Abstract
The quality of pathological assessment is crucial for the safety of patients with cervical cancer if pelvic lymph node dissection is to be replaced by sentinel lymph node (SLN) biopsy. Central pathology review of SLN pathological ultrastaging was conducted in the prospective SENTIX/European Network of Gynaecological Oncological Trial (ENGOT)-CX2 study. All specimens from at least two patients per site were submitted for the central review. For cases with major or critical deviations, the sites were requested to submit all samples from all additional patients for second-round assessment. From the group of 300 patients, samples from 83 cases from 37 sites were reviewed in the first round. Minor, major, critical, and no deviations were identified in 28%, 19%, 14%, and 39% of cases, respectively. Samples from 26 patients were submitted for the second-round review, with only two major deviations found. In conclusion, a high rate of major or critical deviations was identified in the first round of the central pathology review (28% of samples). This reflects a substantial heterogeneity in current practice, despite trial protocol requirements. The importance of the central review conducted prospectively at the early phase of the trial is demonstrated by a substantial improvement of SLN ultrastaging quality in the second-round review.
1. Introduction
SENTIX is an observational prospective study that was designed to evaluate whether a less radical surgical approach comprising sentinel lymph node (SLN) biopsy could replace systematic pelvic lymphadenectomy (PLND) in the management of patients with early-stage cervical cancer [1]. The primary endpoint of the study is the recurrence rate at 24 months after surgery.
Metastatic involvement of pelvic lymph nodes (LN) is the most important prognostic marker in patients with early-stage cervical cancer [2,3,4,5,6,7,8,9,10,11]. Since non-SLN pelvic LNs are not surgically removed in the study, each case with undetected LN involvement can cause a lateral pelvic recurrence, which is often fatal for the patient. SLN pathological ultrastaging improves the sensitivity of LN staging thanks to the more reliable detection of small metastases that would otherwise be missed by standard evaluation [8,9,12,13,14,15,16]. The quality of the SLN pathological evaluation is considered the key prerequisite for the safety of patients in the study. Therefore, the central pathology review was an integral part of the protocol. Samples from two randomly selected patients were submitted for central review from each institution. If the outcome of the review was classified as having major or critical deviations, which could result in missing metastatic involvement, samples from all cases from that institution were requested for the second round of the review.
Standard pelvic lymph node dissection is not performed in the SENTIX trial. Instead, SLNs are removed on both sides of the pelvis, and the major lymphatic channels draining lymph from the lower extremities are preserved. The main advantage of this less radical approach is the substantially lower risk of postoperative complications, such as lower leg lymphedema and pelvic lymphoceles [17,18,19,20]. Lowering the number of removed LNs should, however, be compensated by their more intensive pathology assessment. Protocols for SLN ultrastaging were developed to detect smaller metastases and improve the reliability of SLN staging [12,14,21,22,23]. In the largest retrospective cohort study on sensitivity of SLN ultrastaging, 645 patients underwent SLN biopsy followed by pelvic lymph node dissection [24]. In 23 (3.6%) patients, pathological ultrastaging detected MIC in their SLN, while larger macrometastases were identified in the other pelvic LN. These patients would not be identified as high-risk patients if surgical staging was limited only to SLN biopsy and SLN ultrastaging was not performed. Neither of their pelvic LNs would be removed, nor would they have received adjuvant radiotherapy, with a subsequent significant risk of developing lateral pelvic recurrence with a low chance of curative treatment.
To our knowledge, SENTIX is the first prospective study in patients with cervical cancer that included a prospective review of specimens from SLN. In this paper, we report the final results of the Central Pathology Review for the 300 patients treated per protocol.
2. Results
A total of 47 sites across 18 countries were registered to participate in the SENTIX study. To the date when the first 300 cases were registered and treated according to the protocol, 37 sites enrolled at least two cases, two sites enrolled one case, and eight sites enrolled no patients. The characteristics of the group are shown in Table 1. The outcome of the central review was concluded eight months after the date the 300th patient was enrolled, when all requested samples were received and reviewed.

Table 1.
Preoperative characteristics of patients (N = 300).
Thirty-seven sites were eligible to submit samples for the first-round review. Samples from 83 patients treated in 35 sites were reviewed, including three cases from the trial leading institution. Samples from two Argentinian sites were missing because of customs and transportation challenges. Original pathology reports from two Argentinian sites were, nevertheless, translated into English, and the protocol of SLN assessment reviewed.
