bench to bedside: Molecular pathways and treatment options. Ann. Transl. Med. 2016, 4, 165. [Google Scholar] [CrossRef] [PubMed]
- De Meyts, P.; Whittaker, J. Structural biology of insulin and IGF1 receptors: Implications for drug design. Nat. Rev. Drug Discov. 2002, 1, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Freychet, P.; Roth, J.; Neville, D.M. Insulin Receptors in the Liver: Specific Binding of [125I] Insulin to the Plasma Membrane and Its Relation to Insulin Bioactivity. Proc. Natl. Acad. Sci. USA 1971, 68, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Hao, K.; Qin, C.; Xie, K.; Xie, X.; Yang, Y. Insulin-Like Growth Factor 1 Receptor Promotes the Growth and Chemoresistance of Pancreatic Cancer. Dig. Dis. Sci. 2013, 58, 2705–2712. [Google Scholar] [CrossRef]
- Shimasaki, S.; Ling, N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog. Growth Factor Res. 1991, 3, 243–266. [Google Scholar] [CrossRef]
- Baxter, R.C. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef]
- Murphy, L.O.; Wahab, Y.H.A.; Wang, Q.J.; Knezetic, J.A.; Permert, J.; Larsson, J.; Hollingsworth, A.M.; Adrian, T.E. Receptors and Ligands for Autocrine Growth Pathways Are Upregulated When Pancreatic Cancer Cells Are Adapted to Serum-Free Culture. Pancreas 2001, 22, 293–298. [Google Scholar] [CrossRef]
- Kornmann, M.; Maruyama, H.; Bergmann, U.; Tangvoranuntakul, P.; Beger, H.G.; White, M.F.; Korc, M. Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res. 1998, 58, 4250–4254. [Google Scholar] [PubMed]
- Hirakawa, T.; Yashiro, M.; Doi, Y.; Kinoshita, H.; Morisaki, T.; Fukuoka, T.; Hasegawa, T.; Kimura, K.; Amano, R.; Hirakawa, K. Pancreatic Fibroblasts Stimulate the Motility of Pancreatic Cancer Cells through IGF1/IGF1R Signaling under Hypoxia. PLoS ONE 2016, 11, e0159912. [Google Scholar] [CrossRef] [PubMed]
- Tommelein, J.; De Vlieghere, E.; Verset, L.; Melsens, E.; Leenders, J.; Descamps, B.; Debucquoy, A.; Vanhove, C.; Pauwels, P.; Gespach, C.; et al. Radiotherapy-Activated Cancer-Associated Fibroblasts Promote Tumor Progression through Paracrine IGF1R Activation. Cancer Res. 2017, 78, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Tape, C.J.; Ling, S.; Dimitriadi, M.; McMahon, K.M.; Worboys, J.D.; Leong, H.S.; Norrie, I.C.; Miller, C.J.; Poulogiannis, G.; Lauffenburger, D.A.; et al. Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation. Cell 2016, 165, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Thayer, S.P.; Di Magliano, M.P.; Heiser, P.W.; Nielsen, C.M.; Roberts, U.J.; Lauwers, G.Y.; Qi, Y.P.; Gysin, S.; Castillo, C.F.-D.; Yajnik, V.; et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 2003, 425, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Fendrich, V.; Oh, E.; Bang, S.; Karikari, C.; Ottenhof, N.; Bisht, S.; Lauth, M.; Brossart, P.; Katsanis, N.; Maitra, A.; et al. Ectopic Overexpression of Sonic Hedgehog (Shh) Induces Stromal Expansion and Metaplasia in the Adult Murine Pancreas. Neoplasia 2011, 13, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Héron-Milhavet, L.; Leroith, D. Insulin-like Growth Factor I Induces MDM2-dependent Degradation of p53 via the p38 MAPK Pathway in Response to DNA Damage. J. Biol. Chem. 2002, 277, 15600–15606. [Google Scholar] [CrossRef]
