Prognostic Impact of PD-L1 Expression in Malignant Salivary Gland Tumors as Assessed by Established Scoring Criteria: Tumor Proportion Score (TPS), Combined Positivity Score (CPS), and Immune Cell (IC) Infiltrate
Abstract
1. Introduction
2. Results
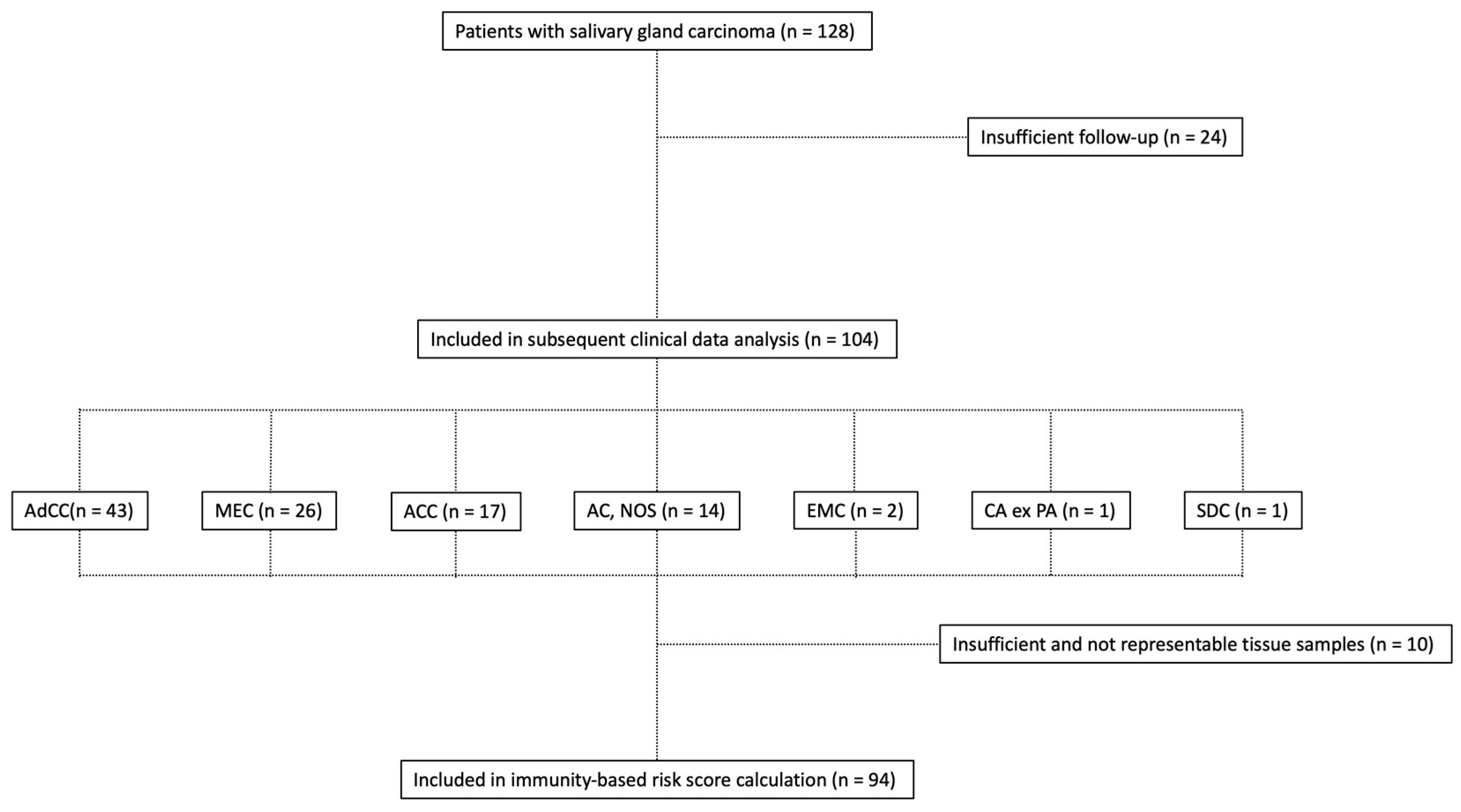
2.1. Clinicopathologic Characteristics
2.2. Histopathological Assessment
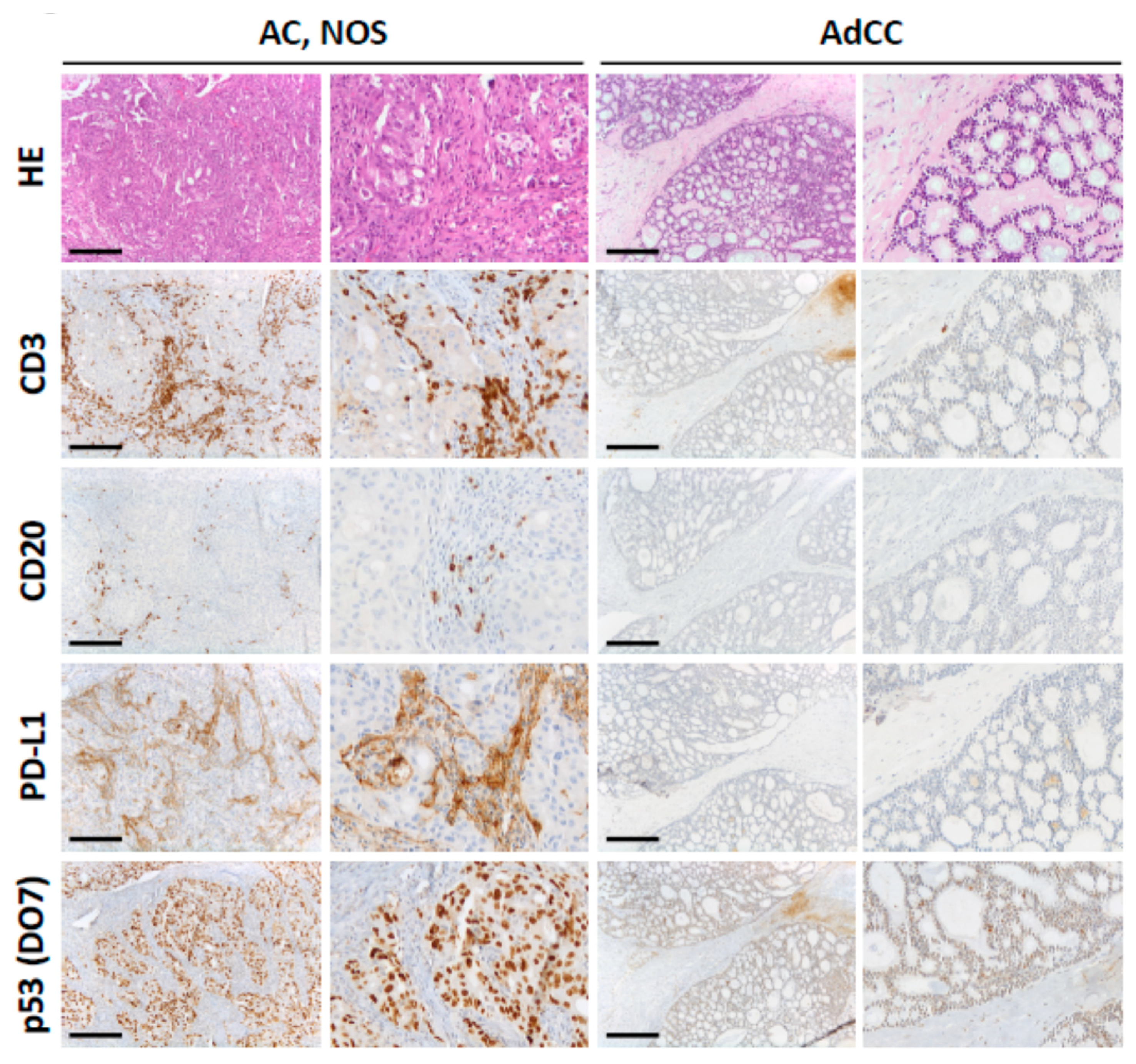
2.3. Characterization of the Inflammatory Infiltrate in Salivary Gland Tumors
2.4. Evaluation of PD-L1 Scoring
2.5. P53 (DO-7) Immunohistochemistry
2.6. Therapeutic Characteristics and Clinical Outcome
3. Discussion
4. Materials and Methods
4.1. Patients and Clinicopathologic Data
4.2. Immunohistochemistry
4.3. Quantification of Inflammatory Cells
4.4. PD-L1 Scoring
4.5. P53 (DO-7) Scoring
4.6. Treatment and Assessment
4.7. Ethics Statement
4.8. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Speight, P.M.; Barrett, A.W. Salivary gland tumours. Oral Dis. 2002, 8, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Mantravadi, A.V.; Moore, M.G.; Rassekh, C.H. AHNS series: Do you know your guidelines? Diagnosis and management of salivary gland tumors. Head Neck 2019, 41, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.A.; Ho, A.L.; Fury, M.G.; Sherman, E.; Pfister, D.G. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: A systematic review. Lancet Oncol. 2011, 12, 815–824. [Google Scholar] [CrossRef]
- Chan, A.T.; Gregoire, V.; Lefebvre, J.L.; Licitra, L.; Hui, E.P.; Leung, S.F.; Felip, E.; Group, E.-E.-E.G.W. Nasopharyngeal cancer: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, vii83–vii85. [Google Scholar] [CrossRef] [PubMed]
- Specenier, P.; Vermorken, J.B. Advances in the systemic treatment of head and neck cancers. Curr. Opin. Oncol. 2010, 22, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Debaere, D.; Vander Poorten, V.; Nuyts, S.; Hauben, E.; Schoenaers, J.; Schöffski, P.; Clement, P. Cyclophosphamide, doxorubicin, and cisplatin in advanced salivary gland cancer. B-ENT 2011, 7, 1. [Google Scholar]