A central pathology review classified findings from the first round as having no deviations in 32 (39%) cases, minor deviations in 23 (28%), major deviations in 16 (19%), and critical in 12 (14%) cases. This corresponds to eight and six sites, respectively, with at least one case with major or critical deviations. SLNs were not processed completely in 40% of cases, and immunohistochemical staining was performed less frequently than required by the protocol in 25% of cases and not at all in 11% of cases. Surprisingly, there were two cases with a higher number of immunohistochemical staining. Other minor issues were found in 16% of cases. These included the use of a different staining sequence or using different immunohistochemical/histochemical staining (i.e., cytokeratin-7 with periodic acid–Schiff or Papanicolaou staining).
For the second-round review, nine sites with major or critical deviations in the first round were asked to submit samples from all enrolled cases. Four sites had not enrolled any other patients at the time of the review, and two centers were prematurely closed. In 26 submitted cases for the second-round review, no deviations were found in nine (35%), while minor deviations were found in 15 (58%), and major deviations in two (8%) cases. One site with major deviations detected in the first and second rounds submitted samples from patients enrolled later in the study for the third-round review, resulting in no deviation. Figure 1 shows the flow chart of the central pathology review. Two sites were prematurely terminated due to critical deviations in the first round, poor communication, and no attempt to resolve the identified issues after repeated requests. Patients from these sites were excluded from the per-protocol analysis.
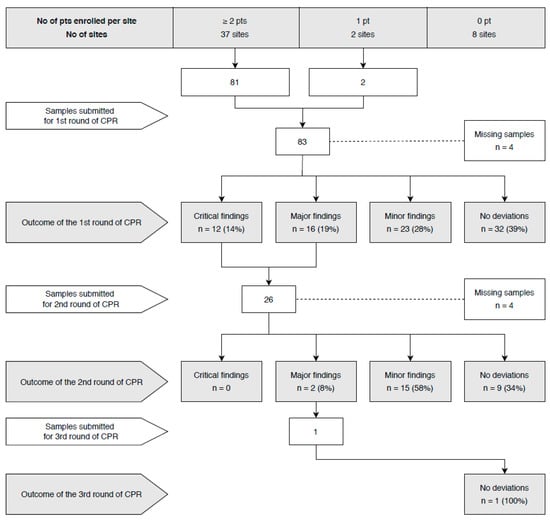
Figure 1.
Flow chart of the central pathology review (CPR).
From the whole cohort of 300 patients, samples from 110 cases (37%) were reviewed in the central laboratory (83 in the first, 26 in the second, and 1 in the third round). Samples from 350 SLNs consisting of 262 in the first round, 85 in the second round, and 3 in the third round were reviewed. Eight micrometastases and two ITCs were found by the initial pathology evaluation at referring institutions. Cases with residual SLN tissue in paraffin blocks, which constituted major or critical deviations, were reprocessed according to the study protocol at the central laboratory, yielding 1782 additional slides. Two additional micrometastases were found in two cases.
3. Discussion
The central review of SLN pathological assessment was an integral part of the ongoing prospective observational SENTIX trial. The main objective of the study was to evaluate the safety of bilateral SLN biopsy instead of systematic pelvic lymph node dissection in patients with early-stage cervical cancer. According to the protocol, the outcome of central pathology review was analyzed in the group of 300 patients treated per protocol. Samples from 110 cases from 37 sites were submitted to the central laboratory. The central review revealed a high number of deviations from the trial protocol for SLN pathological ultrastaging. In the first round, major or critical deviations, defined as those which could potentially result in missing metastatic involvement, were found in 34% of reviewed cases. The most frequent type of deviation was residual tissue found in the paraffin block due to incomplete processing of SLN tissue.
Central review has been conducted continuously throughout the period spanning January 2017 to May 2019. All deviations from the protocol have been reported to the referring sites and communicated to all investigators. We believe that this quality control process led to substantial improvement in pathological SLN ultrastaging quality, demonstrated by the results of the second and third rounds of the review, where only two (8%) major and zero critical deviations were reported. Two sites were terminated due to insufficient quality of pathology examination, and patients from these sites were excluded from the per-protocol analysis.