- Werner, H. Tumor suppressors govern insulin-like growth factor signaling pathways: Implications in metabolism and cancer. Oncogene 2011, 31, 2703–2714. [Google Scholar] [CrossRef]
- Werner, H.; Karnieli, E.; Rauscher, F.J.; Leroith, D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc. Natl. Acad. Sci. USA 1996, 93, 8318–8323. [Google Scholar] [CrossRef]
- Daza, D.O.; Sundström, G.; Bergqvist, C.A.; Duan, C.; Larhammar, D. Evolution of the Insulin-Like Growth Factor Binding Protein (IGFBP) Family. Endocrinology 2011, 152, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Sarfstein, R.; Werner, H. Minireview: Nuclear Insulin and Insulin-like Growth Factor-1 Receptors: A Novel Paradigm in Signal Transduction. Endocrinology 2013, 154, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Firth, S.M.; Baxter, R.C. Cellular Actions of the Insulin-Like Growth Factor Binding Proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A.; Headey, S.; Norton, R. IGF-binding proteins—The pieces are falling into place. Trends Endocrinol. Metab. 2005, 16, 228–234. [Google Scholar] [CrossRef]
- Baxter, R.C. Circulating binding proteins for the insulin like growth factors. Trends Endocrinol. Metab. 1993, 4, 91–96. [Google Scholar] [CrossRef]
- Baxter, R.C. Circulating Levels and Molecular Distribution of the Acid-Labile (alpha) Subunit of the High Molecular Weight Insulin-Like Growth Factor-Binding Protein Complex. J. Clin. Endocrinol. Metab. 1990, 70, 1347–1353. [Google Scholar] [CrossRef]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar]
- Rajaram, S.; Baylink, D.J.; Mohan, S. Insulin-like growth factor-binding proteins in serum and other biological fluids: Regulation and functions. Endocr. Rev. 1997, 18, 801–831. [Google Scholar]
- Rorive, S.; Berton, A.; D’Haene, N.; Takacs, C.N.; Debeir, O.; Decaestecker, C.; Salmon, I. Matrix metalloproteinase-9 interplays with the IGFBP2-IGFII complex to promote cell growth and motility in astrocytomas. Glia 2008, 56, 1679–1690. [Google Scholar] [CrossRef]
- Boldt, H.B.; Conover, C.A. Overexpression of Pregnancy-Associated Plasma Protein-A in Ovarian Cancer Cells Promotes Tumor Growth in Vivo. Endocrinology 2011, 152, 1470–1478. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, H.; Boyle, B.; Rupp, F.; Funk, W.D.; Dedera, D. Identification of a novel insulin-like growth factor binding protein gene homologue with tumor suppressor like properties. Biochem. Biophys. Res. Commun. 2005, 331, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Torng, P.-L.; Lee, Y.-C.; Huang, C.-Y.; Ye, J.-H.; Lin, Y.-S.; Chu, Y.-W.; Huang, S.-C.; Cohen, P.; Wu, C.-W.; Lin, C.-T. Insulin-like growth factor binding protein-3 (IGFBP-3) acts as an invasion-metastasis suppressor in ovarian endometrioid carcinoma. Oncogene 2007, 27, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Azar, W.J.; Živković, S.; Werther, G.A.; Russo, V.C. IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Oncogene 2013, 33, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, A.; Fettscher, O.; Lahm, H.; Blum, W.F.; Kolb, H.J.; Engelhardt, D.; Wolf, E.; Weber, M.M. Overexpression of insulin-like growth factor-binding protein-2 results in increased tumorigenic potential in Y-1 adrenocortical tumor cells. Cancer Res. 2000, 60, 834–838. [Google Scholar]
