Real-Time Visualization of the Mascagni-Sappey Pathway Utilizing ICG Lymphography
Abstract
:1. Introduction
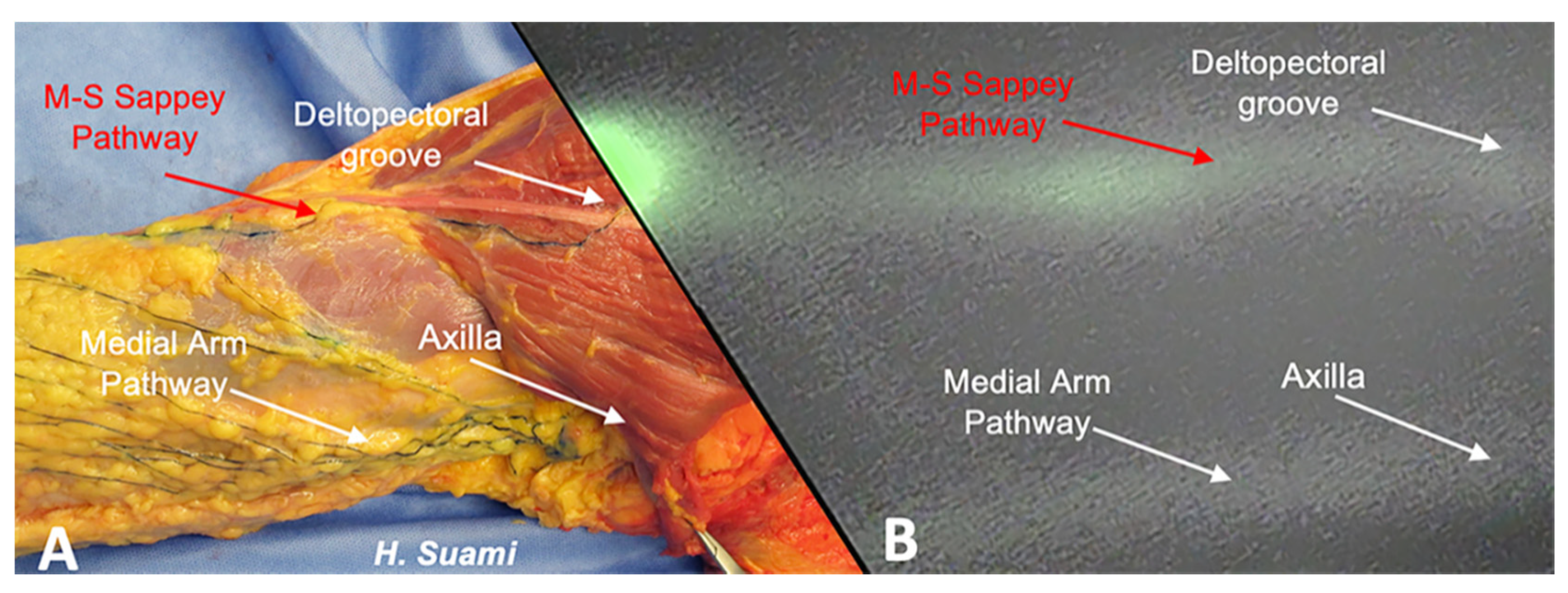
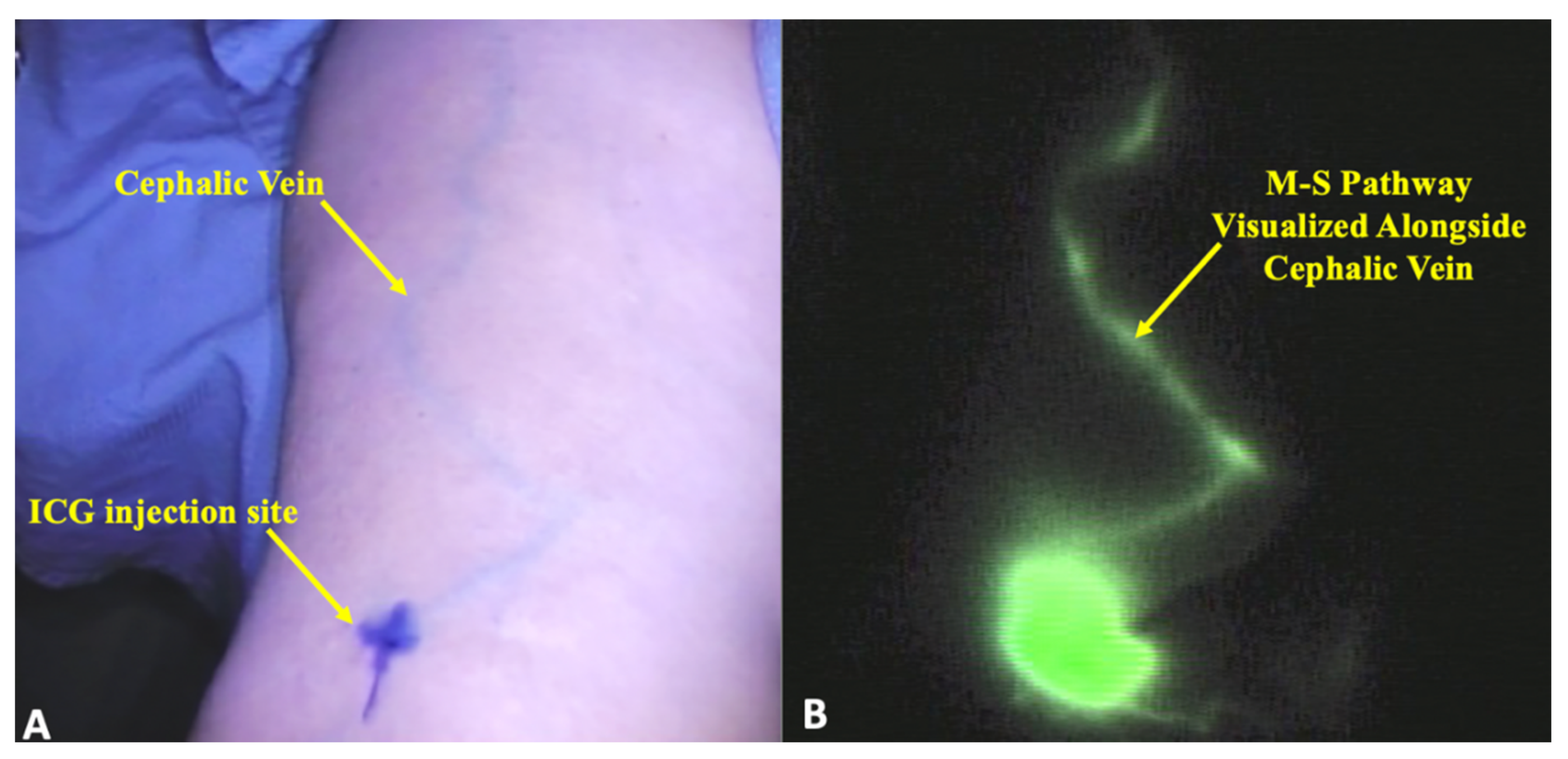
2. Results
3. Discussion
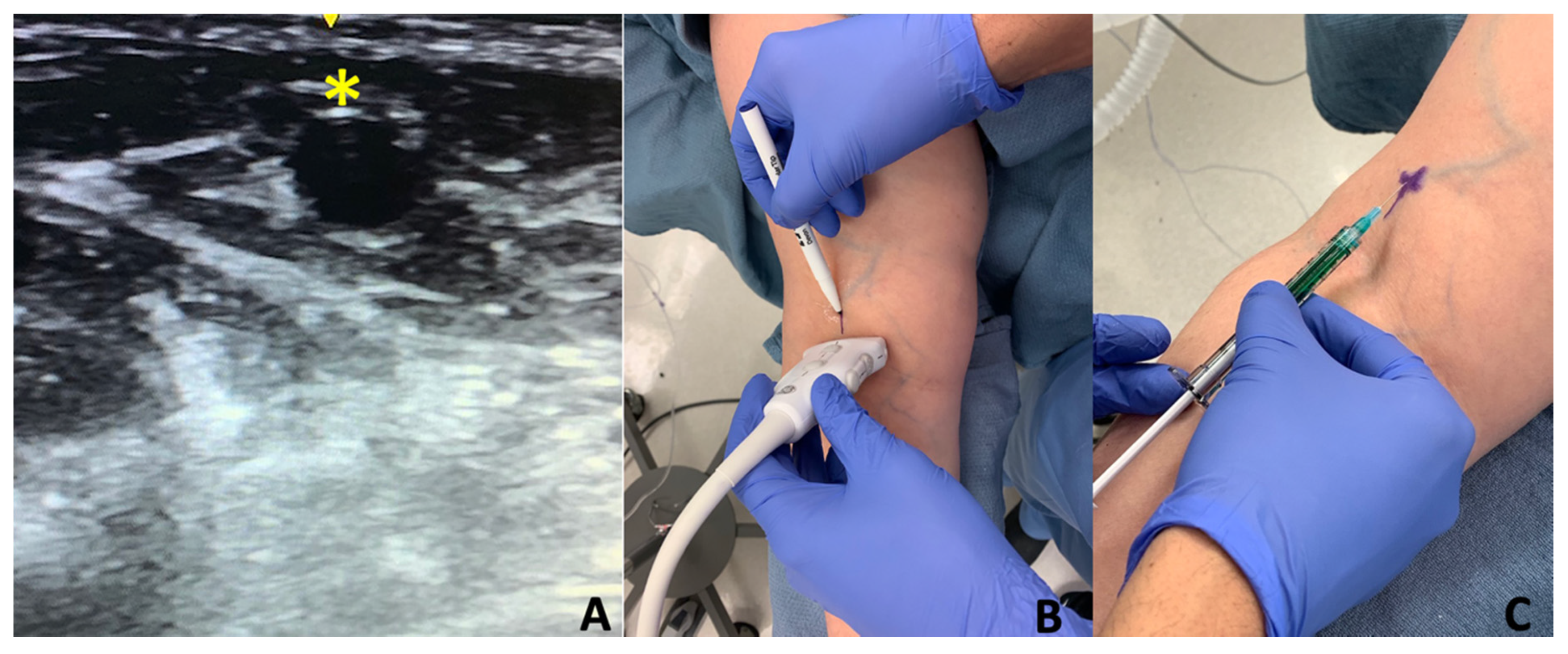
4. Materials and Methods
Injection Technique
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.W.; Lee, S.U.; Lee, N.K.; Jung, S.-Y.; Kim, T.H.; Lee, E.S.; Kang, H.-S.; Shin, K.H. A Model to Estimate the Risk of Breast Cancer-Related Lymphedema: Combinations of Treatment-Related Factors of the Number of Dissected Axillary Nodes, Adjuvant Chemotherapy, and Radiation Therapy. Int. J. Radiat. Oncol. 2013, 86, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Kimball, S.; Epstein, S.; Recht, A.; Lin, S.J.; Lee, B.T.; James, T.A.; Singhal, D. Lymphedema Incidence After Axillary Lymph Node Dissection: Quantifying the Impact of Radiation and the Lymphatic Microsurgical Preventive Healing Approach. Ann. Plast. Surg. 2019, 82, S234–S241. [Google Scholar] [CrossRef] [PubMed]
- Sappey, P.C. Anatomie, Physiologie, Pathologie de Vaisseaux Lymphatiques; Adrain Delahaye: Paris, France, 1874. [Google Scholar]
- Mascagni, P. Vasorum Lymphaticorum Corporis Humani. Historia & Iconographia; P. Carli Edit: Senis, Switzerland, 1787. [Google Scholar]
