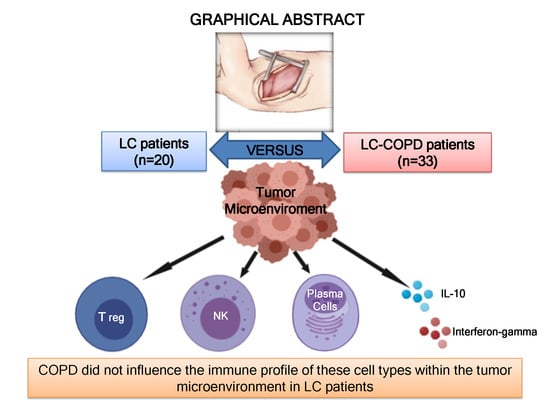
Immune Cell Subtypes and Cytokines in Lung Tumor Microenvironment: Influence of COPD
Abstract
1. Introduction
2. Methods
2.1. Study Design and Ethics
2.2. Clinical Assessment
2.3. Sample Collection and Preservation
2.4. Identification of Treg Cells and Plasma Cells in the Lung Specimens
2.5. Identification of NK Cells in Lung Specimens
2.6. Quantification of Cytokines in Lung Tissue
2.7. Statistical Analyses
3. Results
3.1. Clinical Characteristics
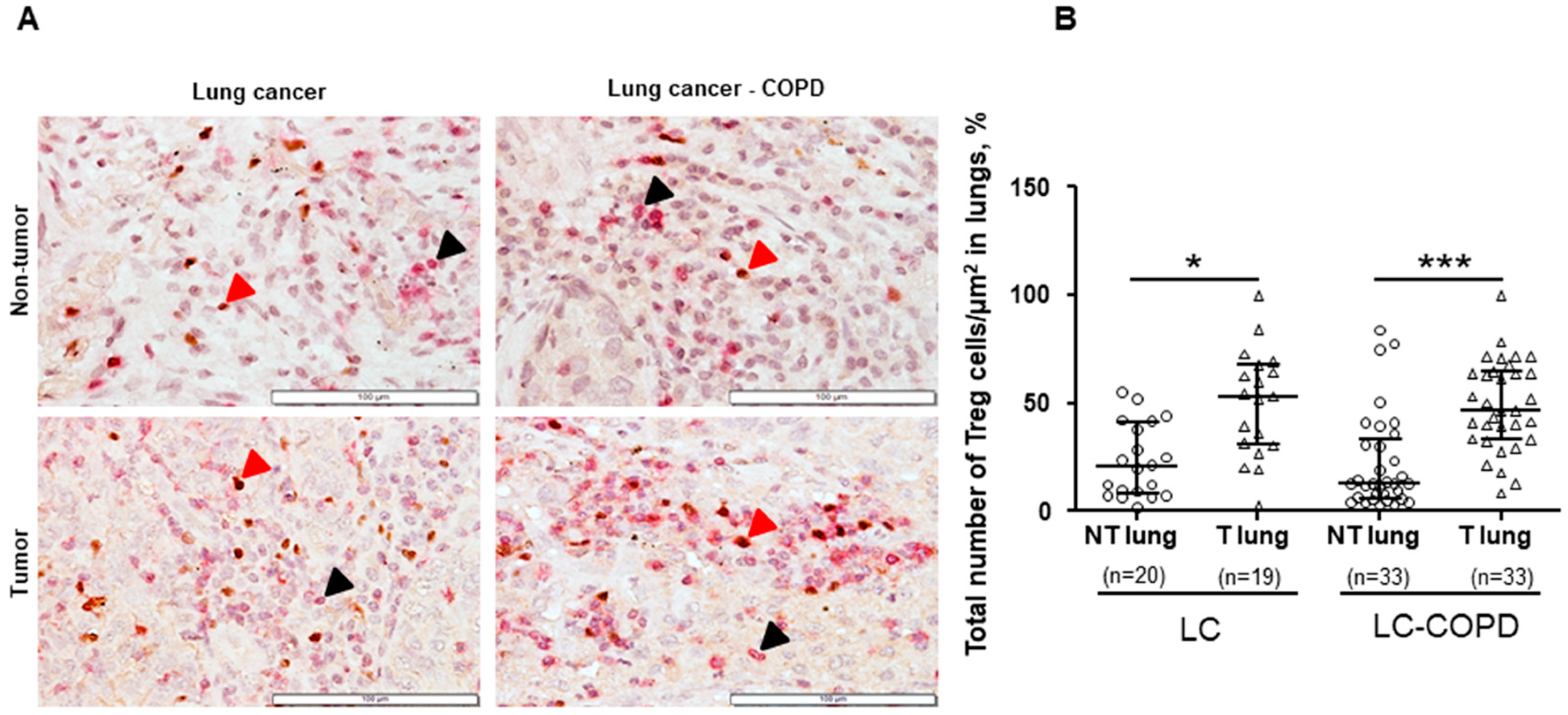
3.2. Treg and NK Cells in Lung Specimens
3.2.1. Differences between LC–COPD and LC in either Tumor Lesions or Non-Tumor Specimens
3.2.2. Differences between Tumor and Non-Tumor Parenchyma in LC–COPD and LC Patients
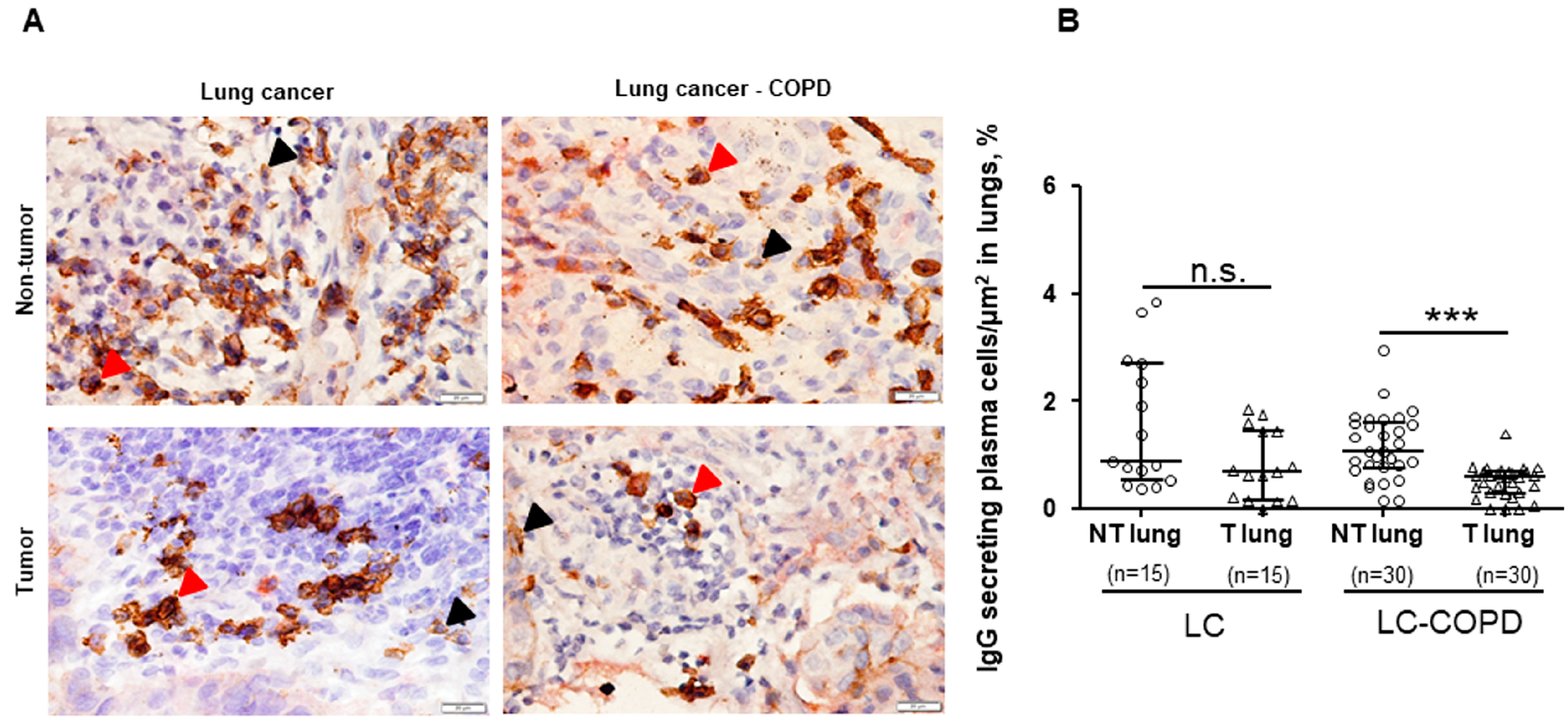
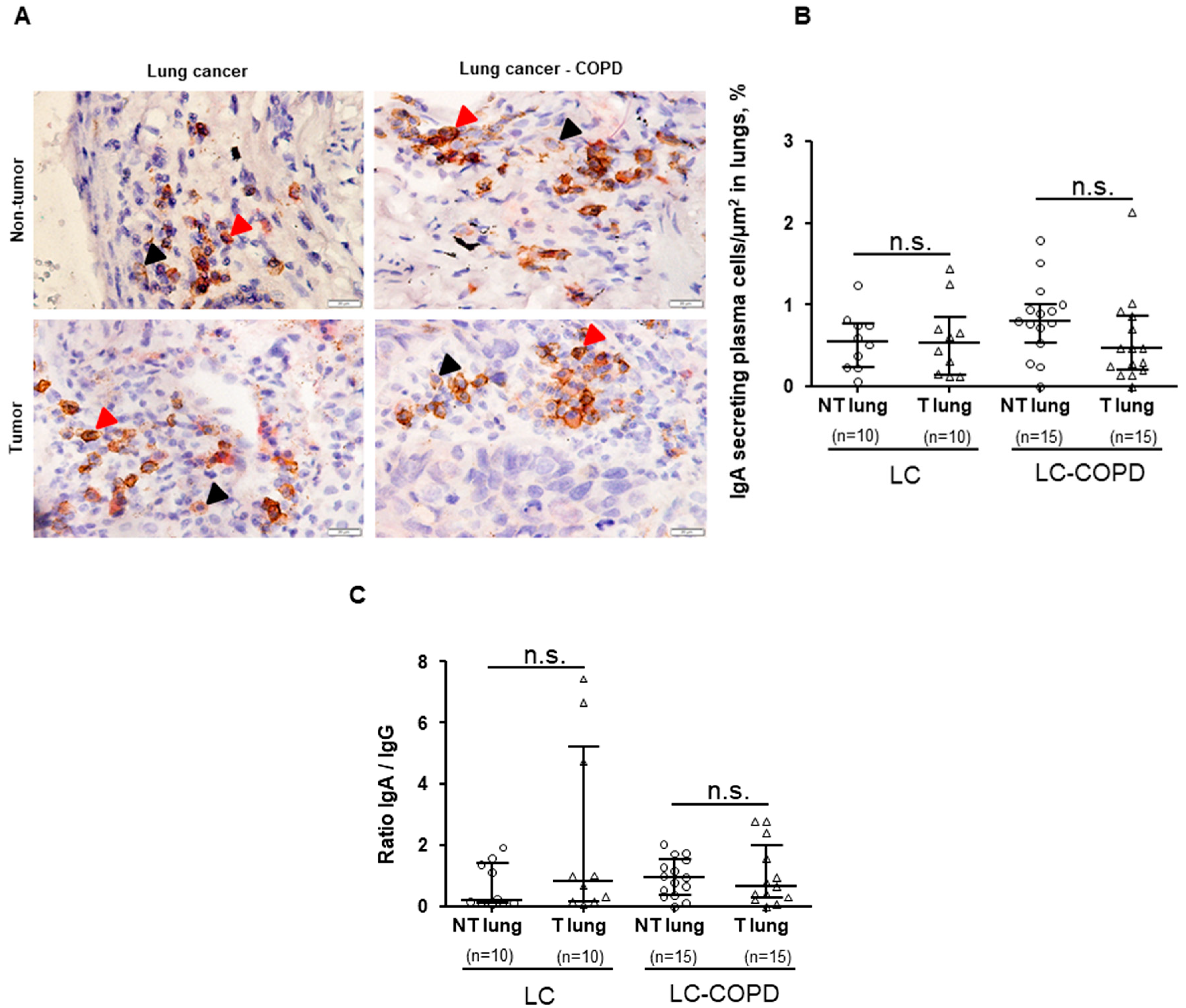
3.3. IgG and IgA Secreting Plasma Cells in Lung Specimens
3.3.1. Differences between LC–COPD and LC in either Tumor Lesions or Non-Tumor Specimens
3.3.2. Differences between Tumor and Non-Tumor Parenchyma in LC–COPD and LC Patients
3.4. Cytokines Levels in Lung Specimens
3.4.1. Differences between LC–COPD and LC in either Tumor Lesions or Non-Tumor Specimens
3.4.2. Differences between Tumor and Non-Tumor Parenchyma in LC–COPD and LC Patients.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Chen, Y.; Yu, T.; Jakopovic, M.; Giaccone, G. Trends and characteristics of young non-small cell lung cancer patients in the United States. Front. Oncol. 2015, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cataluña, J.J.; Novella, L.; Soler, C.; Nieto, M.L.; Esteban, V.; Sánchez-Toril, F.; Miravitlles, M. Clinical Characteristics and Risk of Exacerbations Associated With Different Diagnostic Criteria of Asthma-COPD Overlap. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Pleguezuelos, E.; Gimeno-Santos, E.; Hernandez, C.; del Carmen Mata, M.; Palacios, L.; Pinera, P.; Molina, J.; Chiner, E.; Miravitlles, M. Recommendations on non-Pharmacological Treatment in Chronic Obstructive Pulmonary Disease From the Spanish COPD Guidelines (GesEPOC 2017). Arch. Bronconeumol. 