Prognostic Impact of Tumor-Associated Macrophages on Survival Is Checkpoint Dependent in Classical Hodgkin Lymphoma
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Outcome
2.2. Association of IDO-1, PD-L1, CD68, CD163 Gene Expressions with Outcome
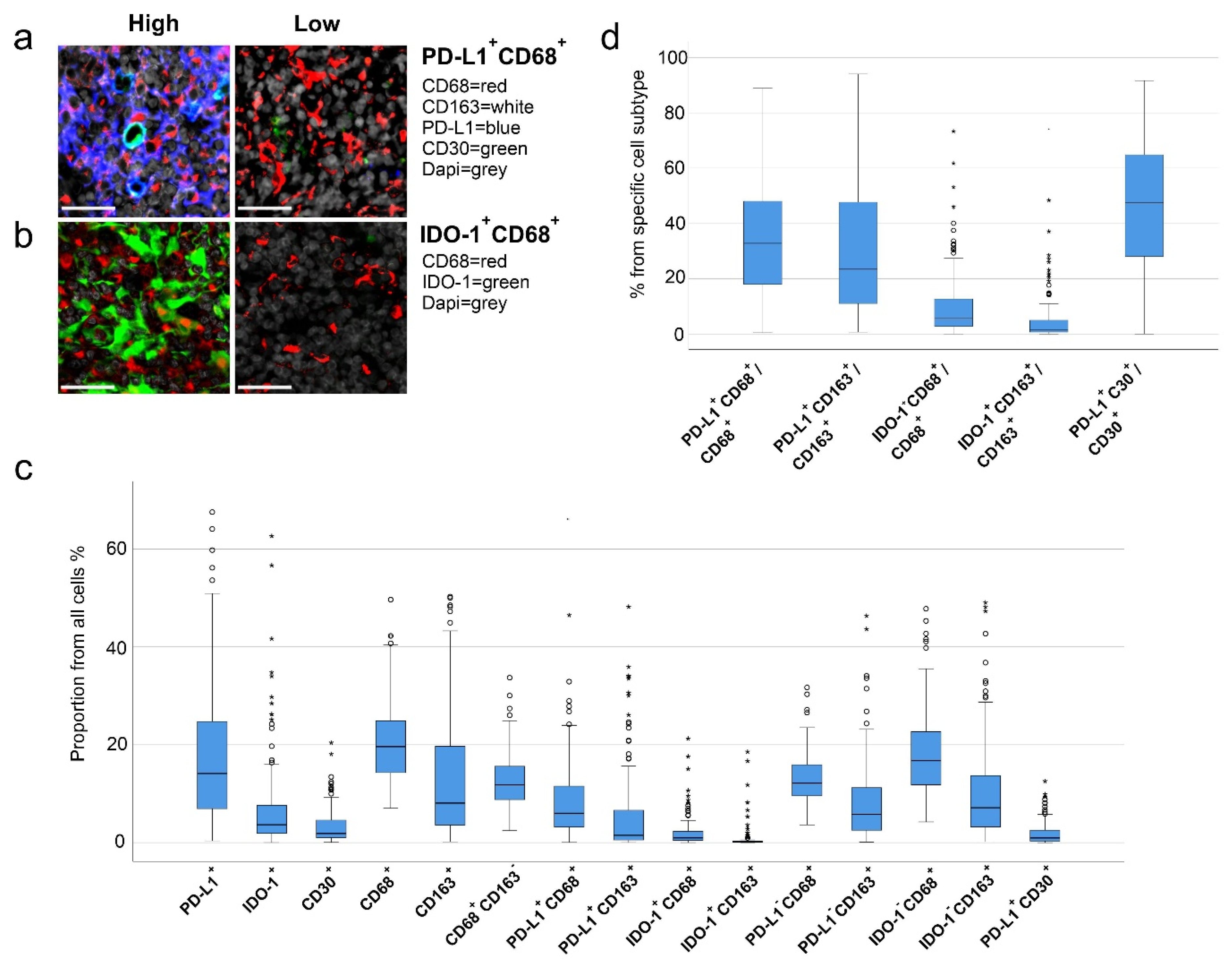
2.3. High Number of PD-L1+ and IDO-1+ Cells Translates to Inferior Outcome
2.4. High Proportions of PD-L1+ and IDO-1+ TAMs Translate to Inferior Outcome
2.5. PD-L1 Expression in HRS Cells
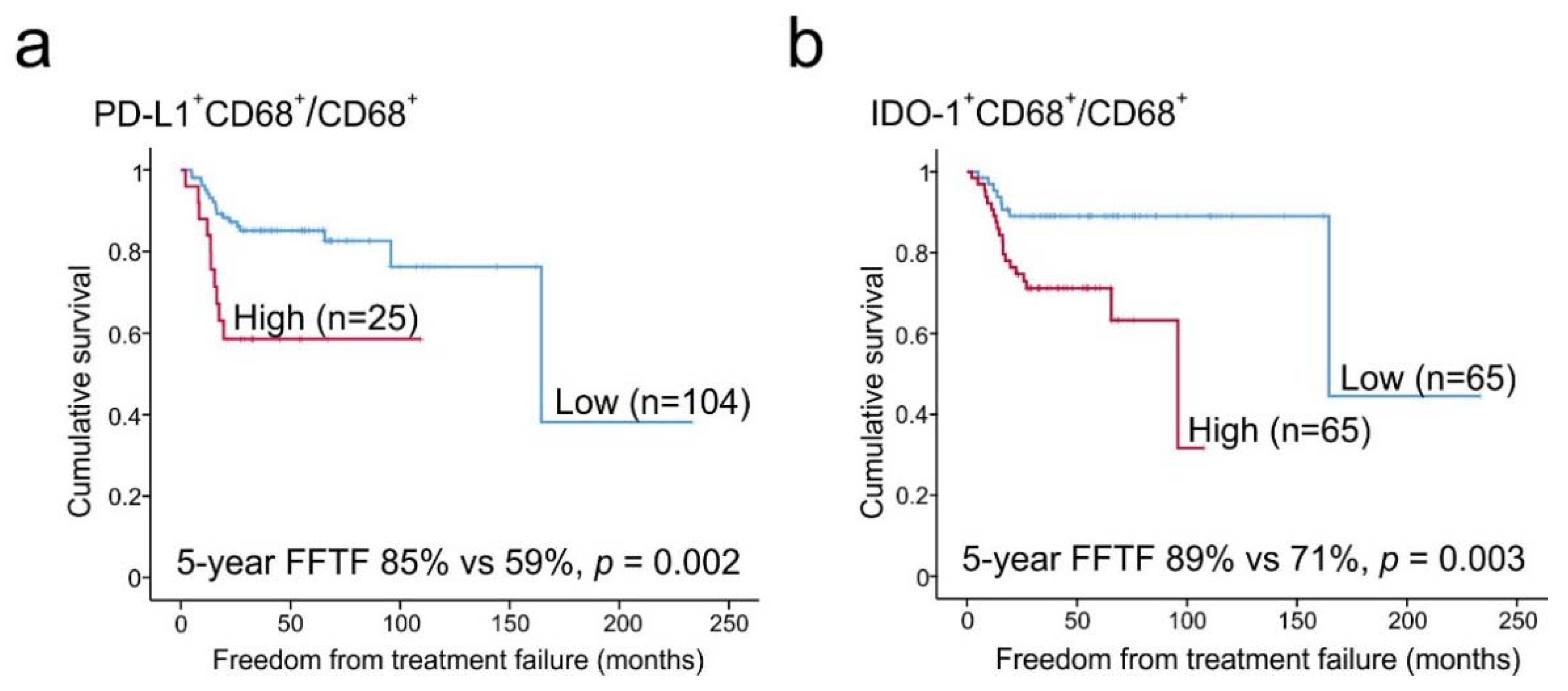
2.6. Prognostic Impact of PD-L1+ and IDO-1+ TAM Proportions
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Gene Expression Analysis
4.3. Multiplex Immunohistochemistry
4.4. Determination of Epstein-Barr Virus Status
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vardhana, S.; Younes, A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica 2016, 101, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Steidl, C.; Connors, J.M.; Gascoyne, R.D. Molecular pathogenesis of Hodgkin’s lymphoma: Increasing evidence of the importance of the microenvironment. J. Clin. Oncol. 2011, 29, 1812–1826. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Celegato, M.; Casagrande, N. Microenvironmental interactions in classical Hodgkin lymphoma and their role in promoting tumor growth, immune escape and drug resistance. Cancer Lett. 2016, 380, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Roemer, M.G.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.R.; Shipp, M.A. Signaling pathways and immune evasion mechanisms in classical Hodgkin lymphoma. Hematol. Am. Soc. Hematol. Educ. Program 2017, 1, 310–316. [Google Scholar] [CrossRef]
- Pedoeem, A.; Azoulay-Alfaguter, I.; Strazza, M.; Silverman, G.J.; Mor, A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014, 153, 145–152. [Google Scholar] [CrossRef]
- Carey, C.D.; Gusenleitner, D.; Lipschitz, M.; Roemer, M.G.M.; Stack, E.C.; Gjini, E.; Hu, X.; Redd, R.; Freeman, G.J.; Neuberg, D.; et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017, 130, 2420–2430. [Google Scholar] [CrossRef]
- Li, F.; Zhang, R.; Li, S.; Liu, J. IDO1: An important immunotherapy target in cancer treatment. Int. Immunopharmacol. 2017, 47, 70–77. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Smith, C.; Thomas, S.; Mandik-Nayak, L.; Laury-Kleintop, L.; Metz, R.; Muller, A.J. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. 2014, 63, 721–735. [Google Scholar] [CrossRef]