We are aware that the quality of pathological SLN evaluation is the critical element for the study outcome and safety of patients. One of the main conditions for site selection demanded experience with SLN biopsy in gynecological malignancies and experience with SLN ultrastaging. Institutional pathologists had to agree with the protocol for SLN ultrastaging and with the central review. Therefore, the high frequency of serious deviations reported in the first round of the review was an unexpected revelation. We can hypothesize that the underlying cause is insufficient communication between clinicians and their pathologists. This outcome also reflects substantial differences in protocols for SLN evaluation between institutions in the absence of internationally accepted recommendations. In a recently published review article, we showed that reported protocols for SLN ultrastaging differ substantially; they are often incompletely described or they are not reported at all [25].
The prevalence of micrometastases varies around 10% in patients with early-stage cervical cancer [24,26]. It approximately corresponds to the results of our study where 10 (9%) cases with micrometastases were found. In 132 SLNs from 40 cases that were reprocessed at the central laboratory, we detected only two additional cases with MIC that were missed at a referring institution. A plausible explanation for the slightly lower than expected occurrence of MIC is preselection of patients. According to the protocol, MRI or expert ultrasound was mandatory in preoperative staging, and all cases with enlarged or suspicious pelvic LN were excluded from the trial. Cases with intraoperatively detected LN involvement were also excluded.
One of the limitations of our protocol for central review in the trial was the timing of central review that started early after the study initiation. Thus, quality control was conducted earlier in sites with faster recruitment. Another weakness is the fact that samples were not reviewed from all enrolled cases. We believe, however, that the algorithm for patient selection was a reasonable compromise which allowed for prospective quality control in the early phase of the trial and improvement of the quality of pathological assessment in the trial.
We would like to emphasize the strong features of the central pathology review design. In the context of other published protocols in cervical cancer, ultrastaging was very intense, with the intention to guarantee reliability in the detection of all metastases ≥200 µm, including small MAC and MIC. The review of samples was performed continuously during the first study period until the first 300 patients were enrolled. If the review had been conducted retrospectively after all patients were registered, the logistics would have been much easier; however, it would not have allowed the real-time communication of findings with sites and quality improvement. Two rounds of the review allowed us to focus attention on sites with difficulties in pathological assessment. The outcome of the second round confirmed a substantial performance improvement. We have developed a meticulous reporting system of review outcomes and a grading of findings. In cases of major deviations, SLN assessment was completed according to the protocol at the central laboratory, without the need of additional sample transportation back to the referring sites. Patients scheduled for fertility-sparing surgery were eligible in the trial since they could profit from intraoperative triage the most [27,28,29]. The threshold for upper tumor size in fertility-sparing treatment was 2 cm, which corresponds to the recent international recommendations [9].
4. Materials and Methods
4.1. Ethics
The protocol was approved by the IRB of the leading institution (General University Hospital in Prague, project number/ethic code: 105/15 IRB of the General Faculty Hospital in Prague) in June 2016, and institutional IRB approval has been a prerequisite for participation for each new institution. Information for patients was available in 17 languages, and informed consent was signed by all patients before preregistration into the study. Study registration: NCT02494063 (ClinicalTrials.gov); European Network of Gynaecological Oncological Trial (ENGOT)-CX2; Central and Eastern European Gynecologic Oncology Group (CEEGOG)-CX1.
4.2. Study Sites
Sites applied individually for participation in the study, and eligibility criteria were evaluated by the SENTIX study office. The minimum requirements for any site to participate included a minimum of 10 patients with early-stage invasive cervical carcinoma treated in the center per year, experience with at least 15 gynecological cancer patients with successful SLN detection, and approval of the protocol for pathological SLN ultrastaging by pathologists.
The SENTIX study was conducted in collaboration with ENGOT (European Network of Gynaecological Oncological Trial groups), according to ENGOT Model A [30].
4.3. Patients
Patients were preregistered into the study if they fulfilled the following inclusion criteria: (a) FIGO classification 2014 [31] stage IA1 + lymphovascular space invasion (LVSI), IA2, IB1; (b) pelvic LNs not enlarged or suspicious on preoperative imaging; (c) squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma; (d) tumor with the largest diameter of ≤4 cm or ≤2 cm in patients scheduled for a fertility-sparing procedure. Patients after neoadjuvant chemotherapy or those with an unusual type of adenocarcinoma (non-HPV-related, e.g., mucinous, clear cell, mesonephric) were excluded. Final registration was provided after the surgery if additional intraoperative criteria were met, such as successful bilateral SLN detection, negative SLN frozen section evaluation, and no intraoperative evidence of more advanced disease (>IB1). The flow chart for patients is displayed in Figure 2.
