Effect of Baseline Characteristics on Cabazitaxel Treatment Duration in Patients with Metastatic Castration-Resistant Prostate Cancer: A Post Hoc Analysis of the Compassionate Use/Expanded Access Programs and CAPRISTANA Registry
Abstract
1. Introduction
2. Results
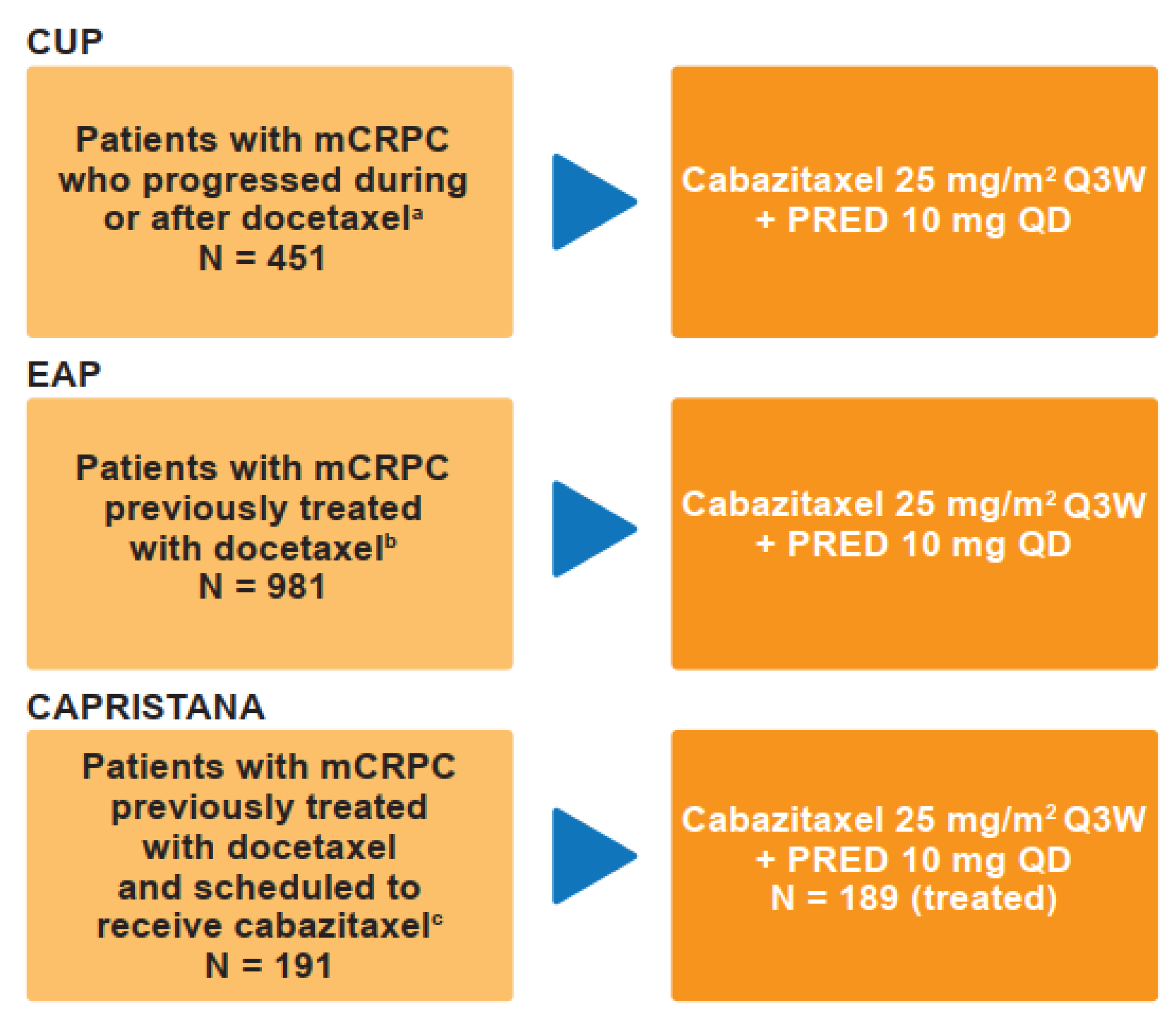
2.1. Patient Population
2.2. Treatment Exposure
2.3. Granulocyte-Colony Stimulating Factor (G-CSF) Use for Neutropenia
2.4. Patient Characteristics That May Impact Cabazitaxel Treatment Duration