- Hollander, P.; Kamper, P.; Smedby, K.E.; Enblad, G.; Ludvigsen, M.; Mortensen, J.; Amini, R.M.; Hamilton-Dutoit, S.; d’Amore, F.; Molin, D.; et al. High proportions of PD-1(+) and PD-L1(+) leukocytes in classical Hodgkin lymphoma microenvironment are associated with inferior outcome. Blood Adv. 2017, 1, 1427–1439. [Google Scholar] [CrossRef]
- Choe, J.Y.; Yun, J.Y.; Jeon, Y.K.; Kim, S.H.; Park, G.; Huh, J.R.; Oh, S.; Kim, J.E. Indoleamine 2,3-dioxygenase (IDO) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: A retrospective cohort study. BMC Cancer 2014, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Steidl, C.; Lee, T.; Shah, S.P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S.J.; Iqbal, J.; Weisenburger, D.D.; et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N. Engl. J. Med. 2010, 362, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Kamper, P.; Bendix, K.; Hamilton-Dutoit, S.; Honore, B.; Nyengaard, J.R.; d’Amore, F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica 2011, 96, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.W.; Park, C.S.; Yoon, D.H.; Suh, C.; Huh, J. CD163 expression was associated with angiogenesis and shortened survival in patients with uniformly treated classical Hodgkin lymphoma. PLoS ONE 2014, 9, e87066. [Google Scholar] [CrossRef]
- Guo, B.; Cen, H.; Tan, X.; Ke, Q. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in adult classical Hodgkin lymphoma. BMC Med. 2016, 14, 159. [Google Scholar] [CrossRef]
- Tan, K.L.; Scott, D.W.; Hong, F.; Kahl, B.S.; Fisher, R.I.; Bartlett, N.L.; Advani, R.H.; Buckstein, R.; Rimsza, L.M.; Connors, J.M.; et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: A correlative study from the E2496 Intergroup trial. Blood 2012, 120, 3280–3287. [Google Scholar] [CrossRef]
- Gotti, M.; Nicola, M.; Lucioni, M.; Fiaccadori, V.; Ferretti, V.; Sciarra, R.; Costanza, M.; Bono, E.; Molo, S.; Maffi, A.; et al. Independent prognostic impact of tumour-infiltrating macrophages in early-stage Hodgkin’s lymphoma. Hematol. Oncol. 2017, 35, 296–302. [Google Scholar] [CrossRef]
- Sanchez-Espiridion, B.; Martin-Moreno, A.M.; Montalban, C.; Medeiros, L.J.; Vega, F.; Younes, A.; Piris, M.A.; Garcia, J.F. Immunohistochemical markers for tumor associated macrophages and survival in advanced classical Hodgkin’s lymphoma. Haematologica 2012, 97, 1080–1084. [Google Scholar] [CrossRef]