2018, 54, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Soler-Cataluña, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Trigueros, J.A.; Cosio, B.G.; Casanova, C.; Antonio, J.R.; et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological Treatment of Stable Phase. Arch. Bronconeumol. 2017, 53, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Mateu-Jimenez, M.; Curull, V.; Pijuan, L.; Sánchez-Font, A.; Rivera-Ramos, H.; Rodríguez-Fuster, A.; Aguiló, R.; Gea, J.; Barreiro, E. Systemic and Tumor Th1 and Th2 Inflammatory Profile and Macrophages in Lung Cancer: Influence of Underlying Chronic Respiratory Disease. J. Thorac. Oncol. 2017, 12, 235–248. [Google Scholar] [CrossRef]
- Mateu-Jimenez, M.; Curull, V.; Rodríguez-Fuster, A.; Aguiló, R.; Sánchez-Font, A.; Pijuan, L.; Gea, J.; Barreiro, E. Profile of epigenetic mechanisms in lung tumors of patients with underlying chronic respiratory conditions. Clin. Epigenet. 2018, 10, 7. [Google Scholar] [CrossRef]
- Mateu-Jiménez, M.; Sánchez-Font, A.; Rodríguez-Fuster, A.; Aguiló, R.; Pijuan, L.; Fermoselle, C.; Gea, J.; Curull, V.; Barreiro, E. Redox Imbalance in Lung Cancer of Patients with Underlying Chronic Respiratory Conditions. Mol. Med. 2016, 22, 85–98. [Google Scholar] [CrossRef]
- Gao, Y.H.; Guan, W.J.; Liu, Q.; Wang, H.Q.; Zhu, Y.N.; Chen, R.C.; Zhang, G.-J. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2016, 21, 269–279. [Google Scholar] [CrossRef]
- Iachina, M.; Jakobsen, E.; Møller, H.; Lüchtenborg, M.; Mellemgaard, A.; Krasnik, M.; Green, A. The Effect of Different Comorbidities on Survival of Non-small Cells Lung Cancer Patients. Lung 2015, 193, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.U.; Yeo, C.D.; Rhee, C.K.; Kang, H.S.; Park, C.K.; Kim, J.S.; Kim, J.W.; Kim, S.J.; Yoon, H.K.; Lee, S.H. Comparison of clinical characteristics and overall survival between spirometrically diagnosed chronic obstructive pulmonary disease (COPD) and non-COPD never-smoking stage I-IV non-small cell lung cancer patients. Int. J. COPD 2019, 14, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; de-Torres, J.P. Cáncer de pulmón y enfisema. Arch. Bronconeumol. 2017, 53, 47–48. [Google Scholar] [CrossRef]