2.5. Disease Progression during the First Two Cycles of Treatment
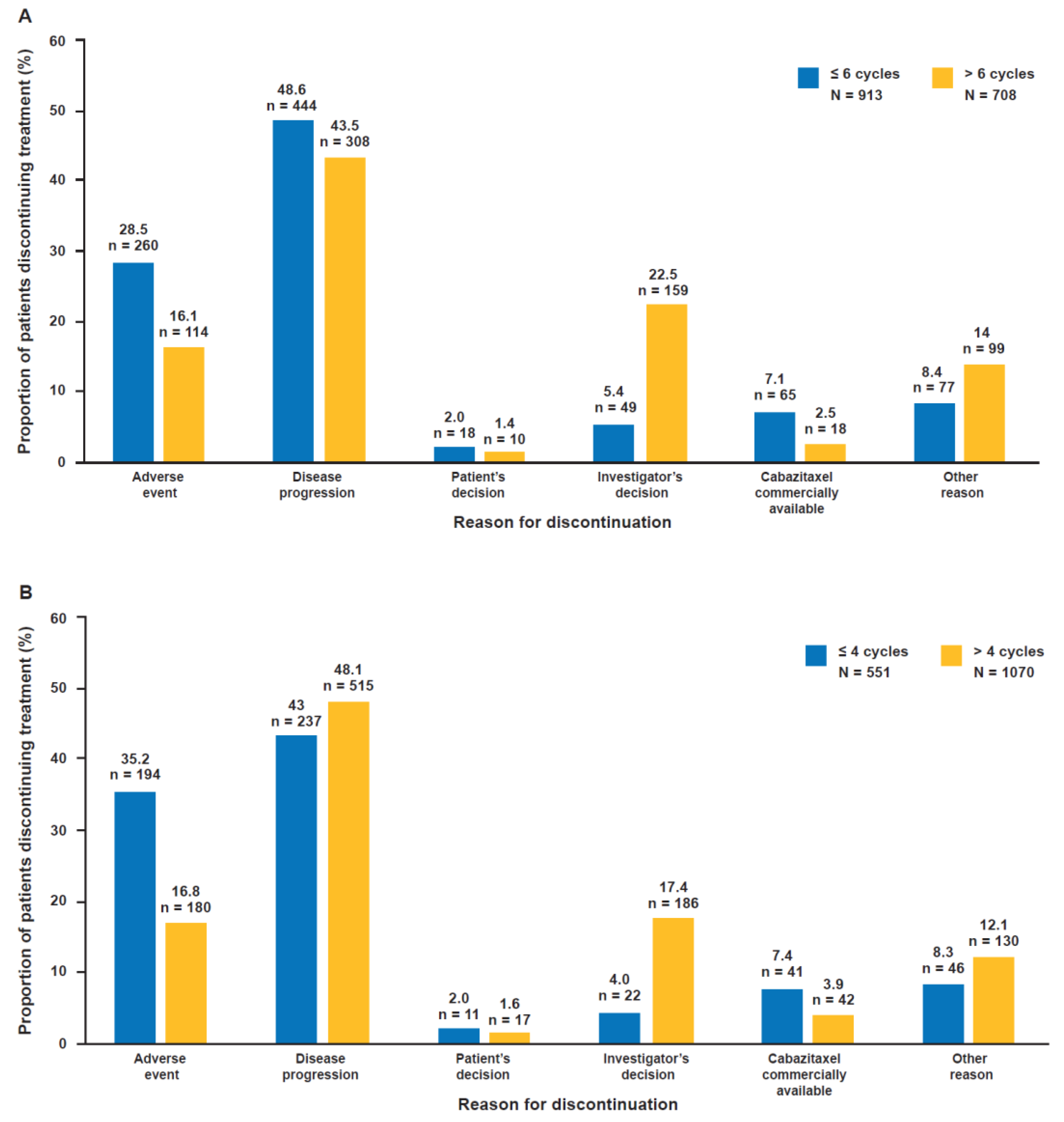
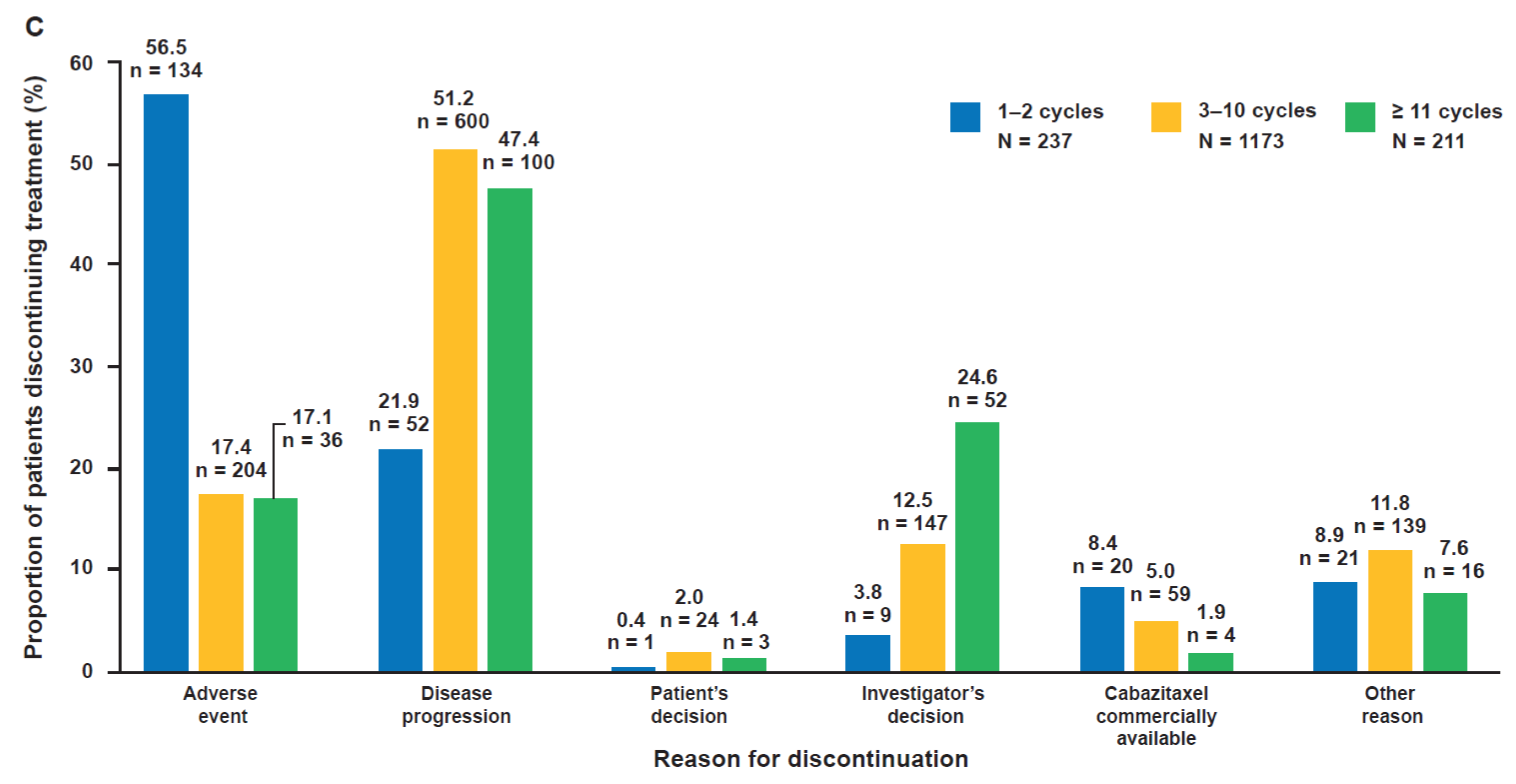
2.6. Reasons for Discontinuation According to Cabazitaxel Treatment Duration
3. Discussion
4. Materials and Methods
4.1. Study Designs and Treatment Protocol
4.2. Patient Population
4.3. Study Objectives and Endpoints
4.4. Safety Assessments
4.5. Statistical Analyses
4.6. Cabazitaxel Treatment Cycles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the Castration-Resistant Prostate Cancer Population: A Systematic Review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Prostate Cancer 2018. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 10 February 2020).
- National Prostate Cancer Adult (NPCA). Second Year Annual Report – further Analysis of Existing Clinical Data and Preliminary Results from the NPCA Prospective Audit 2015. Available online: https://www.npca.org.uk/content/uploads/2018/02/NPCA-2015-Annual-Report_FINAL_301115.pdf (accessed on 18 March 2020).