- Azambuja, D.; Natkunam, Y.; Biasoli, I.; Lossos, I.S.; Anderson, M.W.; Morais, J.C.; Spector, N. Lack of association of tumor-associated macrophages with clinical outcome in patients with classical Hodgkin’s lymphoma. Ann. Oncol. 2012, 23, 736–742. [Google Scholar] [CrossRef]
- Kayal, S.; Mathur, S.; Karak, A.K.; Kumar, L.; Sharma, A.; Bakhshi, S.; Raina, V. CD68 tumor-associated macrophage marker is not prognostic of clinical outcome in classical Hodgkin lymphoma. Leuk. Lymphoma 2014, 55, 1031–1037. [Google Scholar] [CrossRef]
- Cencini, E.; Fabbri, A.; Rigacci, L.; Lazzi, S.; Gini, G.; Cox, M.C.; Mancuso, S.; Abruzzese, E.; Kovalchuk, S.; Goteri, G.; et al. Evaluation of the prognostic role of tumour-associated macrophages in newly diagnosed classical Hodgkin lymphoma and correlation with early FDG-PET assessment. Hematol. Oncol. 2017, 35, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Mittman, S.; Rodriguez, R.; Moskalenko, M.; Pacheco-Sanchez, P.; Yang, Y.; Nickles, D.; Cubas, R. Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019, 79, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.W.; Jeon, Y.K.; Yoon, D.H.; Suh, C.; Huh, J. Programmed death 1 expression in the peritumoral microenvironment is associated with a poorer prognosis in classical Hodgkin lymphoma. Tumour Biol. 2016, 37, 7507–7514. [Google Scholar] [CrossRef] [PubMed]
- Pollari, M.; Bruck, O.; Pellinen, T.; Vahamurto, P.; Karjalainen-Lindsberg, M.L.; Mannisto, S.; Kallioniemi, O.; Kellokumpu-Lehtinen, P.L.; Mustjoki, S.; Leivonen, S.K.; et al. PD-L1(+) tumor-associated macrophages and PD-1(+) tumor-infiltrating lymphocytes predict survival in primary testicular lymphoma. Haematologica 2018, 103, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Steidl, C. The classical Hodgkin lymphoma tumor microenvironment: Macrophages and gene expression-based modeling. Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Chetaille, B.; Bertucci, F.; Finetti, P.; Esterni, B.; Stamatoullas, A.; Picquenot, J.M.; Copin, M.C.; Morschhauser, F.; Casasnovas, O.; Petrella, T.; et al. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood 2009, 113, 2765–3775. [Google Scholar] [CrossRef]
- Vari, F.; Arpon, D.; Keane, C.; Hertzberg, M.S.; Talaulikar, D.; Jain, S.; Cui, Q.; Han, E.; Tobin, J.; Bird, R.; et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018, 131, 1809–1819. [Google Scholar] [CrossRef]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef]