- Licitra, L.; Cavina, R.; Grandi, C.; Pahna, S.D.; Guzzo, M.; Demicheli, R.; Molinari, R. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma: A phase H trial of 22 patients. Ann. Oncol. 1996, 7, 640–642. [Google Scholar] [CrossRef]
- Grünewald, I.; Vollbrecht, C.; Meinrath, J.; Meyer, M.F.; Heukamp, L.C.; Drebber, U.; Quaas, A.; Beutner, D.; Hüttenbrink, K.-B.; Wardelmann, E.; et al. Targeted next generation sequencing of parotid gland cancer uncovers genetic heterogeneity. Oncotarget 2015, 6, 18224. [Google Scholar] [CrossRef]
- Skálová, A.; Stenman, G.; Simpson, R.H.; Hellquist, H.; Slouka, D.; Svoboda, T.; Bishop, J.A.; Hunt, J.L.; Nibu, K.-I.; Rinaldo, A.; et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am. J. Surg. Pathol. 2018, 42, e11–e27. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet. Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Schildhaus, H.-U. Der prädiktive Wert der PD-L1-Diagnostik. Der Pathol. 2018, 39, 498–519. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B.; Delord, J.-P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Algazi, A.P.; Damian, S.; Hong, R.-L.; Le Tourneau, C.; et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: Findings of the phase 1b KEYNOTE-028 study. Am. J. Clin. Oncol. 2018, 41, 1083. [Google Scholar] [CrossRef] [PubMed]
- Muro, K.; Chung, H.C.; Shankaran, V.; Geva, R.; Catenacci, D.; Gupta, S.; Eder, J.P.; Golan, T.; Le, D.T.; Burtness, B. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncology. 2016, 17, 717–726. [Google Scholar] [CrossRef]
- Dolled-Filhart, M.; Roach, C.; Toland, G.; Stanforth, D.; Jansson, M.; Lubiniecki, G.M.; Ponto, G.; Emancipator, K. Development of a companion diagnostic for pembrolizumab in non–small cell lung cancer using immunohistochemistry for programmed death ligand-1. Arch. Pathology. Lab. Med. 2016, 140, 1243–1249. [Google Scholar] [CrossRef]
- Vital, D.; Ikenberg, K.; Moch, H.; Rössle, M.; Huber, G.F. The expression of PD-L1 in salivary gland carcinomas. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Harada, K.; Ferdous, T.; Ueyama, Y. PD-L1 expression in malignant salivary gland tumors. BMC Cancer 2018, 18, 156. [Google Scholar] [CrossRef]