- Varenhorst, E.; Klaff, R.; Berglund, A.; Hedlund, P.O.; Sandblom, G.; Scandinavian Prostate Cancer Group (SPCG) Trial No. 5. Predictors of Early Androgen Deprivation Treatment Failure in Prostate Cancer with Bone Metastases. Cancer Med. 2016, 5, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.M.; Macedo, D.; Fernandes, I.; Costa, L. Castration-Resistant Prostate Cancer: Mechanisms, Targets, and Treatment. Prostate Cancer 2012, 2012, 327253. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen Deprivation Therapy: Progress in Understanding Mechanisms of Resistance and Optimizing Androgen Depletion. Nat. Clin Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lorente, D.; Mateo, J.; Perez-Lopez, R.; de Bono, J.S.; Attard, G. Sequencing of Agents in Castration-Resistant Prostate Cancer. Lancet Oncol. 2015, 16, e279–e292. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel Plus Prednisone Or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Vrignaud, P.; Semiond, D.; Lejeune, P.; Bouchard, H.; Calvet, L.; Combeau, C.; Riou, J.-F.; Commercon, A.; Lavelle, F.; Bissery, M.-C. Preclinical Antitumor Activity of Cabazitaxel, a Semi-Synthetic Taxane Active in Taxane-Resistant Tumors. Clin. Cancer Res. 2013, 19, 2973–2983. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone Plus Cabazitaxel Or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing After Docetaxel Treatment: A Randomised Open-Label Trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Gandaglia, G.; Bray, F.; Cooperberg, M.R.; Karnes, R.J.; Leveridge, M.J.; Moretti, K.; Murphy, D.G.; Penson, D.F.; Miller, D.C. Prostate Cancer Registries: Current Status and Future Directions. Eur. Urol. 2016, 69, 998–1012. [Google Scholar] [CrossRef]
- Carles, J.; Pichler, A.; Korunkova, H.; Tomova, A.; Ghosn, M.; El Karak, F.; Makdessi, J.; Koroleva, I.; Barnes, G.; Bury, D.; et al. An Observational, Multicentre Study of Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer Previously Treated with Docetaxel (CAPRISTANA). BJU Int. 2019, 123, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Heidenreich, A.; Bracarda, S.; Ardavanis, A.; Parente, P.; Scholz, H.; Ozatilgan, A.; Ecstein-Fraisse, E.; Hitier, S.; Di Lorenzo, G.; et al. Real-World Experience with Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer: A Final, Pooled Analysis of the Compassionate use Programme and Early Access Programme. Oncotarget 2019, 10, 4161–4168. [Google Scholar] [PubMed]
- Eisenberger, M.; Hardy-Bessard, A.C.; Kim, C.S.; Geczi, L.; Ford, D.; Mourey, L.; Carles, J.; Parente, P.; Font, A.; Kacso, G.; et al. Phase III Study Comparing a Reduced Dose of Cabazitaxel (20 mg/M2) and the Currently Approved Dose (25 mg/M2) in Postdocetaxel Patients with Metastatic Castration-Resistant Prostate Cancer-PROSELICA. J. Clin. Oncol. 2017, 35, 3198–3206. [Google Scholar] [CrossRef] [PubMed]
- Caffo, O.; Maines, F.; Rizzo, M.; Kinspergher, S.; Veccia, A. Metastatic Castration-Resistant Prostate Cancer in very Elderly Patients: Challenges and Solutions. Clin. Interv. Aging 2016, 12, 19–28. [Google Scholar] [CrossRef]
- Sanofi. JEVTANA® (Cabazitaxel) Injection, Summary of Product Characteristics, EMA; Sanofi-Aventis U.S. LLC: Toronto, ON, Canada, 2017. [Google Scholar]
- Sanofi. JEVTANA® (Cabazitaxel) Injection, Prescribing Information; FDA. Revised; Sanofi-Aventis U.S. LLC: Toronto, ON, Canada, 2017. [Google Scholar]
- Castagneto, B.; Stevani, I.; Bortolus, R.; Bearz, A.; Buosi, R.; Chimienti, E.; Guglielmi, A.; Guglielmini, P.; Santarossa, S.; Fratino, L. Weekly Cabazitaxel in Elderly Patients (EP) with Metastatic Castration Resistant Prostate Cancer (mCRPC) Progressing After Docetaxel Treatment: WeCabE, a Phase II Study. J. Clin. Oncol. 2018, 36, 300. [Google Scholar] [CrossRef]
- Clement-Zhao, A.; Auvray, M.; Aboudagga, H.; Blanc-Durand, F.; Angelergues, A.; Vano, Y.A.; Mercier, F.; El Awadly, N.; Verret, B.; Thibault, C.; et al. Safety and Efficacy of 2-Weekly Cabazitaxel in Metastatic Castration-Resistant Prostate Cancer. BJU Int. 2018, 121, 203–208. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Trial Evaluating the Safety of Two Schedules of Cabazitaxel in Elderly Men with mCRPC Previously Treated with Docetaxel (CABASTY). Available online: https://clinicaltrials.gov/ct2/show/NCT02961257 (accessed on 20 March 2020).