- Armand, P.; Shipp, M.A.; Ribrag, V.; Michot, J.M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016, 34, 3733–3739. [Google Scholar] [CrossRef]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Brockelmann, P.J.; Engert, A. Checkpoint inhibition in Hodgkin lymphoma–A review. Oncol. Res. Treat. 2017, 40, 654–660. [Google Scholar] [CrossRef]
- Gay, N.D.; Okada, C.Y.; Chen, A.I.; Scott, E.C. Targeting the programmed cell death 1 pathway in Hodgkin lymphoma: The place of nivolumab. Ther. Adv. Hematol. 2017, 8, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Meti, N.; Esfahani, K.; Johnson, N.A. The role of immune checkpoint inhibitors in classical Hodgkin lymphoma. Cancers (Basel) 2018, 10, e.204. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Komiya, T.; Huang, C.H. Updates in the clinical development of epacadostat and other indoleamine 2,3-dioxygenase 1 inhibitors (IDO1) for human cancers. Front. Oncol. 2018, 8, 423. [Google Scholar] [CrossRef]
- Leivonen, S.K.; Pollari, M.; Bruck, O.; Pellinen, T.; Autio, M.; Karjalainen-Lindsberg, M.L.; Mannisto, S.; Kellokumpu-Lehtinen, P.L.; Kallioniemi, O.; Mustjoki, S.; et al. T-cell inflamed tumor microenvironment predicts favorable prognosis in primary testicular lymphoma. Haematologica 2019, 104, 338–346. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef]
| Characteristic | n = 130 (%) |
|---|---|
| Median follow-up time, months (range) | 55 (7–229) |
| Age (years) | |
| Median (range) | 29 (16–83) |
| <60 | 116 (89) |
| ≥60 | 14 (11) |
| Sex | |
| Male | 59 (45) |
| Female | 71 (55) |
| Histologic subtype | |
| Nodular sclerosis | 102 (78) |
| Mixed cellularity | 21 (16) |
| Lymphocyte-rich | 6 (5) |
| Unclassified cHL | 1 (1) |
| Stage | |
| I-IIA | 56 (43) |
| IIB-IV | 73 (56) |
| NA | 1 (1) |
| EBV status | |
| Negative | 89 (69) |
| Positive | 34 (26) |
| NA | 7 (5) |
| IPS | |
| 0–3 | 86 (66) |
| 4–7 | 7 (5) |
| NA | 37 (29) |
| Treatment | |
| ABVD | 41 (32) |
| ABVD + radiotherapy | 70 (53) |
| BEACOPPesc | 5 (4) |
| BEACOPPesc + radiotherapy | 4 (3) |
| ABVD + BEACOPPesc | 4 (3) |
| CHOP | 4 (3) |
| Other | 2 (2) |
| Radiotherapy * | 77 (59) |
| Relapses | 28 (22) |
| Deaths | 10 (8) |
| cHL related deaths | 6 (60) |
| 5-year FFTF | 80% |
| 5-year DSS | 94% |
| 5-year OS | 91% |