- González, J.; Henschke, C.I.; Yankelevitz, D.F.; Seijo, L.M.; Reeves, A.P.; Yip, R.; Xie, Y.; Chung, M.; Sánchez-Salcedo, P.; Alcaide, A.B.; et al. Emphysema phenotypes and lung cancer risk. PLoS ONE 2019, 14, e0219187. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.C.; Sigel, K.; De-Torres, J.P.; Mhango, G.; Kale, M.; Kong, C.Y.; Zulueta, J.J.; Wilson, D.; Brown, S.-A.W.; Slatore, C.; et al. The effect of radiographic emphysema in assessing lung cancer risk. Thorax 2019, 74, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Seijo, L.M.; Trujillo, J.C.; Zulueta, J.J. Screening in Lung Cancer: The Latest Evidence. Arch. Bronconeumol. 2020, 56, 7–8. [Google Scholar] [CrossRef] [PubMed]
- González-Marrón, A.; Martín-Sánchez, J.C.; Garcia-Alemany, F.; Martínez-Martín, E.; Matilla-Santander, N.; Cartanyà-Hueso, À.; Vidal, C.; García, M.; Martínez-Sánchez, J.M. Estimation of the Risk of Lung Cancer in Women Participating in a Population-Based Breast Cancer Screening Program. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Mao, K.; Guo, X. Clinical significance of serum T helper 1/T helper 2 cytokine shift in patients with non-small cell lung cancer. Oncol. Lett. 2014, 8, 1682–1686. [Google Scholar] [CrossRef]
- Conway, E.M.; Pikor, L.A.; Kung, S.H.Y.; Hamilton, M.J.; Lam, S.; Lam, W.L.; Bennewith, K.L. Macrophages, Inflammation, and Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 116–130. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Scurr, M.; Pembroke, T.; Bloom, A.; Roberts, D.; Thomson, A.; Smart, K.; Bridgeman, H.; Adams, R.; Brewster, A.; Jones, R.; et al. Low-dose cyclophosphamide induces antitumor T-cell responses, which associate with survival in metastatic colorectal cancer. Clin. Cancer Res. 2017, 23, 6771–6780. [Google Scholar] [CrossRef] [PubMed]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.-F.; Joller, N.; Tan, D.J.; Teng, M.W.L.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, K.M.; Bae, J.S.; Park, H.S.; Lee, H.; Chung, M.J.; Moon, W.S.; Lee, D.G.; Jang, K.Y. Tumor-infiltrating PD1-positive lymphocytes and FoxP3-positive regulatory T cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl. Oncol. 2013, 6, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kusnadi, A.; Lee, E.-J.; Kim, W.W.; Cho, B.C.; Lee, I.J.; Seong, J.; Ha, S.-J. Tumor-infiltrating regulatory T cells delineated by upregulation of PD-1 and inhibitory receptors. Cell. Immunol. 2012, 278, 76–83. [Google Scholar] [CrossRef]