- Droz, J.P.; Albrand, G.; Gillessen, S.; Hughes, S.; Mottet, N.; Oudard, S.; Payne, H.; Puts, M.; Zulian, G.; Balducci, L.; et al. Management of Prostate Cancer in Elderly Patients: Recommendations of a Task Force of the International Society of Geriatric Oncology. Eur. Urol. 2017, 72, 521–531. [Google Scholar] [CrossRef]
- Heidenreich, A.; Scholz, H.J.; Rogenhofer, S.; Arsov, C.; Retz, M.; Muller, S.C.; Albers, P.; Gschwend, J.; Wirth, M.; Steiner, U.; et al. Cabazitaxel Plus Prednisone for Metastatic Castration-Resistant Prostate Cancer Progressing After Docetaxel: Results from the German Compassionate-use Programme. Eur. Urol. 2013, 63, 977–982. [Google Scholar] [CrossRef]
- Bracarda, S.; Gernone, A.; Gasparro, D.; Marchetti, P.; Ronzoni, M.; Bortolus, R.; Fratino, L.; Basso, U.; Mazzanti, R.; Messina, C.; et al. Real-World Cabazitaxel Safety: The Italian Early-Access Program in Metastatic Castration-Resistant Prostate Cancer. Future Oncol. 2014, 10, 975–983. [Google Scholar] [CrossRef]
- Castellano, D.; Anton Aparicio, L.M.; Esteban, E.; Sanchez-Hernandez, A.; Germa, J.R.; Batista, N.; Maroto, P.; Perez-Valderrama, B.; Luque, R.; Mendez-Vidal, M.J.; et al. Cabazitaxel for Metastatic Castration-Resistant Prostate Cancer: Safety Data from the Spanish Expanded Access Program. Expert Opin. Drug Saf. 2014, 13, 1165–1173. [Google Scholar] [CrossRef]
- Wissing, M.D.; van Oort, I.M.; Gerritsen, W.R.; van den Eertwegh, A.J.; Coenen, J.L.; Bergman, A.M.; Gelderblom, H. Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer: Results of a Compassionate use Program in the Netherlands. Clin. Genitourin. Cancer 2013, 11, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bracarda, S.; Mason, M.; Ozen, H.; Sengelov, L.; Van Oort, I.; Papandreou, C.; Fossa, S.; Hitier, S.; Climent, M.A.; et al. Safety of Cabazitaxel in Senior Adults with Metastatic Castration-Resistant Prostate Cancer: Results of the European Compassionate-use Programme. Eur. J. Cancer 2014, 50, 1090–1099. [Google Scholar] [CrossRef]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Sokol, E.S.; Moch, H.; Mileshkin, L.; Baciarello, G.; Losa, F.; Beringer, A.; Thomas, M.; Foser, S.; Elvin, J.; et al. Comprehensive Genomic Profiling (CGP) of Carcinoma Of Unknown Primary Origin (CUP): Retrospective Molecular Classification of Potentially Eligible Patients (Pts) for Targeted or Immunotherapy Treatment (Tx) Using the Prospective Cupisco Trial’s Criteria. Ann. Oncol. 2019, 30 (Suppl. 5), 934. [Google Scholar] [CrossRef]
- Fizazi, K.; Maillard, A.; Penel, N.; Baciarello, G.; Allouache, D.; Daugaard, G.; Van de Wouw, G.; Soler, G.; Vauleon, E.; Chaigneau, L.; et al. A Phase 3 Trial of Empiric Chemotherapy with Cisplatin and Gemcitabine or Systemic Treatment Tailored by Molecular Gene Expression Analysis in Patients with Carcinomas of an Unknown Primary (Cup) Site (GEFCAPI 04). Ann. Oncol. 2019, 30 (Suppl. 5), 851. [Google Scholar] [CrossRef]
- Losa, F.; Soler, G.; Casado, A.; Estival, A.; Fernández, I.; Giménez, S.; Longo, F.; Pazo-Cid, R.; Salgado, J.; Seguí, M.Á. SEOM clinical guideline on unknown primary cancer (2017). Clin. Transl. Oncol. 2018, 20, 89–96. [Google Scholar] [CrossRef]
- Argentiero, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Pantano, F.; Iuliani, M.; Santini, D.; Silvestris, N.; Vacca, A. Skeletal Metastases of Unknown Primary: Biological Landscape and Clinical Overview. Cancers (Basel) 2019, 11, 1270. [Google Scholar] [CrossRef]
- Hamid, A.; Wang, X.V.; Chen, Y.-H.; Feng, F.Y.; Den, R.B.; Attard, G.; Van Allen, E.M.; Huang, H.-C.; Karns, A.; Dittamore, R.; et al. Luminal B Subtype as a Predictive Biomarker of Docetaxel Benefit for Newly Diagnosed Metastatic Hormone Sensitive Prostate Cancer (Mhspc): A Correlative Study of E3805 CHAARTED. J. Clin. Oncol. 2020, 38 (Suppl. 6), 162. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. 2010. Available online: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed on 16 March 2020).