| Gene Symbol | FFTF | DSS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| CD274 (PD-L1) | 1.607 | 1.027–2.513 | 0.038 | 2.091 | 0.871–5.024 | 0.099 | 1.791 | 0.816–3.931 | 0.146 |
| IDO-1 | 1.465 | 1.069–2.009 | 0.018 | 2.234 | 1.327–3.762 | 0.003 | 2.107 | 1.311–3.388 | 0.002 |
| CD68 | 1.256 | 0.768–2.054 | 0.364 | 2.319 | 0.923–5.826 | 0.074 | 2.405 | 1.056–5.475 | 0.037 |
| CD163 | 0.895 | 0.691–1.161 | 0.404 | 1.217 | 0.771–1.923 | 0.399 | 1.364 | 0.908–2.050 | 0.135 |
| Cell Immunophenotype (proportion from all cells) | FFTF | DSS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| PD-L1+ | 1.027 | 1.003–1.051 | 0.025 | 1.069 | 1.018–1.123 | 0.007 | 1.054 | 1.012–1.098 | 0.011 |
| IDO-1+ | 1.048 | 1.020–1.076 | 0.001 | 1.082 | 1.042–1.123 | <0.001 | 1.074 | 1.039–1.111 | <0.001 |
| CD30+ | 1.059 | 0.967–1.160 | 0.217 | 1.147 | 1.001–1.315 | 0.049 | 1.087 | 0.949–1.245 | 0.227 |
| CD68+ | 1.012 | 0.970–1.055 | 0.594 | 1.045 | 0.956–1.143 | 0.331 | 1.061 | 0.989–1.139 | 0.098 |
| CD163+ | 1.009 | 0.981–1.037 | 0.541 | 1.035 | 0.981–1.091 | 0.206 | 1.041 | 0.997–1.086 | 0.066 |
| CD68+CD163- | 1.022 | 0.959–1.088 | 0.506 | 1.009 | 0.877–1.161 | 0.899 | 0.994 | 0.883–1.118 | 0.914 |
| PD-L1+CD68+ | 1.042 | 1.002–1.084 | 0.040 | 1.109 | 1.031–1.194 | 0.006 | 1.093 | 1.026–1.164 | 0.006 |
| PD-L1+CD163+ | 1.029 | 0.993–1.066 | 0.114 | 1.099 | 1.030–1.172 | 0.004 | 1.088 | 1.031–1.148 | 0.002 |
| IDO-1+CD68+ | 1.107 | 1.021–1.201 | 0.014 | 1.235 | 1.107–1.378 | <0.001 | 1.221 | 1.105–1.348 | <0.001 |
| IDO-1+CD163+ | 1.181 | 1.070–1.304 | 0.001 | 1.319 | 1.163–1.495 | <0.001 | 1.290 | 1.151–1.447 | <0.001 |
| PD-L1−CD68+ | 0.980 | 0.914–1.051 | 0.576 | 0.918 | 0.763–1.104 | 0.363 | 0.987 | 0.872–1.117 | 0.837 |
| PD-L1−CD163+ | 0.991 | 0.947–1.037 | 0.703 | 0.962 | 0.848–1.091 | 0.543 | 1.001 | 0.940–1.087 | 0.770 |
| IDO-1−CD68+ | 0.976 | 0.928–1.025 | 0.330 | 0.884 | 0.755–1.036 | 0.129 | 0.982 | 0.898–1.074 | 0.690 |
| IDO-1−CD163+ | 0.993 | 0.956–1.032 | 0.732 | 0.987 | 0.902–1.080 | 0.775 | 1.015 | 0.960–1.074 | 0.595 |
| PD-L1+CD30+ | 1.063 | 0.922–1.225 | 0.402 | 1.192 | 0.928–1.532 | 0.169 | 1.093 | 0.854–1.401 | 0.480 |
| Cell immunophenotype (proportion from specific cell subtype) | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p |
| PD-L1+CD68+/CD68+ | 1.021 | 1.003–1.039 | 0.024 | 1.047 | 1.005–1.090 | 0.027 | 1.034 | 1.001–1.068 | 0.042 |
| PD-L1⁺CD163+/CD163+ | 1.020 | 1.006–1.035 | 0.005 | 1.038 | 1.005–1.072 | 0.022 | 1.028 | 1.002–1.054 | 0.036 |