- Nam, T.; Moralez, A.; Clemmons, D. Vitronectin binding to IGF binding protein-5 (IGFBP-5) alters IGFBP-5 modulation of IGF-I actions. Endocrinology 2002, 143, 30–36. [Google Scholar] [CrossRef][Green Version]
- Jones, J.I.; Gockerman, A.; Busby, W.H.; Wright, G.; Clemmons, D.R. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc. Natl. Acad. Sci. USA 1993, 90, 10553–10557. [Google Scholar] [CrossRef]
- Schedlich, L.J.; Le Page, S.L.; Firth, S.M.; Briggs, L.J.; Jans, D.A.; Baxter, R.C. Nuclear Import of Insulin-like Growth Factor-binding Protein-3 and -5 Is Mediated by the Importin β Subunit. J. Biol. Chem. 2000, 275, 23462–23470. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Zhao, Y.; Maures, T.J.; Yin, P.; Duan, C. Evidence That IGF Binding Protein-5 Functions as a Ligand-Independent Transcriptional Regulator in Vascular Smooth Muscle Cells. Circ. Res. 2004, 94, e46–e54. [Google Scholar] [CrossRef]
- Zhu, W.; Shiojima, I.; Ito, Y.; Li, Z.; Ikeda, H.; Yoshida, M.; Naito, A.T.; Nishi, J.-I.; Ueno, H.; Umezawa, A.; et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature 2008, 454, 345–349. [Google Scholar] [CrossRef]
- Oh, Y.; Müller, H.L.; Lamson, G.; Rosenfeld, R.G. Insulin-like growth factor (IGF)-independent action of IGF-binding protein-3 in Hs578T human breast cancer cells. Cell surface binding and growth inhibition. J. Biol. Chem. 1993, 268, 14964–14971. [Google Scholar]
- Leal, S.M.; Liu, Q.; Huang, S.S.; Huang, J.S. The type V transforming growth factor beta receptor is the putative insulin-like growth factor-binding protein 3 receptor. J. Biol. Chem. 1997, 272, 20572–20576. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Wai, C.; DeMambro, V.; Rosen, C.J.; Clemmons, D.R. IGFBP-2 directly stimulates osteoblast differentiation. J. Bone Miner. Res. 2014, 29, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.; Honselmann, K.C.; Liss, A. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers 2018, 10, 316. [Google Scholar] [CrossRef]
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 2019, 18, 14. [Google Scholar] [CrossRef]
- Waghray, M.; Yalamanchili, M.; Di Magliano, M.P.; Simeone, D.M. Deciphering the role of stroma in pancreatic cancer. Curr. Opin. Gastroenterol. 2013, 29, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Bauer, C.A.; Öhlund, D.; Lauth, M.; Buchholz, M.; Michl, P.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut 2018, 68, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Q.; Liu, L.; Xu, J.-Z.; Yu, X.-J. Reflections on depletion of tumor stroma in pancreatic cancer. Biochim. Biophys. Acta (BBA) Bioenerg. 2019, 1871, 267–272. [Google Scholar] [CrossRef]
- Yang, J.; Waldron, R.T.; Su, H.-Y.; Moro, A.; Chang, H.-H.; Eibl, G.; Ferreri, K.; Kandeel, F.R.; Lugea, A.; Li, L.; et al. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am. J. Physiol. Liver Physiol. 2016, 311, G675–G687. [Google Scholar] [CrossRef]
- Zang, G.; Sandberg, M.; Carlsson, P.-O.; Welsh, N.; Jansson, L.; Barbu, A. Activated pancreatic stellate cells can impair pancreatic islet function in mice. Upsala J. Med Sci. 2015, 120, 169–180. [Google Scholar] [CrossRef]