| Treatment Characteristics | CUP/EAP/CAPRISTANA | |||
|---|---|---|---|---|
| N = 1621 | ||||
| Cabazitaxel Cycles Received | ||||
| ≤6 n = 913 | >6 n = 708 | |||
| Median cabazitaxel cycles, n (range) | 4 (1–6) | 10 (7–49) | ||
| Median duration of cabazitaxel exposure, months (range) | 2.8 (1–6) | 6.9 (5–35) | ||
| G-CSF during Cycle 1, n (%) Prophylactic Therapeutic Both | n = 499 (54.7) 385 (42.2) 69 (7.6) 45 (4.9) | n = 380 (53.7) 314 (44.4) 33 (4.7) 33 (4.7) | ||
| Cabazitaxel cycles received | ||||
| ≤4 n = 551 | >4 n = 1070 | |||
| Median cabazitaxel cycles, n (range) | 3 (1–4) | 8 (5–49) | ||
| Median duration of cabazitaxel exposure, months (range) | 2.1 (1–5) | 5.8 (3–35) | ||
| G-CSF during Cycle 1, n (%) Prophylactic Therapeutic Both | n = 297 (53.9) 224 (40.7) 43 (7.8) 30 (5.4) | n = 582 (54.4) 475 (44.4) 59 (5.5) 48 (4.5) | ||
| Cabazitaxel cycles received | ||||
| 1–2 n = 237 | 3–10 n = 1173 | ≥11 n = 211 | ||
| Median cabazitaxel cycles, n (range) | 1 (1–2) | 6 (3–10) | 14 (11–49) | |
| Median duration of cabazitaxel exposure, months (range) | 0.7 (1–2) | 4.3 (2–11) | 10.3 (8–35) | |
| G-CSF during Cycle 1, n (%) Prophylactic Therapeutic Both | n = 141 (59.5) 100 (42.2) 25 (10.5) 16 (6.8) | n = 632 (53.9) 515 (43.9) 66 (5.6) 51 (4.3) | n = 106 (50.2) 84 (39.8) 11 (5.2) 11 (5.2) | |
| Patient Baseline Characteristics | CUP/EAP/CAPRISTANA N = 1621 | ||
|---|---|---|---|
| Cabazitaxel Cycles Received | |||
| ≤6 n = 913 | >6 n = 708 | p Value | |
| Median age, years (range) | 68.0 (42–89) | 68.0 (43–89) | 0.0855 |
| Age, n (%) <65 years 65–75 years ≥75 years | 271 (29.7) 433 (47.4) 209 (22.9) | 230 (32.5) 339 (47.9) 139 (19.6) | 0.2227 |
| ECOG PS, n (%) 0–1 2 a | n = 912 816 (89.5) 96 (10.5) | n = 708 665 (93.9) 43 (6.1) | 0.0015 |
| Frailty, n (%) ECOG PS <2 and ≤75 years ECOG PS <2 and >75 years ECOG PS ≥2 and ≤75 years ECOG PS ≥2 and >75 years | n = 912 660 (72.4) 156 (17.1) 73 (8.0) 23 (2.5) | n = 708 566 (79.9) 99 (14.0) 30 (4.2) 13 (1.8) | 0.0016 |
| Median time from prostate cancer diagnosis, years (range) | 4.5 (0–22) | 4.7 (0–20) | 0.0659 |
| Median time from mCRPC diagnosis, years (range) | 1.7 (0–14) | 1.8 (0–12) | 0.1298 |
| Median docetaxel cycles at last administration, n (range) | 7 (1–69) | 8 (1–58) | <0.0001 |
| Median cumulative dose of last docetaxel administration, mg/m2 (range) | 600 (50–5145) | 675 (105–8700) | 0.0005 |
| Metastatic sites, n (%) Bone Regional lymph nodes Lungs Liver Visceral, other soft tissue | n = 912 829 (90.8) 282 (30.9) 111 (12.2) 105 (11.5) 47 (5.1) | n = 707 630 (89.0) 214 (30.2) 68 (9.6) 46 (6.5) 23 (3.2) | - |
| Number of metastatic sites, n (%) 0 1 ≥2 | n = 913 1 (0.1) 298 (32.6) 614 (67.3) | n = 708 1 (0.1) 272 (38.4) 435 (61.4) | 0.0254 |