- Jonges, L.E.; Albertsson, P.; Van Vlierberghe, R.L.P.; Johansson, B.R.; Van De Velde, C.J.H.; Fleuren, G.J.; Nannmark, U.; Kuppen, P.J. The phenotypic heterogeneity of human natural killer cells: Presence of at least 48 different subsets in the peripheral blood. Scand. J. Immunol. 2001, 53, 103–110. [Google Scholar] [CrossRef]
- Ferlazzo, G.; Thomas, D.; Lin, S.-L.; Goodman, K.; Morandi, B.; Muller, W.A.; Moretta, A.; Münz, C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J. Immunol. 2004, 172, 1455–1462. [Google Scholar] [CrossRef]
- Deniz, G.; Van De Veen, W.; Akdis, M. Natural killer cells in patients with allergic diseases. J. Allergy Clin. Immunol. 2013, 132, 527–535. [Google Scholar] [CrossRef]
- Hodge, G.; Barnawi, J.; Jurisevic, C.; Moffat, D.; Holmes, M.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-γ by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clin. Exp. Immunol. 2014, 178, 79–85. [Google Scholar] [CrossRef]
- Aktaş, O.N.; Öztürk, A.B.; Erman, B.; Erus, S.; Tanju, S.; Dilege, Ş. Role of natural killer cells in lung cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 997–1003. [Google Scholar] [CrossRef]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, H.; Abe, Y.; Kamiya, T.; Morino, F.; Nagata, J.; Tokunaga, T.; Oshika, Y.; Suemizu, H.; Kijima, H.; Tsuchida, T.; et al. Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2000, 11, 815–819. [Google Scholar] [CrossRef]
- Huang, A.L.; Liu, S.G.; Qi, W.J.; Zhao, Y.F.; Li, Y.M.; Lei, B.; Sheng, W.-J.; Shen, H. TGF-β1 protein expression in non-small cell lung cancers is correlated with prognosis. Asian Pac. J. Cancer Prev. 2014, 15, 8143–8147. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Kimpfler, S.; Warth, A.; Schnabel, P.A.; Dienemann, H.; Schadendorf, D.; Hoffmann, H.; Umansky, V. Foxp3+ regulatory T cells and natural killer cells distinctly infiltrate primary tumors and draining lymph nodes in pulmonary adenocarcinoma. J. Thorac. Oncol. 