- Rosendahl, A.H.; Gundewar, C.; Hilmersson, K.S.; Ni, L.; Saleem, M.; Andersson, R. Conditionally immortalized human pancreatic stellate cell lines demonstrate enhanced proliferation and migration in response to IGF-I. Exp. Cell Res. 2015, 330, 300–310. [Google Scholar] [CrossRef]
- McCaig, C. Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: Inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. J. Cell Sci. 2002, 115, 4293–4303. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.; Allan, G.J.; Lochrie, J.D.; Flint, D.J. Insulin-like growth factor-binding protein-5 (IGFBP-5): A critical member of the IGF axis. Biochem. J. 2006, 395, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Andress, D.L.; Birnbaum, R.S. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J. Biol. Chem. 1992, 267, 22467–22472. [Google Scholar] [PubMed]
- Arai, T.; Parker, A.; Busby, W.; Clemmons, D.R. Heparin, heparan sulfate, and dermatan sulfate regulate formation of the insulin-like growth factor-I and insulin-like growth factor-binding protein complexes. J. Biol. Chem. 1994, 269, 20388–20393. [Google Scholar] [PubMed]
- Clarke, J.B. Identification of the Extracellular Matrix Binding Sites for Insulin-like Growth Factor-binding Protein 5. J. Biol. Chem. 1996, 271, 13523–13529. [Google Scholar] [CrossRef]
- Ceyhan, G.O.; Schäfer, K.-H.; Kerscher, A.G.; Rauch, U.; Demir, I.E.; Kadihasanoglu, M.; Böhm, C.; Müller, M.W.; Büchler, M.W.; Giese, N.A.; et al. Nerve Growth Factor and Artemin Are Paracrine Mediators of Pancreatic Neuropathy in Pancreatic Adenocarcinoma. Ann. Surg. 2010, 251, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Rajah, R.; Bhala, A.; Nunn, S.E.; Peehl, D.M.; Cohen, P. 7S nerve growth factor is an insulin-like growth factor-binding protein protease. Endocrinology 1996, 137, 2676–2682. [Google Scholar] [CrossRef][Green Version]
- Jakubowska, K.; Pryczynicz, A.; Januszewska, J.; Sidorkiewicz, I.; Kemona, A.; Niewiński, A.; Lewczuk, Ł.; Kędra, B.; Guzińska-Ustymowicz, K. Expressions of Matrix Metalloproteinases 2, 7, and 9 in Carcinogenesis of Pancreatic Ductal Adenocarcinoma. Dis. Markers 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yano, K.; Sugimoto, S.; Ishii, G.; Hasebe, T.; Endoh, Y.; Kodama, K.; Goya, M.; Chiba, T.; Ochiai, A. Matrix metalloproteinase-7 facilitates insulin-like growth factor bioavailability through its proteinase activity on insulin-like growth factor binding protein 3. Cancer Res. 2004, 64, 665–671. [Google Scholar] [CrossRef]
- Miyamoto, S.; Nakamura, M.; Yano, K.; Ishii, G.; Hasebe, T.; Endoh, Y.; Sangai, T.; Maeda, H.; Shi-Chuang, Z.; Chiba, T.; et al. Matrix metalloproteinase-7 triggers the matricrine action of insulin-like growth factor-II via proteinase activity on insulin-like growth factor binding protein 2 in the extracellular matrix. Cancer Sci. 2007, 98, 685–691. [Google Scholar] [CrossRef]
- Braulke, T.; Claussen, M.; Saftig, P.; Wendland, M.; Neifer, K.; Schmidt, B.; Zapf, J.; Figura, K.B.; Peters, C. Proteolysis of IGFBPs by cathepsin D in vitro and in cathepsin D-deficient mice. Prog. Growth Factor Res. 1995, 6, 265–271. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Meyerhardt, J.A.; Chan, A.T.; Ng, K.; Chan, J.A.; Wu, K.; Pollak, M.N.; Giovannucci, E.L.; Fuchs, C.S. Insulin, the Insulin-Like Growth Factor Axis, and Mortality in Patients With Nonmetastatic Colorectal Cancer. J. Clin. Oncol. 