- Rodriguez, C.P.; Wu, Q.V.; Voutsinas, J.; Fromm, J.R.; Jiang, X.; Pillarisetty, V.G.; Lee, S.M.; Santana-Davila, R.; Goulart, B.; Baik, C.S.; et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin. Cancer Res. 2020, 26, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Boukheris, H.; Curtis, R.E.; Land, C.E.; Dores, G.M. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: A population-based study in the United States. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Terhaard, C.H.; Lubsen, H.; Van der Tweel, I.; Hilgers, F.; Eijkenboom, W.; Marres, H.; Tjho-Heslinga, R.; De Jong, J.; Roodenburg, J. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck J. Sci. Spec. Head Neck 2004, 26, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Mukaigawa, T.; Hayashi, R.; Hashimoto, K.; Ugumori, T.; Hato, N.; Fujii, S. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J. Surg. Oncol. 2016, 114, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.H.; Lelkaitis, G.; Håkansson, K.; Vogelius, I.R.; Johannesen, H.H.; Fischer, B.M.; Bentzen, S.M.; Specht, L.; Kristensen, C.A.; von Buchwald, C.; et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br. J. Cancer 2019, 120, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Kiyoshima, T.; Shima, K.; Kobayashi, I.; Matsuo, K.; Okamura, K.; Komatsu, S.; Rasul, A.; Sakai, H. Expression of p53 tumor suppressor gene in adenoid cystic and mucoepidermoid carcinomas of the salivary glands. Oral Oncol. 2001, 37, 315–322. [Google Scholar] [CrossRef]
- Ross, J.; Gay, L.; Wang, K.; Vergilio, J.-A.; Suh, J.; Ramkissoon, S.; Somerset, H.; Johnson, J.; Russell, J.; Ali, S. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann. Oncol. 2017, 28, 2539–2546. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Seethala, R.R.; Stenman, G. Update from the 4th edition of the World Health Organization classification of head and neck tumours: tumors of the salivary gland. Head Neck Pathol. 2017, 11, 55–67. [Google Scholar] [CrossRef]
- Yemelyanova, A.; Vang, R.; Kshirsagar, M.; Lu, D.; Marks, M.A.; Shih, I.M.; Kurman, R.J. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod. Pathol. 2011, 24, 1248. [Google Scholar] [CrossRef]