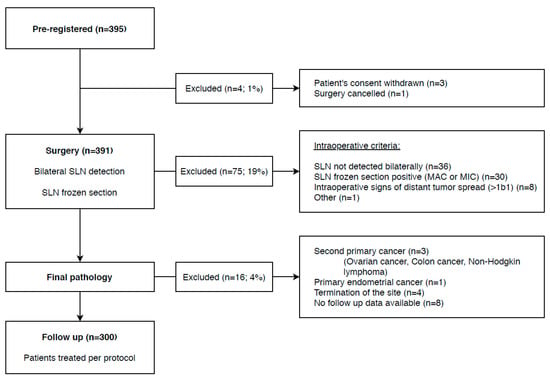
Figure 2.
Flow chart for patients registered into the SENTIX trial.
4.4. SLN Detection
All surgical approaches and all 3 main, currently available techniques for SLN detection, such as blue dye, radiocolloid, or indocyanine green, were eligible by the protocol. However, preferred techniques were either a combination of blue dye and radiocolloid or indocyanine green [10,14,32,33,34].
4.5. SLN Ultrastaging Protocol
After intraoperative processing, all SLNs were fixed in 10% neutral buffered formalin, sliced at 2 mm intervals, and embedded in paraffin. The tissue sections were then processed for ultrastaging. Pairs of tissue sections (4 μm thick) were cut at 150 μm intervals in a serial manner from each paraffin block until there was no lymph node tissue left. The first section of each pair was stained with hematoxylin and eosin (HE), and the second section was examined immunohistochemically after staining with anticytokeratin AE1/AE3 antibodies (Figure 3). The protocol was mandatory for all participating institutions.
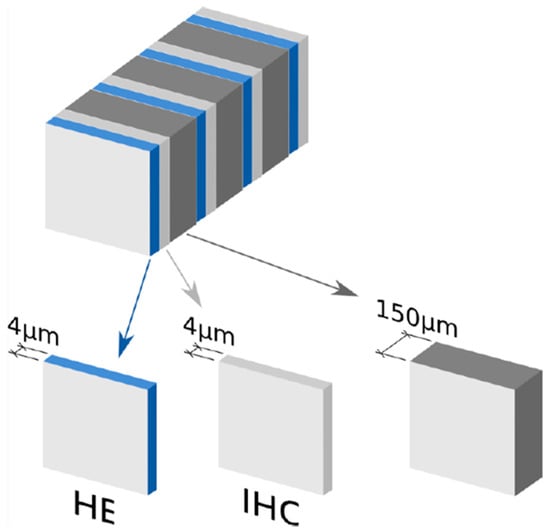
Figure 3.
Sentinel lymph node (SLN) ultrastaging protocol in SENTIX trial protocol.
The type of metastasis was classified according to the TNM system [35]. Macrometastases were defined as metastases >2 mm in diameter, micrometastases as metastasis of >0.2 to ≤2 mm in diameter, and ITCs as individual cells or small clusters of cells up to 0.2 mm in diameter (<200 cells).
4.6. Central Pathology Review
According to the protocol, all SLN slides with corresponding paraffin blocks and the full pathology report from 2 patients per site were requested by the trial coordinator for central review. Cases were selected randomly from patients without MAC, reported by referring sites using a random number generator. The trial coordinator was responsible for communication with sites, random selection of cases, logistics of sample transportation, and review report finalization and submission to the sites. Samples were reviewed by the central laboratory at the Department of Pathology of the SENTIX leading institution at the General University Hospital in Prague, Czech Republic. All samples were reviewed by one of the two senior gynecological pathologists. The review process included examination of all slides, review of the original pathology report, correlation of the number of slides with a size and number of lymph nodes, checking the number of immunohistochemical slides, and examination of paraffin blocks for any residual unprocessed lymph node tissue.
The outcome of each review was summarized in a report composed of: (a) description of received samples and original findings at the referring institution, (b) description of deviations from the protocol, (c) grading of deviations, (d) description and findings of SLN evaluation completed according to the protocol at the central laboratory (in cases with major or critical deviation), (e) final SLN status combining results from SLN evaluation at the referring institution and at the central laboratory, (f) recommendation for quality improvement if any deviations were identified. The report also contained a copy of SLN protocol from the SENTIX trial and a description of the grading system of deviations.