- Singhal, D.; Tran, B.N.; Angelo, J.P.; Lee, B.T.; Lin, S.J. Technological Advances in Lymphatic Surgery: Bringing to Light the Invisible. Plast. Reconstr. Surg. 2019, 143, 283. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Scaglioni, M.F. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin. Plast. Surg. 2018, 32, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Leduc, A.; Caplan, I.; Leduc, O. Lymphatic drainage of the upper limb. Substitution lymphatic pathways. Eur. J. Lymphology 1993, 4, 11–18. [Google Scholar]
- Kubik, S. The role of the lateral upper arm bundle and the lymphatic watersheds in the formation of collateral pathways in lymphedema. Acta Biol. Acad. Sci. Hung. 1980, 31, 191–200. [Google Scholar] [PubMed]
- Tashiro, K.; Yamashita, S.; Koshima, I.; Miyamoto, S. Visualization of Accessory Lymphatic Pathways in Secondary Upper Extremity Lymphedema Using Indocyanine Green Lymphography. Ann. Plast. Surg. 2017, 79, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Nakamura, R.; Yamamoto, N.; Tokumoto, H.; Ishigaki, T.; Yamaji, Y.; Sasahara, Y.; Kubota, Y.; Mitsukawa, N.; Satoh, K. Early Detection of Lymphatic Disorder and Treatment for Lymphedema following Breast Cancer. Plast. Reconstr. Surg. 2016, 138, 192e–202e. [Google Scholar] [CrossRef] [PubMed]