- Franklin, H.R.; Simonetti, G.P.; Dubbelman, A.C.; ten Bokkel Huinink, W.W.; Taal, B.G.; Wigbout, G.; Mandjes, I.A.; Dalesio, O.B.; Aaronson, N.K. Toxicity grading systems. A comparison between the WHO scoring system and the common toxicity criteria when used for nausea and vomiting. Ann. Oncol. 1994, 5, 113–117. [Google Scholar] [CrossRef]
- Trotti, A.; Byhardt, R.; Stetz, J.; Gwede, C.; Corn, B.; Fu, K.; Gunderson, L.; McCormick, B.; Morrisintegral, M.; Rich, T.; et al. Common toxicity criteria: Version 2.0. An improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 13–47. [Google Scholar] [CrossRef]


| Attribute | Overall Study Group (n = 94) | AdCC (n = 41) | MEC (n = 21) | ACC (n = 16) | AC, NOS (n = 12) | EMC (n = 2) | Ca ex PA* (n = 1) | SDC (n = 1) |
|---|---|---|---|---|---|---|---|---|
| Male/female | 44 (46.8%)/ 50 (53.2%) | 20 (48.8%)/ 21 (51.2%) | 9 (42.9%)/ 12 (57.1%) | 7 (43.8%)/ 9 (56.2%) | 6 (50.0%)/ 6 (50.0%) | 1 (50.0%)/ 1 (50.0%) | - 1 (100.0%) | 1 (100.0%)/- |
| Median age (range), years | 56.5 (18–90) | 56.0 (20–90) | 55.0 (32–83) | 49.0 (18–69) | 74.0 (53–82) | 46.0 (18–74) | 74 | 77 |
| BMI (median, range) | 26.2 (17.0–45.5) | 26.5 (18.7–34.2) | 25.4 (21.3–35.0) | 26.1 (22.0–34.9) | 24.8 (20.8–45.5) | 22.1 (17.0–27.2) | 26.3 | 26.3 |
| ECOG PS | ||||||||
| 0 to 2 | 89 (94.8%) | 39 (95.1%) | 19 (90.5%) | 16 (100.0%) | 11 (91.7%) | 2 (100.0%) | 1 (100.0%) | 1 (100.0%) |
| 3 + 4 | 5 (5.2%) | 2 (4.9%) | 2 (9.5%) | - | 1 (8.3%) | - | - | - |
| CCI (median, range) | 4.5 (0–9) | 4.0 (0–8) | 4.0 (0–8) | 2.0 (0–6) | 6.5 (4–9) | 3.0 (2–4) | 4 | 9 |
| LDH level | ||||||||
| <240 U/L | 62 (65.9%) | 28 (68.3%) | 12 (57.1%) | 12 (75.0%) | 6 (50.0%) | 2 (100.0%) | 1 (100.0%) | 1 (100.0%) |
| >240 U/L | 32 (34.1%) | 13 (31.7%) | 9 (42.9%) | 4 (25.0%) | 6 (50.0%) | - | - | - |
| B symptoms** | ||||||||
| Yes | 8 (8.5%) | 3 (7.3%) | 2 (9.6%) | - | 3 (25.0%) | - | - | - |
| No | 86 (91.5%) | 38 (92.7%) | 19 (90.4%) | 16 (100.0%) | 9 (75.0%) | 2 (100.0%) | 1 (100.0%) | 1 (100.0%) |
| UICC/AJCC | ||||||||
| I | 20 (21.3%) | 9 (22.0%) | 5 (23.8%) | 5 (31.3%) | - | 1 (50.0%) | - | - |
| II | 15 (16.0%) | 5 (12.2%) | 4 (19.0%) | 5 (31.3%) | - | 1 (50.0%) | - | - |