The outcome of the central review was graded as follows: (1) No deviations, if all SLNs were processed according to the study protocol. (2) Minor deviations, if samples were processed with high quality with minor deviations carrying no risk to miss metastatic involvement (MAC or MIC), i.e., a lower number of IHC staining. (3) Major deviations, if deviations were found which could result in failure to detect metastasis and if the assessment could be completed by additional assessment at the central laboratory (i.e., SLN processed incompletely or no IHC staining). (4) Critical deviations, if major deviations were found but the assessment could not be completed in the central laboratory due to incomplete sample submission. In cases with major deviations, residual SLN tissues in paraffin blocks were reprocessed according to the study protocol at the central laboratory. If critical deviations were identified, sites were requested to complete SLN evaluation according to the protocol and return these samples for the second or, if necessary, the third review round. All major and critical deviations were discussed with the site investigator and pathologist.
All sites with major or critical deviations were asked to submit samples and pathology reports from all enrolled patients for the second round of assessment. The same grading classification and reporting were applied to the second-round assessment. A summary of the outcomes and recommendations for the improvement of SLN assessment was also reported in quarterly newsletters distributed to all study investigators.
According to the original protocol, the central pathology review was planned in the first 300 cases registered into the trial and treated by the protocol. This milestone was achieved on 27 September 2018. By that date, 395 patients were preregistered. The patient flow chart is shown in Figure 2.
5. Conclusions
The high number of severe deviations from the SLN ultrastaging protocol was an unexpected outcome of the first round of the central pathology review in the SENTIX trial. The central review and an intensive communication of its outcome with investigators resulted in a substantial improvement in the quality of pathological assessments, as demonstrated by the outcomes of the second-round review. The results of this study highlight the need for a central pathology review in similar studies involving multiple sites in multiple countries, where different protocols may otherwise be used in routine clinical practice.
Author Contributions
The protocol of the study was designed by D.C. and K.N. with support from P.D., who processed and reviewed all histological material from all trial sites and guaranteed quality of the central pathologic evaluation. K.N. wrote the first draft of the manuscript which was then critically reviewed and revised by the other authors. All authors (K.N., R.K., C.K., J.K., P.D., A.G., A.P. (Andrea Plaikner), S.B., N.M.-G., E.L., A.B. (Alberto Berjon), B.G.-I., F.M., M.P., P.K., M.R., L.C.S., M.M. (Marcin Misiek), C.Z., S.T., A.V., I.V., M.M. (Martin Michal), B.S., A.P. (Almerinda Petiz), R.P. (Radovan Pilka), O.A.S., J.P., A.B. (Alessandro Buda), L.v.L., L.M., M.B., D.W., W.S., P.H., B.K., G.S., R.T., J.C.S., F.J.d.S.G., P.J.C.M., R.P. (Robert Poka), K.T., M.L., M.F., J.J., D.C.) contributed with patient enrollment, data acquisition, pathological evaluation, as well as writing and revising of the draft and the final approval of the manuscript. All authors approved the submission of the study. J.J. did the data cleaning, interpretation, and final statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by two grants from the Czech Research Council (No 16-31643A; NV19-03-00023) and a further one from the Ministry of Health, Czech Republic (Project RVO 64165).