- Turfe, Z.; Pettinga, J.; Leduc, O.; Leduc, A.; Komorowska-Timek, E. Chemotherapy port related lymphedema after axillary lymph node dissection. Breast Edinb. Scotl. 2016, 28, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Bravo, M.G.; James, T.A.; Suami, H.; Lee, B.T.; Singhal, D. The All but Forgotten Mascagni-Sappey Pathway: Learning from Immediate Lymphatic Reconstruction. J. Reconstr. Microsurg. 2020, 36, 28–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suami, H.; Koelmeyer, L.; Mackie, H.; Boyages, J. Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: A review of animal and clinical imaging studies. Surg. Oncol. 2018, 27, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Pissas, A.; Rzal, K.; Math, M.L.; Dubois, J.B. Prevention of secondary lymphedema. Ann. Ital. Chir. 2002, 73, 489–492. [Google Scholar] [PubMed]
- Johnson, A.R.; Granoff, M.D.; Lee, B.T.; Padera, T.P.; Bouta, E.M.; Singhal, D. The Impact of Taxane-based Chemotherapy on the Lymphatic System. Ann. Plast. Surg. 2019, 82, S173–S178. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Demographics and Cancer Treatment Characteristics n = 23 | |
|---|---|
| Age, mean (SD ^) | 51.6 (17) |
| BMI, kg/m2, mean (SD) | 28.2 (6) |
| Race, n (%) | |
| Caucasian | 17 (74) |
| Black/African American | 4 (17) |
| Asian | 2 (9) |
| Ethnicity, n (%) | |
| Hispanic | 4 (17) |
| Non-Hispanic | 19 (83) |