| III | 19 (20.2%) | 9 (22.0%) | 4 (19.0%) | 4 (25.0%) | 1 (8.3%) | - | - | 1 (100.0%) |
| IVA | 15 (16.0%) | 8 (19.5%) | 3 (14.3%) | 1 (6.3%) | 2 (16.7%) | - | 1 (100.0%) | - |
| IVB | 8 (8.5%) | 6 (14.6%) | 1 (4.8%) | - | - | - | - | - |
| IVC | 17 (18.0%) | 4 (9.7%) | 3 (14.3%) | 1 (6.3%) | 9 (75.0%) | - | - | - |
| Primary localization | ||||||||
| GP | 41 (43.6%) | 6 (14.6%) | 9 (42.9%) | 14 (87.5%) | 9 (75.0%) | 2 (100.0%) | - | 1 (100.0%) |
| GSM | 15 (16.0%) | 9 (22.0%) | 4 (19.0%) | 1 (6.3%) | 1 (8.3%) | - | - | - |
| GSL | 4 (4.3%) | 2 (4.9%) | 2 (9.6%) | - | - | - | - | - |
| P | 17 (18.0%) | 14 (34.1%) | 2 (9.6%) | - | - | - | 1 (100.0%) | - |
| NC | 5 (5.3%) | 4 (9.7%) | - | - | 1 (8.3%) | - | - | - |
| others | 12 (12.8%) | 6 (14.6%) | 4 (19.0%) | 1 (6.3%) | 1 (8.3%) | - | - | - |
| Nodal disease | ||||||||
| N0 | 66 (70.2%) | 36 (87.8%) | 16 (76.2%) | 11 (68.7%) | - | 2 (100.0%) | 1 (100.0%) | - |
| N+ | 28 (29.8%) | 5 (12.2%) | 5 (23.8%) | 5 (31.3%) | 12 (100.0%) | - | - | 1 (100.0%) |
| Metastatic disease | ||||||||
| M0 | 77 (82.0%) | 37 (90.3%) | 18 (85.7%) | 15 (93.7%) | 3 (25.0%) | 2 (100.0%) | 1 (100.0%) | 1 (100.0%) |
| M+ | 17 (18.0%) | 4 (9.7%) | 3 (14.3%) | 1 (6.3%) | 9 (75.0%) | - | - | - |
| Second malignancy | ||||||||
| Yes | 10 (10.6%) | 3 (7.3%) | 3 (14.3%) | - | 4 (33.3%) | - | - | - |
| No | 84 (89.4%) | 38 (92.7%) | 18 (85.7%) | 16 (100.0%) | 8 (66.7%) | 2 (100.0%) | 1 (100.0%) | 1 (100.0%) |
| Attribute | Overall Study Group (n = 94) | AdCC (n = 41) | MEC (n = 21) | ACC (n = 16) | AC, NOS (n = 12) | EMC (n = 2) | Ca ex PA (n = 1) | SDC (n = 1) |
|---|---|---|---|---|---|---|---|---|
| Ki-67 (median, range) | 15% (1%–80%) | 20% (5%–80%) | 5% (2%–15%) | 5% (1%–50%) | 30% (10%–60%) | 7.5% | 15% | 15% |
| p53 (DO7) | ||||||||
| null/ + + | 5 | 1 | 0 | 0 | 4 | n.a. | n.a. | n.a. |
| + | 89 | 40 | 21 | 16 | 8 | n.a. | n.a | n.a. |
| Inflammatory cells(median, range) | ||||||||
| CD3 + | 31.5 (0–547.7) | 22.7 (0–148) | 5.3 (0–151) | 37.0 (1–158) | 66.8 (0.7–547.7) | 6 | 44.3 | 73.3 |
| CD20 + | 0.0 (0–75.3) | 0.3 (0–31) | 0.3 (0–40.7) | 1.8 (0–75.3) | 1.8 (0–10.3) | 0 | 0.7 | 0.7 |