| IDO-1+CD68⁺/CD68+ | 1.032 | 1.009–1.057 | 0.007 | 1.066 | 1.031–1.102 | <0.001 | 1.059 | 1.028–1.091 | <0.001 |
| IDO-1+CD163+/CD163+ | 1.040 | 1.015–1.066 | 0.002 | 1.062 | 1.030–1.094 | <0.001 | 1.057 | 1.028–1.087 | <0.001 |
| PD-L1+CD30+/CD30+ | 1.008 | 0.992–1.024 | 0.323 | 1.016 | 0.981–1.053 | 0.369 | 1.008 | 0.981–1.036 | 0.555 |
| Characteristic | PD-L1+CD68+/CD68+ | IDO-1+CD68+/CD68+ | ||||
|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | |
| Number of Patients (%) | 104 | 25 | 65 | 65 | ||
| Sex | ||||||
| Male | 45 (43) | 13 (52) | 0.431 | 29 (45) | 30 (46) | 0.86 |
| Female | 59 (57) | 12 (48) | 36 (55) | 35 (54) | ||
| Age (years) | 0.837 | |||||
| <60 | 93 (89) | 22 (88) | 60 (92) | 56 (86) | 0.258 | |
| ≥60 | 11 (11) | 3 (12) | 5 (8) | 9 (14) | ||
| Subtype | ||||||
| NS | 86 (83) | 16 (64) | 0.039 | 57 (88) | 45 (69) | 0.01 |
| Other * | 8 (17) | 9 (36) | 8 (12) | 20 (31) | ||
| Stage | ||||||
| I-IIA | 49 (48) | 6 (24) | 0.033 | 34 (53) | 22 (34) | 0.027 |
| IIB-IV | 54 (52) | 19 (76) | 30 (47) | 43 (66) | ||
| EBV status | ||||||
| Negative | 75 (76.5) | 13 (54) | 0.029 | 48 (81) | 23 (64) | 0.032 |
| Positive | 23 (23.5) | 11 (46) | 11 (19) | 41 (36) | ||
| IPS | ||||||
| 0–3 | 66 (92) | 20 (95) | 0.585 | 42 (93) | 44 (92) | 0.761 |
| 4–7 | 6 (8) | 1 (5) | 3 (7) | 4 (8) | ||
| Characteristic | FFTF | DSS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| PD-L1+CD68+/CD68+ (high) | 3.222 | 1.46–7.09 | 0.004 | 11.958 | 2.15–66.63 | 0.005 | 4.646 | 1.23–17.54 | 0.023 |
| IDO-1+CD68+/CD68+ (high) | 3.537 | 1.47–8.50 | 0.005 | 6.050 | 0.70–52.21 | 0.102 | 4.237 | 0.88–20.52 | 0.073 |
| PD-L1+CD68+/CD68+ (high) Stage adjusted (I-IIA vs. IIB-IV) | 2.525 | 1.12–5.68 | 0.025 | 8.243 | 1.46–46.64 | 0.017 | 3.348 | 0.87–12.86 | 0.078 |
| IDO-1+CD68+/CD68+ (high) Stage adjusted (I-IIA vs. IIB-IV) | 2.586 | 1.08–6.17 | 0.032 | 4.154 | 0.48–35.77 | 0.195 | 3.301 | 0.67–16.38 | 0.144 |
| PD-L1+CD68+/CD68+ (high) Subtype adjusted (NS vs. others) | 3.455 | 1.54–7.75 | 0.003 | 11.123 | 1.93–64.09 | 0.007 | 4.202 | 1.08–16.36 | 0.038 |
| IDO-1+CD68+/CD68+ (high) Subtype adjusted (NS vs. others) | 3.419 | 1.41–8.32 | 0.007 | 5.711 | 0.65–51.57 | 0.117 | 3.957 | 0.79–19.86 | 0.095 |
| PD-L1+CD68+/CD68+ (high) EBV status adjusted (neg vs. pos) | 3.715 | 1.64–8.41 | 0.002 | 11.071 | 1.91–64.06 | 0.007 | 3.450 | 0.88–13.50 | 0.075 |