2011, 6, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, O.I.; Sharonov, G.V.; Serebrovskaya, E.O.; Turchaninova, M.A.; Zaretsky, A.R.; Shugay, M.; Chudakov, D.M. Intratumoral immunoglobulin isotypes predict survival in lung adenocarcinoma subtypes. J. Immunother. Cancer 2019, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar]
- Slatore, C.G.; Horeweg, N.; Jett, J.R.; Midthun, D.E.; Powell, C.A.; Wiener, R.S.; Wisnivesky, J.P.; Gould, M.K. An Official American Thoracic Society research statement: A research framework for pulmonary nodule evaluation and management. Am. J. Respir. Crit. Care Med. 2015, 192, 500–514. [Google Scholar] [CrossRef]
- Kozower, B.D.; Larner, J.M.; Detterbeck, F.C.; Jones, D.R. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e369S–e399S. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martínez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch. Bronconeumol. 2017, 53, 128–149. [Google Scholar] [CrossRef]
- Buchwalow, I.; Boecker, W.; Wolf, E.; Samoilova, V.; Tiemann, M. Signal amplification in immunohistochemistry: Loose-jointed deformable heteropolymeric HRP conjugates vs. linear polymer backbone HRP conjugates. Acta Histochem. 2013, 115, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, K.; Silva-Santos, B.; Mavilio, D. Natural cytotoxicity receptors: Broader expression patterns and functions in innate and adaptive immune cells. Front. Immunol. 2013, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Pazina, T.; Shemesh, A.; Brusilovsky, M.; Porgador, A.; Campbell, K.S. Regulation of the functions of natural cytotoxicity receptors by interactions with diverse ligands and alterations in splice variant expression. Front. Immunol. 2017, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology 2016, 5, e1093722. [Google Scholar] [CrossRef]
- Hirashima, T.; Kanai, T.; Suzuki, H.; Yoshida, H.; Matsushita, A.; Kawasumi, H.; Samejima, Y.; Noda, Y.; Nasu, S.; Tanaka, A.; et al. The Levels of Interferon-gamma Release as a Biomarker for Non-small-cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Anticancer Res. 2019, 39, 6231–6240. [Google Scholar] [CrossRef]