| Pain at baseline (CAPRISTANA study only), n (%) None Moderate Severe | n = 86 15 (17.4) 63 (73.3) 8 (9.3) | n = 68 18 (26.5) 47 (69.1) 3 (4.4) | 0.2457 |
| Patient Baseline Characteristics | CUP/EAP/CAPRISTANA N = 1621 | ||
|---|---|---|---|
| Cabazitaxel Cycles Received | |||
| ≤4 n = 551 | >4 n = 1070 | p Value | |
| Median age, years (range) | 69.0 (42–89) | 68.0 (42–89) | 0.2033 |
| Age, n (%) < 65 years 65–75 years ≥ 75 years | 165 (29.9) 258 (46.8) 128 (23.2) | 336 (31.4) 514 (48.0) 220 (20.6) | 0.4562 |
| ECOG PS, n (%) 0–1 2 a | n = 550 476 (86.5) 74 (13.5) | n = 1070 1005 (93.9) 65 (6.1) | <0.0001 |
| Frailty, n (%) ECOG PS <2 and ≤75 years ECOG PS <2 and >75 years ECOG PS ≥2 and ≤75 years ECOG PS ≥2 and >75 years | n = 550 386 (70.2) 90 (16.4) 56 (10.2) 18 (3.3) | n = 1070 840 (78.5) 165 (15.4) 47 (4.4) 18 (1.7) | <0.0001 |
| Median time from prostate cancer diagnosis, years (range) | 4.6 (0–22) | 4.7 (0–20) | 0.1603 |
| Median time from mCRPC diagnosis, years (range) | 1.7 (0–11) | 1.8 (0–14) | 0.1359 |
| Median docetaxel cycles at last administration, n (range) | 7 (1–69) | 8 (1–68) | 0.0061 |
| Median cumulative dose of last docetaxel administration, mg/m2 (range) | 600 (50–5145) | 675 (50–8700) | 0.0063 |
| Metastatic sites, n (%) Bone Regional lymph nodes Lungs Liver Visceral, other soft tissue | n = 550 497 (90.2) 173 (31.4) 80 (14.5) 75 (13.6) 29 (5.3) | n = 106 962 (89.9) 323 (30.2) 99 (9.3) 76 (7.1) 41 (3.8) | - |
| Number of metastatic sites, n (%) 0 1 ≥2 | n = 551 1 (0.2) 171 (31.0) 379 (68.8) | n = 1070 1 (<0.1) 399 (37.3) 670 (62.6) | 0.0190 |
| Pain at baseline (CAPRISTANA study only), n (%) None Moderate Severe | n = 48 6 (12.5) 36 (75.0) 6 (12.5) | n = 106 27 (25.5) 74 (69.8) 5 (4.7) | 0.0737 |
| Patient Baseline Characteristics | CUP/EAP/CAPRISTANA N = 1621 | |||
|---|---|---|---|---|
| Cabazitaxel Cycles Received | ||||
| 1–2 n = 237 | 3–10 n = 1173 | ≥11 n = 211 | p Value | |
| Median age, years (range) | 70.0 (42–89) | 68.0 (42–89) | 68.0 (49–87) | 0.0114 |
| Age, n (%) <65 years 65–75 years ≥75 years | 64 (27.0) 103 (43.5) 70 (29.5) | 377 (32.1) 562 (47.9) 234 (19.9) | 60 (28.4) 107 (50.7) 44 (20.9) | 0.0177 |
| ECOG PS, n (%) 0–1 2 a | n = 237 197 (83.1) 40 (16.9) | n = 1172 1082 (92.3) 90 (7.7) | n = 211 202 (95.7) 9 (4.3) | 0.0001 |
| Frailty, n (%) ECOG PS <2 and ≤75 years ECOG PS <2 and >75 years ECOG PS ≥2 and ≤75 years ECOG PS ≥2 and >75 years | n = 237 149 (62.9) 48 (20.3) 26 (11.0) 14 (5.9) | n = 1172 906 (77.3) 176 (15.0) 70 (6.0) 20 (1.7) | n = 211 171 (81.0) 31 (14.7) 7 (3.3) 2 (0.9) | <0.0001 |
| Median time from prostate cancer diagnosis, years (range) | 4.9 (1–22) | 4.4 (0–20) | 5.5 (1–18) | 0.0002 |