| IDO-1+CD68+/CD68+ (high) EBV status adjusted (neg vs. pos) | 3.937 | 1.54–10.06 | 0.004 | 5.390 | 0.61–47.52 | 0.129 | 3.237 | 0.65–16.03 | 0.150 |
| Age (≥60y) | 3.799 | 1.60–9.00 | 0.002 | 12.708 | 2.46–65.55 | 0.002 | 16.516 | 4.30–63.37 | <0.001 |
| Stage (IIB-IV) | 7.791 | 2.35–25.82 | 0.001 | 55.058 | 0.07–41007.16 | 0.235 | 6.631 | 0.83–53.06 | 0.075 |
| Female | 0.676 | 0.32–1.42 | 0.303 | 0.746 | 0.15–3.71 | 0.721 | 0.596 | 0.16–2.23 | 0.442 |
| EBV-status (positive) | 0.811 | 0.33–2.02 | 0.653 | 1.608 | 0.29–8.84 | 0.585 | 4.100 | 1.09–15.38 | 0.036 |
| IPS (4–7) | 1.157 | 0.27–4.95 | 0.854 | 4.603 | 0.48–44.65 | 0.188 | 9.725 | 1.61–58.77 | 0.013 |
| Other cHL subtype than NS | 1.106 | 0.44–2.76 | 0.829 | 2.527 | 0.45–14.35 | 0.295 | 2.582 | 0.63–10.61 | 0.188 |
| Risk Factor | HR | 95% CI | p |
|---|---|---|---|
| PD-L1+CD68+/CD68+ (high) | 2.625 | 1.173–5.876 | 0.019 |
| Stage (IIB-IV) | 5.834 | 1.720–19.786 | 0.005 |
| Age (≥60 years) | 2.631 | 1.095–6.319 | 0.031 |
| IDO-1+CD68+/CD68+ (high) | 2.480 | 1.034–5.947 | 0.042 |
| Stage (IIB-IV) | 5.799 | 1.705–19.717 | 0.005 |
| Age (≥60 years) | 2.221 | 0.921–5.356 | 0.076 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karihtala, K.; Leivonen, S.-K.; Brück, O.; Karjalainen-Lindsberg, M.-L.; Mustjoki, S.; Pellinen, T.; Leppä, S. Prognostic Impact of Tumor-Associated Macrophages on Survival Is Checkpoint Dependent in Classical Hodgkin Lymphoma. Cancers 2020, 12, 877. https://doi.org/10.3390/cancers12040877
Karihtala K, Leivonen S-K, Brück O, Karjalainen-Lindsberg M-L, Mustjoki S, Pellinen T, Leppä S. Prognostic Impact of Tumor-Associated Macrophages on Survival Is Checkpoint Dependent in Classical Hodgkin Lymphoma. Cancers. 2020; 12(4):877. https://doi.org/10.3390/cancers12040877
Chicago/Turabian StyleKarihtala, Kristiina, Suvi-Katri Leivonen, Oscar Brück, Marja-Liisa Karjalainen-Lindsberg, Satu Mustjoki, Teijo Pellinen, and Sirpa Leppä. 2020. "Prognostic Impact of Tumor-Associated Macrophages on Survival Is Checkpoint Dependent in Classical Hodgkin Lymphoma" Cancers 12, no. 4: 877. https://doi.org/10.3390/cancers12040877
APA StyleKarihtala, K., Leivonen, S.-K., Brück, O., Karjalainen-Lindsberg, M.-L., Mustjoki, S., Pellinen, T., & Leppä, S. (2020). Prognostic Impact of Tumor-Associated Macrophages on Survival Is Checkpoint Dependent in Classical Hodgkin Lymphoma. Cancers, 12(4), 877. https://doi.org/10.3390/cancers12040877