- Jin, S.; Deng, Y.; Hao, J.W.; Li, Y.; Liu, B.; Yu, Y.; Shi, F.-D.; Zhou, Q.-H. NK cell phenotypic modulation in lung cancer environment. PLoS ONE 2014, 9, e109976. [Google Scholar] [CrossRef]
- Platonova, S.; Cherfils-Vicini, J.; Damotte, D.; Crozet, L.; Vieillard, V.; Validire, P.; Alifano, M.; Sautès-Fridman, C.; Cremer, I.; André, P.; et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011, 71, 5412–5422. [Google Scholar] [CrossRef]
- Selitsky, S.R.; Mose, L.E.; Smith, C.C.; Chai, S.; Hoadley, K.A.; Dittmer, D.P.; Moschos, S.J.; Parker, J.S.; Vincent, B.G. Prognostic value of B cells in cutaneous melanoma. Genome Med. 2019, 11, 36. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Bomze, D.; Ring, S.S.; Berner, F.; Fässler, M.; Diem, S.; Abdou, M.-T.; Hammers, C.; Emtenani, S.; Braun, A.; et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J. Am. Acad. Dermatol. 2020, 82, 854–861. [Google Scholar] [CrossRef]
- Pan, J.; Yu, L.; Wu, Q.; Lin, X.; Liu, S.; Hu, S.; Rosa, C.; Eichinger, D.J.; Pino, I.; Zhu, H.; et al. Integration of IgA and IgG Autoantigens Improves Performance of Biomarker Panels for Early Diagnosis of Lung Cancer. Mol. Cell. Proteom. 2020, 19, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Lin, X.J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017, 551, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Welinder, C.; Jirström, K.; Lehn, S.; Nodin, B.; Marko-Varga, G.; Blixt, O.; Danielsson, L.; Jansson, B. Intra-tumour IgA1 is common in cancer and is correlated with poor prognosis in bladder cancer. Heliyon 2016, 2, e00143. [Google Scholar] [CrossRef] [PubMed]


| Anthropometric Variables | Lung Cancer (n = 20) | Lung Cancer-COPD (n = 33) |
|---|---|---|
| Age, years | 65 (14) | 67 (8) |
| Male, n/Female, N | 10/10 | 29/4 ** |
| BMI, kg/m2 | 28 (4) | 25 (4) * |
| Smoking History | ||
| Current: n, % | 8, 40 | 23, 70 * |
| Ex-smoker: n, % | 3, 15 | 9, 27 |
| Never smoker: n, % | 9, 45 | 1, 3 *** |
| Pack-years | 18 (22) | 56 (27) *** |
| Lung Function Parameters | ||
| FEV1, % | 88 (9) | 68 (15) *** |
| FEV1/FVC, % | 77 (5) | 63 (8) *** |
| DLCO, % | 87 (15) | 72 (20) ** |
| KCO, % | 89 (13) | 73 (18) ** |
| GOLD Stage | ||
| GOLD Stage I: n, % | NA | 10, 30 |