2009, 27, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.; Shun, C.-T.; Liang, J.-T.; Chiu, H.-M.; Chen, M.-J.; Chen, C.; Wang, H.-P.; Wu, M.-S.; Lin, J.-T. Plasma Insulin-Like Growth Factor-Binding Protein-2 Levels as Diagnostic and Prognostic Biomarker of Colorectal Cancer. J. Clin. Endocrinol. Metab. 2010, 95, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Lu, H.; Gao, W.; Wang, L.; Lu, K.; Wu, S.; Pataer, A.; Huang, M.; El-Zein, R.; Lin, T.; et al. Insulin-Like Growth Factor Binding Protein-2 Level Is Increased in Blood of Lung Cancer Patients and Associated with Poor Survival. PLoS ONE 2013, 8, e74973. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Ellis, R.; Howard, B.; Boufraqech, M.; Gara, S.K.; Zhang, L.; Quezado, M.M.; Nilubol, N.; Kebebew, E. Analysis of IGF and IGFBP as Prognostic Serum Biomarkers for Adrenocortical Carcinoma. Ann. Surg. Oncol. 2014, 21, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Mircean, C.; Shmulevich, I.; Wang, H.; Liu, J.; Niemistö, A.; Kavanagh, J.J.; Lee, J.-H.; Zhang, W. Insulin-like growth factor binding protein 2 promotes ovarian cancer cell invasion. Mol. Cancer 2005, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Baron-Hay, S.; Boyle, F.; Ferrier, A.; Scott, C. Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clin. Cancer Res. 2004, 10, 1796–1806. [Google Scholar] [CrossRef]
- Sharma, J.; Gray, K.P.; Evan, C.; Nakabayashi, M.; Fichorova, R.; Rider, J.R.; Mucci, L.; Kantoff, P.W.; Sweeney, C.J. Elevated insulin-like growth factor binding protein-1 (IGFBP-1) in men with metastatic prostate cancer starting androgen deprivation therapy (ADT) is associated with shorter time to castration resistance and overall survival. Prostate 2013, 74, 225–234. [Google Scholar] [CrossRef]
- Dai, B.; Ruan, B.; Wu, J.; Wang, J.; Shang, R.; Sun, W.; Li, X.; Dou, K.; Wang, D.; Li, Y. Insulin-like growth factor binding protein-1 inhibits cancer cell invasion and is associated with poor prognosis in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 5645–5654. [Google Scholar]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. IGF Binding Protein-5 Induces Cell Senescence. Front. Endocrinol. 2018, 9, 53. [Google Scholar] [CrossRef]
- Nguyen, X.-X.; Muhammad, L.; Nietert, P.J.; Feghali-Bostwick, C. IGFBP-5 Promotes Fibrosis via Increasing Its Own Expression and That of Other Pro-fibrotic Mediators. Front. Endocrinol. 2018, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Wang, C.-Y.; Neuhouser, M.L.; Xiao, L.; Smith, A.W.; Reding, K.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R.; et al. Associations of insulin-like growth factor and insulin-like growth factor binding protein-3 with mortality in women with breast cancer. Int. J. Cancer 2012, 132, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-I.; Wang, Y.-H.; Wu, T.-F.; Wu, W.-R.; Liao, A.C.; Shen, K.-H.; Hsing, C.-H.; Shiue, Y.-L.; Huang, H.-Y.; Hsu, H.-P.; et al. IGFBP-5 overexpression as a poor prognostic factor in patients with urothelial carcinomas of upper urinary tracts and urinary bladder. J. Clin. Pathol. 2013, 66, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Seligson, D.B.; Yu, H.; Tze, S.; Said, J.; Pantuck, A.J.; Cohen, P.; Lee, K.-W. IGFBP-3 nuclear localization predicts human prostate cancer recurrence. Horm. Cancer 2012, 4, 12–23. [Google Scholar] [CrossRef]