Acknowledgments
We would like to acknowledge investigators from all 47 sites of the SENTIX trial (Adamik Zdenek, KNTB a.s Zlin, Czech Republic; Almeida Marycell Cardona, Hospital Español de Buenos Aires, Caba, Argentina, and Hospital de Alta Complejidad de Formosa, Formosa, Argentina; Baurain Jean Francois, Saint-Luc University Clinics, Brussels, Belgium; Bader Arnim, Medical University of Graz, Graz, Austria; Blecharz Pawel, M.Sklodowska-Curie Memorial Institute, Krakow, Poland; Cadron Isabelle, AZ Turnhout, Turnhout, Belgium; Cornez Nathalie, CHU Ambroise Pare, Mons, Belgium; Dostalek Lukas, Gynecologic Oncology Center, Department of Obstetrics and Gynecology, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic; Dusek Ladislav, Institute of Biostatistics and Analyses, Masaryk University, Brno, Czech Republic; Fischerova Daniela, Gynecologic Oncology Center, Department of Obstetrics and Gynecology, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic; Fruhauf Filip, Gynecologic Oncology Center, Department of Obstetrics and Gynecology, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic; Gresova Andrea, Oncology Institute of East Slovakia, Košice, Slovakia; Gunthert Andreas, Cantonal Hospital of Lucerne, Lucerne, Switzerland; Haidopoulos Dimitrios, Alexandra Hospital, Athens, Greece; Hambalek Josef, Department of Obstetrics and Gynecology, Faculty of Medicine and Dentistry, Palacky University, University Hospital Olomouc, Czech Republic; Hryhorenki Andriy, Podilskyy Regional Oncological Centre, Vinnytsia, Ukraine; Krasznai Zoard, University of Debrecen, Deparment of Obstetrics and Gynaecology, Debrecen, Hungary; Kridelka Frederic, CHU de Liege, Notre Dame des Bruyeres, Belgium; Laky Rene, Medical University of Graz, Graz, Austria; Lonkhuijzen Luc van, Academic Medical Centre, Amsterdam, The Netherlands; Francesco Raspagliesi, IRCCS Foundation National Cancer Institute in Milan, Milan, Italy; Martinez Alicia Martin, University Hospital of the Canary Islands, Las Palmas de Gran Canaria, Spain; Meili Gesine, Lucerne Cantonal Hospital, New Women’s Hospital, Luzern, Switzerland; Novotny Zdenek, University Hospital Pilsen, Pilsen, Czech Republic; Ostojich Marcela, Department of Gynecology, Institute of Oncology Angel H Roffo University of Buenos Aires, Buenos Aires Autonomous City, Argentina; Petiz Almerinda, Porto Institute of Oncology, Porto, Portugal; Santos Javier Garcia, Hospital Clinico San Carlos, Madrid, Spain; Segovia Myriam Gracia, Hospital Clinico San Carlos, Madrid, Spain; Sawicki Sambor, University Clinical Centre in Gdansk, Gdansk, Poland; Sebastia Jordi Ponce, Hospital Universitari de Bellvitge, Barcelona, Spain; Slama Jiri, Gynecologic Oncology Center, Department of Obstetrics and Gynecology, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic; Smrkolj Spela, University Medical Centre Ljubljana, Ljubljana, Slovenia; Squifflet Jean, Saint-Luc University Clinics, Brussels, Belgium; Torne Aureli, Institute Clinic of Gynecology, Obstetrics and Neonatology (ICGON), Hospital Clinic of Barcelona, Barcelona, Spain; Tummers Philippe, University Hospital Gent, Belgium; Weinberger Vit, University Hospital Brno, Brno, Czech Republic; Zalewski Kamil, Department of Gynecologic Oncology, Holycross Cancer Center, Kielce, Poland; Zapardiel Ignacio, La Paz University Hospital, Madrid, Spain; Michal Zikan, Hospital Na Bulovce, Prague, Czech Republic) and members of the Steering Committee (Kucukmetin Ali from Northern Gynaecological Oncology Centre, Queen Elizabeth Hospital, Gateshead, UK; Querleu Denis from Institut Bergonié, Bordeaux, France; van der Zee Ate from University Medical Center, Groningen, The Netherlands). We thank all medical specialists, data and case managers, secretaries, study coordinators, and all other people who are involved in the Sentix trial.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cibula, D.; Dusek, J.; Jarkovsky, J.; Dundr, P.; Querleu, D.; van der Zee, A.; Kucukmetin, A.; Kocian, R. A prospective multicenter trial on sentinel lymph node biopsy in patients with early-stage cervical cancer (SENTIX). Int. J. Gynecol. Cancer 2019, 29, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.; Bundy, B.; Zaino, R.; Sevin, B.U.; Creasman, W.T.; Major, F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol. Oncol. 1990, 38, 352–357. [Google Scholar] [CrossRef]