| GOLD Stage II: n, % | NA | 20, 60 |
| GOLD Stage III: n, % | NA | 3, 10 |
| TNM Staging | ||
| Stage 0+ II: n, % | 17, 85 | 28, 84.8 |
| Stage III: n, % | 3, 15 | 3, 9.1 |
| Stage IV: n, % | 0, 0 | 2, 6.1 |
| Histological Diagnosis | ||
| Squamous cell carcinoma: n, % | 4, 20 | 7, 21 |
| Adenocarcinoma: n, % | 15, 75 | 25, 76 |
| Others: n, % | 1, 5 | 1, 3 |
| Blood Parameters | ||
| Total leucocytes/μL | 6.39 (1.77) × 103 | 9.52 (2.70) × 103 *** |
| Total neutrophils/μL | 3.72 (1.37) × 103 | 6.64 (2.42) × 103 *** |
| Total lymphocytes/μL | 1.97 (0.71) × 103 | 2.02 (0.76) × 103 |
| Albumin (g/dL) | 4.4 (0.2) | 4.0 (0.6) ** |
| Total proteins (g/dL) | 7.0 (0.4) | 6.8 (1.0) |
| Fibrinogen (mg/dL) | 443 (126) | 427 (83) |
| CRP (mg/dL) | 3.5 (5.6) | 10.5 (19.5) |
| GSV (mm/h) | 23 (10) | 26 (16) |
| Body Weight Loss, kg | ||
| 0, n, % | 20, 100 | 30, 91 |
| 1–5, n, % | 0, 0 | 1, 3 |
| 6–10, n, % | 0, 0 | 2, 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Ramis-Cabrer, D.; Curull, V.; Wang, X.; Qin, L.; Mateu-Jiménez, M.; Duran, X.; Pijuan, L.; Rodríguez-Fuster, A.; Espases, R.A.; et al. Immune Cell Subtypes and Cytokines in Lung Tumor Microenvironment: Influence of COPD. Cancers 2020, 12, 1217. https://doi.org/10.3390/cancers12051217
Tang J, Ramis-Cabrer D, Curull V, Wang X, Qin L, Mateu-Jiménez M, Duran X, Pijuan L, Rodríguez-Fuster A, Espases RA, et al. Immune Cell Subtypes and Cytokines in Lung Tumor Microenvironment: Influence of COPD. Cancers. 2020; 12(5):1217. https://doi.org/10.3390/cancers12051217
Chicago/Turabian StyleTang, Jun, Daniel Ramis-Cabrer, Víctor Curull, Xuejie Wang, Liyun Qin, Mercé Mateu-Jiménez, Xavier Duran, Lara Pijuan, Alberto Rodríguez-Fuster, Rafael Aguiló Espases, and et al. 2020. "Immune Cell Subtypes and Cytokines in Lung Tumor Microenvironment: Influence of COPD" Cancers 12, no. 5: 1217. https://doi.org/10.3390/cancers12051217
APA StyleTang, J., Ramis-Cabrer, D., Curull, V., Wang, X., Qin, L., Mateu-Jiménez, M., Duran, X., Pijuan, L., Rodríguez-Fuster, A., Espases, R. A., & Barreiro, E. (2020). Immune Cell Subtypes and Cytokines in Lung Tumor Microenvironment: Influence of COPD. Cancers, 12(5), 1217. https://doi.org/10.3390/cancers12051217