| Neoadjuvant chemotherapy, n (%) | 13 (57) |
| Taxane-based, n (%) | 12 (92) |
| Axillary intervention | |
| Sentinel lymph node biopsy (SLNB) †, n (%) | 14 (61) |
| Nodes removed in SLNB, median (IQR *) | 4 (2–5) |
| Axillary lymph node dissection (ALND) ‡, n (%) | 23 (100) |
| Positive nodes removed, median (IQR) | 1 (0–3) |
| Total nodes removed in ALND, median (IQR) | 15 (9–22) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, A.R.; Granoff, M.D.; Suami, H.; Lee, B.T.; Singhal, D. Real-Time Visualization of the Mascagni-Sappey Pathway Utilizing ICG Lymphography. Cancers 2020, 12, 1195. https://doi.org/10.3390/cancers12051195
Johnson AR, Granoff MD, Suami H, Lee BT, Singhal D. Real-Time Visualization of the Mascagni-Sappey Pathway Utilizing ICG Lymphography. Cancers. 2020; 12(5):1195. https://doi.org/10.3390/cancers12051195
Chicago/Turabian StyleJohnson, Anna Rose, Melisa D. Granoff, Hiroo Suami, Bernard T. Lee, and Dhruv Singhal. 2020. "Real-Time Visualization of the Mascagni-Sappey Pathway Utilizing ICG Lymphography" Cancers 12, no. 5: 1195. https://doi.org/10.3390/cancers12051195
APA StyleJohnson, A. R., Granoff, M. D., Suami, H., Lee, B. T., & Singhal, D. (2020). Real-Time Visualization of the Mascagni-Sappey Pathway Utilizing ICG Lymphography. Cancers, 12(5), 1195. https://doi.org/10.3390/cancers12051195