| TPS | ||||||||
| mean/median | 4.3/1 | 0.98/ 1 | 3.29/ 0.33 | 4.14/1 | 14.67/5 | 0.1/1 | 1 | 10 |
| (range) | (0–60) | (0–10) | (0–35) | (0–20) | (0–60) | |||
| TPS < 1 | 33 (35.1%) | 16 | 10 | 6 | - | 1 | - | - |
| TPS 1 - 5 | 42 (44.7%) | 20 | 8 | 6 | 6 | 1 | 1 | - |
| TPS > 5 | 19 (20.2%) | 5 | 3 | 4 | 6 | - | - | 1 |
| CPS | ||||||||
| mean/median | 9.8/3.5 | 3.63/2 | 6.16/0.5 | 9.2/3.5 | 28.92/11 | 2/2 | 3 | 56 |
| (range) | (0–86) | (0–25) | (0–71) | (0–40) | (1–86) | |||
| CPS < 1 | 19 (20.2%) | 10 | 6 | 3 | - | - | - | - |
| CPS 1 - 10 | 52 (55.3%) | 25 | 8 | 9 | 7 | 2 | 1 | - |
| CPS > 10 | 23 (24.5%) | 6 | 7 | 4 | 5 | - | - | 1 |
| IC | ||||||||
| 0–1 | 68 (73.1%) | 34 | 14 | 14 | 4 | 1 | 1 | - |
| 2–3 | 25 (26.9%) | 7 | 7 | 2 | 8 | - | - | 1 |
| Attribute | Overall Study Group (n = 94) | AdCC (n = 41) | MEC (n = 21) | ACC (n = 16) | AC, NOS (n = 12) | Others (n = 4) |
|---|---|---|---|---|---|---|
| 1st line therapy | ||||||
| Surgical resection | 85 (90.4%) | 35 (85.4%) | 19 (90.5%) | 16 (100.0%) | 11 (91.7%) | 4 (100.0%) |
| Radiotherapy | 44 (46.8%) | 21 (51.2%) | 13 (61.9%) | 3 (18.7%) | 8 (66.7%) | 2 (50.0%) |
| Chemotherapy (CTX) | 11 (11.7%) | 3 (7.3%) | 3 (14.3%) | 2 (12.5%) | 4 (33.3%) | - |
| - CAP | 9 | 3 | 2 | 1 | 3 | - |
| - MFP | - | - | - | - | - | - |
| - others | 2 | - | 1 | - | 1 | - |
| Best response 1st line | ||||||
| CR | 61 (64.9%) | 31 (75.6%) | 14 (66.7%) | 13 (81.3%) | 1 (8.3%) | 2 (50.0%) |
| PR | 32 (34.0%) | 10 (24.4%) | 7 (33.3%) | 3 (18.7%) | 10 (83.3%) | 2 (50.0%) |
| SD | 1 (1.1%) | - | - | - | 1 (8.3%) | - |
| PD | - | - | - | - | - | - |
| Lines of therapy | 1.81 | 1.81 | 1.62 | 1.24 | 2.29 | 1.5 |
| (mean, range) | (1–4) | (1–4) | (1–3) | (1–4) | (1–4) | (1–3) |
| Treatment of relapses | ||||||
| Surgical resection | 22 (23.4%) | 12 (29.3%) | 5 (23.8%) | - | 4 (33.3%) | 1 (25.0%) |
| Radiotherapy | 15 (15.9%) | 4 (9.7%) | 1 (4.8%) | 1 (6.3%) | 8 (66.7%) | 1 (25.0%) |
| Chemotherapy | 34 (36.2%) | 18 (43.9%) | 5 (23.8%) | 3 (18.7%) | 7 (58.3%) | 1 (25.0%) |
| - CAP | 12 | 8 | 1 | 1 | 1 | 1 |
| - MFP | 10 | 5 | 3 | 1 | 1 | - |
| - others | 12 | 5 | 1 | 1 | 5 | - |