- McCaffery, I.; Tudor, Y.; Deng, H.; Tang, R.; Suzuki, S.; Badola, S.; Kindler, H.L.; Fuchs, C.S.; Loh, E.; Patterson, S.D.; et al. Putative Predictive Biomarkers of Survival in Patients with Metastatic Pancreatic Adenocarcinoma Treated with Gemcitabine and Ganitumab, an IGF1R Inhibitor. Clin. Cancer Res. 2013, 19, 4282–4289. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Michaud, D.S.; Giovannucci, E.L.; Schernhammer, E.; Stampfer, M.J.; Manson, J.E.; Cochrane, B.B.; Rohan, T.E.; Ma, J.; Pollak, M.N.; et al. Circulating Insulin-Like Growth Factor Binding Protein-1 and the Risk of Pancreatic Cancer. Cancer Res. 2007, 67, 7923–7928. [Google Scholar] [CrossRef]
- Dahlem, C.; Barghash, A.; Puchas, P.; Haybaeck, J.; Kessler, S.M. The Insulin-Like Growth Factor 2 mRNA Binding Protein IMP2/IGF2BP2 is Overexpressed and Correlates with Poor Survival in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 3204. [Google Scholar] [CrossRef]
- Kendrick, Z.W.; Firpo, M.A.; Repko, R.C.; Scaife, C.L.; Adler, D.G.; Boucher, K.M.; Mulvihill, S.J. Serum IGFBP2 and MSLN as diagnostic and prognostic biomarkers for pancreatic cancer. HPB 2014, 16, 670–676. [Google Scholar] [CrossRef]
- Yoneyama, T.; Ohtsuki, S.; Honda, K.; Kobayashi, M.; Iwasaki, M.; Uchida, Y.; Okusaka, T.; Nakamori, S.; Shimahara, M.; Ueno, T.; et al. Identification of IGFBP2 and IGFBP3 As Compensatory Biomarkers for CA19-9 in Early-Stage Pancreatic Cancer Using a Combination of Antibody-Based and LC-MS/MS-Based Proteomics. PLoS ONE 2016, 11, e0161009. [Google Scholar] [CrossRef]
- Nixon, A.B.; Pang, H.; Starr, M.D.; Friedman, P.N.; Bertagnolli, M.M.; Kindler, H.L.; Goldberg, R.M.; Venook, A.P.; Hurwitz, H.I.; Oncology, A.F.C.T.I. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: Results from CALGB80303 (Alliance). Clin. Cancer Res. 2013, 19, 6957–6966. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yashiro, M.; Murata, A.; Hirata, K.; Kimura, K.; Amano, R.; Yamada, N.; Nakata, B.; Hirakawa, K. IGF-1 receptor and IGF binding protein-3 might predict prognosis of patients with resectable pancreatic cancer. BMC Cancer 2013, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Bollig-Fischer, A.; Wu, J.; Liao, D.J. Gene expression profiles in primary pancreatic tumors and metastatic lesions of Ela-c-myc transgenic mice. Mol. Cancer 2008, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chen, P.-Y.; Lin, Y.-Y.; Feng, L.-Y.; Chen, S.-H.; Huang, Y.-C.; Huang, C.-Y.; Jung, S.-M.; Chen, L.Y.; Wei, K.-C.; et al. Suppression of tumor growth via IGFBP3 depletion as a potential treatment in glioma. J. Neurosurg. 2020, 132, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Berberich, A.; Kessler, T.; Thomé, C.M.; Pusch, S.; Hielscher, T.; Sahm, F.; Oezen, I.; Schmitt, L.-M.; Ciprut, S.; Hucke, N.; et al. Targeting Resistance against the MDM2 Inhibitor RG7388 in Glioblastoma Cells by the MEK Inhibitor Trametinib. Clin. Cancer Res. 2018, 25, 253–265. [Google Scholar] [CrossRef]