- Derks, M.; van der Velden, J.; de Kroon, C.D.; Nijman, H.W.; van Lonkhuijzen, L.; van der Zee, A.G.J.; Zwinderman, A.H.; Kenter, G.G. Surgical Treatment of Early-Stage Cervical Cancer: A Multi-Institution Experience in 2124 Cases in The Netherlands Over a 30-Year Period. Int. J. Gynecol. Cancer 2018, 28, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Chien, T.Y.; Huang, S.H.; Wu, C.J.; Shih, B.Y.; Chang, S.C. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol. Oncol. 2004, 93, 458–464. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Tian, J.; Fu, X.; Ren, X.; Hao, Q. Significance of the absolute number and ratio of metastatic lymph nodes in predicting postoperative survival for the International Federation of Gynecology and Obstetrics stage IA2 to IIA cervical cancer. Int. J. Gynecol. Cancer 2013, 23, 157–163. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.Y.; Lee, S.W.; Park, J.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. A postoperative scoring system for distant recurrence in node-positive cervical cancer patients after radical hysterectomy and pelvic lymph node dissection with para-aortic lymph node sampling or dissection. Gynecol. Oncol. 2017, 144, 536–540. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Wang, W.; Xia, B.; Li, K.; Sun, F.; Hou, Y. A Prognostic Nomogram for Cervical Cancer after Surgery from SEER Database. J. Cancer 2018, 9, 3923–3928. [Google Scholar] [CrossRef]
- Cibula, D.; McCluggage, W.G. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol. Oncol. 2019, 152, 202–207. [Google Scholar] [CrossRef]
- Cibula, D.; Potter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Kohler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef]
- Darai, E.; Rouzier, R.; Ballester, M.; Barranger, E.; Coutant, C. Sentinel lymph node biopsy in gynaecological cancers: The importance of micrometastases in cervical cancer. Surg. Oncol. 2008, 17, 227–235. [Google Scholar] [CrossRef]
- Ferrandina, G.; Pedone Anchora, L.; Gallotta, V.; Fagotti, A.; Vizza, E.; Chiantera, V.; De Iaco, P.; Ercoli, A.; Corrado, G.; Bottoni, C.; et al. Can We Define the Risk of Lymph Node Metastasis in Early-Stage Cervical Cancer Patients? A Large-Scale, Retrospective Study. Ann. Surg. Oncol. 2017, 24, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Slama, J.; Zikan, M.; Zaal, A.; Sevcik, L.; Kenter, G.; Querleu, D.; Jach, R.; et al. Bilateral ultrastaging of sentinel lymph node in cervical cancer: Lowering the false-negative rate and improving the detection of micrometastasis. Gynecol. Oncol. 2012, 127, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodayan, S.; Hasanzadeh, M.; Treglia, G.; Azad, A.; Yousefi, Z.; Zarifmahmoudi, L.; Sadeghi, R. Sentinel node biopsy for lymph nodal staging of uterine cervix cancer: A systematic review and meta-analysis of the pertinent literature. Eur. J. Surg. Oncol. 2015, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lecuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Darai, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: Results of the SENTICOL study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Tax, C.; Rovers, M.M.; de Graaf, C.; Zusterzeel, P.L.; Bekkers, R.L. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol. Oncol. 2015, 139, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Fang, F.; Li, Y.F. Sentinel-lymph-node procedures in early stage cervical cancer: A systematic review and meta-analysis. Med. Oncol. 2015, 32, 385. [Google Scholar] [CrossRef]
- Biglia, N.; Librino, A.; Ottino, M.C.; Panuccio, E.; Daniele, A.; Chahin, A. Lower limb lymphedema and neurological complications after lymphadenectomy for gynecological cancer. Int. J. Gynecol. Cancer 2015, 25, 521–525. [Google Scholar] [CrossRef]
- Hareyama, H.; Hada, K.; Goto, K.; Watanabe, S.; Hakoyama, M.; Oku, K.; Hayakashi, Y.; Hirayama, E.; Okuyama, K. Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic malignancies: A retrospective study. Int. J. Gynecol. Cancer 2015, 25, 751–757. [Google Scholar] [CrossRef]
- Weinberger, V.; Cibula, D.; Zikan, M. Lymphocele: Prevalence and management in gynecological malignancies. Expert Rev. Anticancer Ther. 2014, 14, 307–317. [Google Scholar] [CrossRef]