| Targeted therapy | 14 (14.9%) | 7 (17.1%) | 2 (9.5%) | 1 (6.3%) | 4 (33.3%) | - |
| - mTor inhibition | 4 | 1 | 1 | - | 2 | - |
| - EGFR inhibition | 3 | 2 | 1 | - | -2 | - |
| - Immunotherapy | 3 | 2 | - | - | 1 | - |
| - others | 4 | 2 | - | 1 | 1 | - |
| CTX associated toxicity profile | ||||||
| Cytopenia grade III/IV | 12 (35.3%) | 4 (22.2%) | 3 (60.0%) | 1 (66.7%) | 4 (57.1%) | - |
| Acute kidney disease | 8 (23.5%) | 4 (22.2%) | 3 (60.0%) | - | 1 (14.3%) | - |
| Sepsis | 3 (8.8%) | 1 (5.6%) | 1 (20.0%) | - | 1 (14.3%) | - |
| Cardiotoxicity | 1 (2.9%) | 1 (5.6%) | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witte, H.M.; Gebauer, N.; Lappöhn, D.; Umathum, V.G.; Riecke, A.; Arndt, A.; Steinestel, K. Prognostic Impact of PD-L1 Expression in Malignant Salivary Gland Tumors as Assessed by Established Scoring Criteria: Tumor Proportion Score (TPS), Combined Positivity Score (CPS), and Immune Cell (IC) Infiltrate. Cancers 2020, 12, 873. https://doi.org/10.3390/cancers12040873
Witte HM, Gebauer N, Lappöhn D, Umathum VG, Riecke A, Arndt A, Steinestel K. Prognostic Impact of PD-L1 Expression in Malignant Salivary Gland Tumors as Assessed by Established Scoring Criteria: Tumor Proportion Score (TPS), Combined Positivity Score (CPS), and Immune Cell (IC) Infiltrate. Cancers. 2020; 12(4):873. https://doi.org/10.3390/cancers12040873
Chicago/Turabian StyleWitte, Hanno M., Niklas Gebauer, Daniela Lappöhn, Vincent G. Umathum, Armin Riecke, Annette Arndt, and Konrad Steinestel. 2020. "Prognostic Impact of PD-L1 Expression in Malignant Salivary Gland Tumors as Assessed by Established Scoring Criteria: Tumor Proportion Score (TPS), Combined Positivity Score (CPS), and Immune Cell (IC) Infiltrate" Cancers 12, no. 4: 873. https://doi.org/10.3390/cancers12040873
APA StyleWitte, H. M., Gebauer, N., Lappöhn, D., Umathum, V. G., Riecke, A., Arndt, A., & Steinestel, K. (2020). Prognostic Impact of PD-L1 Expression in Malignant Salivary Gland Tumors as Assessed by Established Scoring Criteria: Tumor Proportion Score (TPS), Combined Positivity Score (CPS), and Immune Cell (IC) Infiltrate. Cancers, 12(4), 873. https://doi.org/10.3390/cancers12040873