- Liao, B.; Hu, Y.; Brewer, G. RNA-binding Protein Insulin-like Growth Factor mRNA-binding Protein 3 (IMP-3) Promotes Cell Survival via Insulin-like Growth Factor II Signaling after Ionizing Radiation. J. Biol. Chem. 2011, 286, 31145–31152. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Mok, M.T.S.; Kang, W.; Yang, W.; Tang, W.; Wu, F.; Xu, L.; Yan, M.; Yu, Z.; Lee, S.-D.; et al. Loss of tumor suppressor IGFBP4 drives epigenetic reprogramming in hepatic carcinogenesis. Nucleic Acids Res. 2018, 46, 8832–8847. [Google Scholar] [CrossRef]
- Mahapatra, L.; Andruska, N.; Mao, C.; Le, J.; Shapiro, D.J. A Novel IMP1 Inhibitor, BTYNB, Targets c-Myc and Inhibits Melanoma and Ovarian Cancer Cell Proliferation. Transl. Oncol. 2017, 10, 818–827. [Google Scholar] [CrossRef]
- Wang, W.; Lim, K.G.; Feng, M.; Bao, Y.; Lee, P.L.; Cai, Y.; Chen, Y.; Zhang, H.; Marzese, D.; Hoon, D.S.; et al. KDM6B Counteracts EZH2-Mediated Suppression of IGFBP5 to Confer Resistance to PI3K/AKT Inhibitor Treatment in Breast Cancer. Mol. Cancer Ther. 2018, 17, 1973–1983. [Google Scholar] [CrossRef]
- Martin, J.L.; De Silva, H.C.; Lin, M.Z.; Scott, C.; Baxter, R.C. Inhibition of Insulin-like Growth Factor-Binding Protein-3 Signaling through Sphingosine Kinase-1 Sensitizes Triple-Negative Breast Cancer Cells to EGF Receptor Blockade. Mol. Cancer Ther. 2013, 13, 316–328. [Google Scholar] [CrossRef]
- Marzec, K.; Baxter, R.C.; Martin, J.L. Targeting Insulin-Like Growth Factor Binding Protein-3 Signaling in Triple-Negative Breast Cancer. BioMed Res. Int. 2015, 2015, 638526. [Google Scholar] [CrossRef]
- Julovi, S.; Martin, J.L.; Baxter, R.C. Nuclear Insulin-Like Growth Factor Binding Protein-3 As a Biomarker in Triple-Negative Breast Cancer Xenograft Tumors: Effect of Targeted Therapy and Comparison With Chemotherapy. Front. Endocrinol. 2018, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Koochekpour, S.; Strup, S.E.; Kyprianou, N. Reversion of epithelial-mesenchymal transition by a novel agent DZ-50 via IGF binding protein-3 in prostate cancer cells. Oncotarget 2017, 8, 78507–78519. [Google Scholar] [CrossRef] [PubMed]
| Properties | IGFBP1 | IGFBP2 | IGFBP3 | IGFBP4 | IGFBP5 | IGFBP6 |
|---|---|---|---|---|---|---|
| Molecular weight (KDa) | 25–30 | 31 | 28–31 | 25-28 | 29 | 22–25 |
| No. of amino acids | 243 | 289 | 264 | 237 | 252 | 216 |
| No. of cysteines | 18 | 18 | 18 | 20 | 18 | 16 |
| Chromosomal localization | 7p | 2q | 7p | 17q | 2q | 12 |
| Glycosylation sites | No | No | Yes | Yes | Yes | Yes |
| Heparin-binding domain | No | Yes | Yes | No | Yes | Yes |
| Binds to ECM or cell surface | No | Yes | Yes | No | Yes | No |
| Binds to ALS | No | No | Yes | No | Yes | No |
| Nuclear localization sequence | No | Yes | Yes | No | Yes | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, D.; Radhakrishnan, P. Role of Tumor and Stroma-Derived IGF/IGFBPs in Pancreatic Cancer. Cancers 2020, 12, 1228. https://doi.org/10.3390/cancers12051228
Thomas D, Radhakrishnan P. Role of Tumor and Stroma-Derived IGF/IGFBPs in Pancreatic Cancer. Cancers. 2020; 12(5):1228. https://doi.org/10.3390/cancers12051228
Chicago/Turabian StyleThomas, Divya, and Prakash Radhakrishnan. 2020. "Role of Tumor and Stroma-Derived IGF/IGFBPs in Pancreatic Cancer" Cancers 12, no. 5: 1228. https://doi.org/10.3390/cancers12051228
APA StyleThomas, D., & Radhakrishnan, P. (2020). Role of Tumor and Stroma-Derived IGF/IGFBPs in Pancreatic Cancer. Cancers, 12(5), 1228. https://doi.org/10.3390/cancers12051228