- Zikan, M.; Fischerova, D.; Pinkavova, I.; Slama, J.; Weinberger, V.; Dusek, L.; Cibula, D. A prospective study examining the incidence of asymptomatic and symptomatic lymphoceles following lymphadenectomy in patients with gynecological cancer. Gynecol. Oncol. 2015, 137, 291–298. [Google Scholar] [CrossRef]
- Bats, A.S.; Clement, D.; Larousserie, F.; Lefrere-Belda, M.A.; Faraggi, M.; Froissart, M.; Lecuru, F. Sentinel lymph node biopsy improves staging in early cervical cancer. Gynecol. Oncol. 2007, 105, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Popa, I.; Plante, M.; Renaud, M.C.; Roy, M.; Tetu, B. Negative sentinel lymph node accurately predicts negative status of pelvic lymph nodes in uterine cervix carcinoma. Gynecol. Oncol. 2006, 103, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Ramirez, P.T.; Levenback, C.F.; Munsell, M.F.; Euscher, E.D.; Soliman, P.T.; Frumovitz, M. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol. Oncol. 2017, 145, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Zikan, M.; Zaal, A.; Sevcik, L.; Kenter, G.G.; Querleu, D.; Jach, R.; Bats, A.S.; et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol. Oncol. 2012, 124, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Dundr, P.; Cibula, D.; Nemejcova, K.; Ticha, I.; Bartu, M.; Jaksa, R. Pathologic Protocols for Sentinel Lymph Nodes Ultrastaging in Cervical Cancer. Arch. Pathol. Lab. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Delomenie, M.; Bonsang-Kitzis, H.; Bats, A.S.; Ngo, C.; Balaya, V.; Xuan, H.T.N.; Koual, M.; Mathevet, P.; Lecuru, F. The clinical implication of lymph nodes micrometastases and isolated tumor cells in patients with cervical cancer: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 241, 71–76. [Google Scholar] [CrossRef]
- La Rosa, V.L.; Shah, M.; Kahramanoglu, I.; Cerentini, T.M.; Ciebiera, M.; Lin, L.T.; Dirnfeld, M.; Minona, P.; Tesarik, J. Quality of life and fertility preservation counseling for women with gynecological cancer: An integrated psychological and clinical perspective. J. Psychosom. Obstet. Gynaecol. 2019, 1–7. [Google Scholar] [CrossRef]
- Vitale, S.G.; La Rosa, V.L.; Rapisarda, A.M.C.; Lagana, A.S. The Importance of Fertility Preservation Counseling in Patients with Gynecologic Cancer. J. Reprod. Infertil. 2017, 18, 261–263. [Google Scholar]
- Nezhat, C.; Roman, R.A.; Rambhatla, A.; Nezhat, F. Reproductive and oncologic outcomes after fertility-sparing surgery for early stage cervical cancer: A systematic review. Fertil. Steril. 2020, 113, 685–703. [Google Scholar] [CrossRef]
- Vergote, I.; Pujade-Lauraine, E.; Pignata, S.; Kristensen, G.B.; Ledermann, J.; Casado, A.; Sehouli, J.; Mirza, M.; Fossati, R.; Marth, C.; et al. European Network of Gynaecological Oncological Trial Groups’ requirements for trials between academic groups and pharmaceutical companies. Int. J. Gynecol. Cancer 2010, 20, 476–478. [Google Scholar] [CrossRef]
- FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynaecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef]
- Buda, A.; Papadia, A.; Zapardiel, I.; Vizza, E.; Ghezzi, F.; De Ponti, E.; Lissoni, A.A.; Imboden, S.; Diestro, M.D.; Verri, D.; et al. From Conventional Radiotracer Tc-99(m) with Blue Dye to Indocyanine Green Fluorescence: A Comparison of Methods Towards Optimization of Sentinel Lymph Node Mapping in Early Stage Cervical Cancer for a Laparoscopic Approach. Ann. Surg. Oncol. 2016, 23, 2959–2965. [Google Scholar] [CrossRef] [PubMed]
- Jewell, E.L.; Huang, J.J.; Abu-Rustum, N.R.; Gardner, G.J.; Brown, C.L.; Sonoda, Y.; Barakat, R.R.; Levine, D.A.; Leitao, M.M., Jr. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol. Oncol. 2014, 133, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Luhrs, O.; Ekdahl, L.; Lonnerfors, C.; Geppert, B.; Persson, J. Combining Indocyanine Green and Tc(99)-nanocolloid does not increase the detection rate of sentinel lymph nodes in early stage cervical cancer compared to Indocyanine Green alone. Gynecol. Oncol. 2019. [Google Scholar] [CrossRef]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C.H. International Union against Cancer (UICC). TNM Classification of Malignant Tumours, 7th ed.; Wiley: New York, NY, USA, 2009. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).