| Median time from mCRPC diagnosis, years (range) | 1.8 (0–10) | 1.7 (0–14) | 1.8 (0–10) | 0.2483 |
| Median docetaxel cycles at last administration, n (range) | 8 (1–34) | 8 (1–69) | 10 (2–49) | <0.0001 |
| Median cumulative dose of last docetaxel administration, mg/m2 (range) | 600 (50–2850) | 610.6 (50–8700) | 750 (120–2830) | <0.0123 |
| Metastatic sites, n (%) Bone Regional lymph nodes Lungs Liver Visceral, other soft tissue | n = 236 211 (89.0) 75 (31.6) 42 (17.7) 39 (16.5) 9 (3.8) | n = 1173 1062 (90.5) 361 (30.8) 119 (10.1) 101 (8.6) 54 (4.6) | n = 210 186 (88.2) 60 (28.4) 18 (8.5) 11 (5.2) 7 (3.3) | - |
| Number of metastatic sites, n (%) 0 1 ≥2 | n = 237 1 (0.4) 71 (30.0) 165 (69.6) | n = 1173 0 415 (35.4) 758 (64.6) | n = 211 1 (0.5) 84 (39.8) 126 (59.7) | 0.0203 |
| Pain at baseline (CAPRISTANA study only), n (%) None Moderate Severe | n = 8 3 (37.5) 4 (50.0) 1 (12.5) | n = 127 24 (18.9) 93 (73.2) 10 (7.9) | n = 19 6 (31.6) 13 (68.4) 0 | 0.2260 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, Z.; Di Lorenzo, G.; Pichler, A.; De Giorgi, U.; Hitier, S.; Ecstein-Fraisse, E.; Ozatilgan, A.; Carles, J. Effect of Baseline Characteristics on Cabazitaxel Treatment Duration in Patients with Metastatic Castration-Resistant Prostate Cancer: A Post Hoc Analysis of the Compassionate Use/Expanded Access Programs and CAPRISTANA Registry. Cancers 2020, 12, 995. https://doi.org/10.3390/cancers12040995
Malik Z, Di Lorenzo G, Pichler A, De Giorgi U, Hitier S, Ecstein-Fraisse E, Ozatilgan A, Carles J. Effect of Baseline Characteristics on Cabazitaxel Treatment Duration in Patients with Metastatic Castration-Resistant Prostate Cancer: A Post Hoc Analysis of the Compassionate Use/Expanded Access Programs and CAPRISTANA Registry. Cancers. 2020; 12(4):995. https://doi.org/10.3390/cancers12040995
Chicago/Turabian StyleMalik, Zafar, Giuseppe Di Lorenzo, Angelika Pichler, Ugo De Giorgi, Simon Hitier, Evelyne Ecstein-Fraisse, Ayse Ozatilgan, and Joan Carles. 2020. "Effect of Baseline Characteristics on Cabazitaxel Treatment Duration in Patients with Metastatic Castration-Resistant Prostate Cancer: A Post Hoc Analysis of the Compassionate Use/Expanded Access Programs and CAPRISTANA Registry" Cancers 12, no. 4: 995. https://doi.org/10.3390/cancers12040995
APA StyleMalik, Z., Di Lorenzo, G., Pichler, A., De Giorgi, U., Hitier, S., Ecstein-Fraisse, E., Ozatilgan, A., & Carles, J. (2020). Effect of Baseline Characteristics on Cabazitaxel Treatment Duration in Patients with Metastatic Castration-Resistant Prostate Cancer: A Post Hoc Analysis of the Compassionate Use/Expanded Access Programs and CAPRISTANA Registry. Cancers, 12(4), 995. https://doi.org/10.3390/cancers12040995







