Landscape and Future Perspectives of Immunotherapy in Neuroendocrine Neoplasia
Abstract
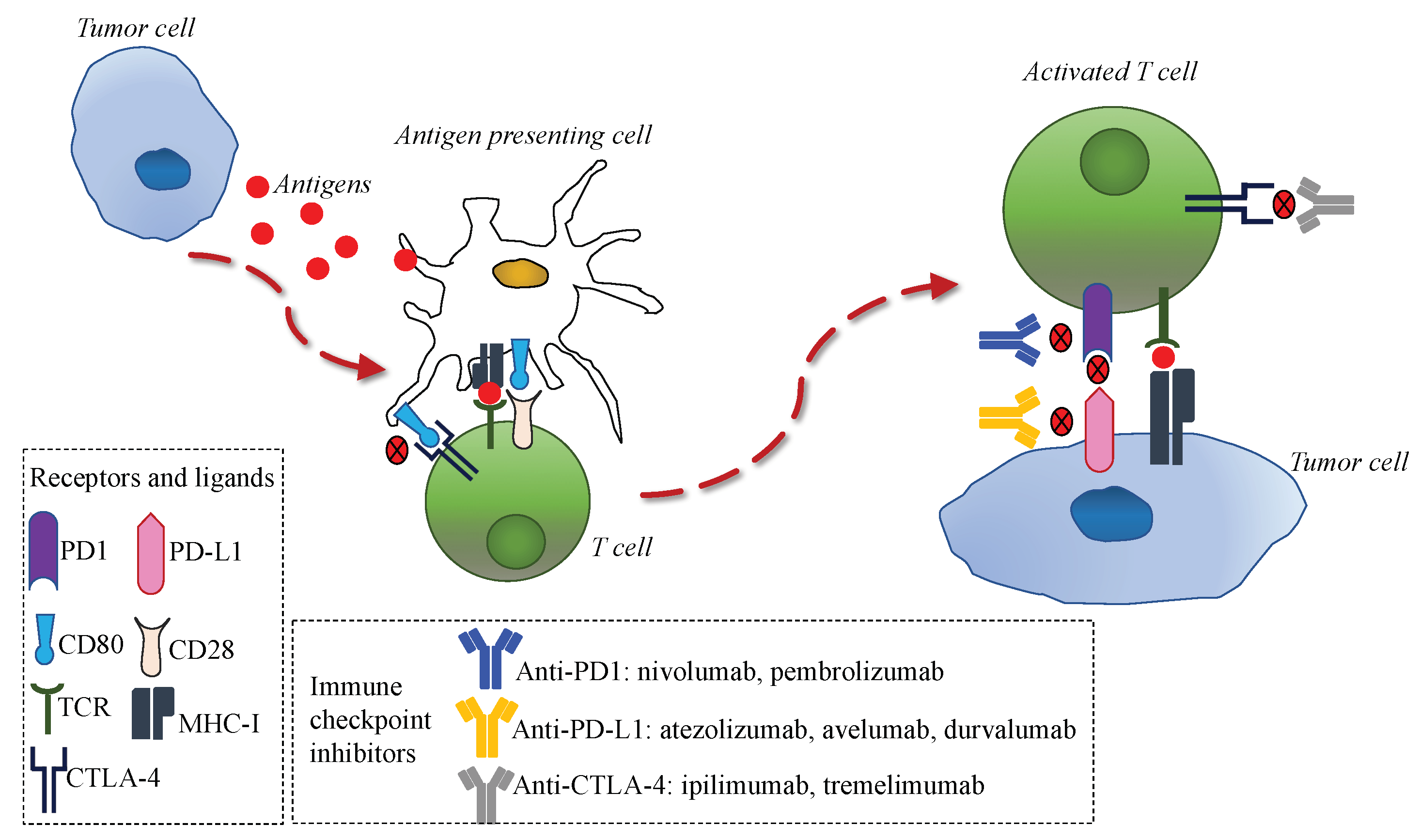
1. Introduction
2. Materials and Methods
3. Immunotherapy in Human Cancers and Rationale in NENs
4. Predictive Biomarkers for Immunotherapy
5. Immunotherapy in Lung NENs
5.1. SCLC First Line and Maintenance
5.2. SCLC Second and Third Line
5.3. LCNEC, Typical and Atypical Carcinoid
6. Immunotherapy in Merkel Cell Carcinoma
7. Immunotherapy in Gastroenteropancreatic NENs
8. Ongoing Clinical Trials and Future Perspectives
8.1. Pulmonary High-Grade NENs
8.2. Well-Differentiated GEP- and Lung NENs
8.3. Merkel Cell Carcinoma
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, e1335. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.R.J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Brighi, N.; Lamberti, G.; Manuzzi, L.; Maggio, I.; Campana, D. Therapeutic options in lung neuroendocrine tumors. Anticancer Drugs 2019, 30, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Müller-Hermelink, H.K.; Harris, C.C. Tumours of the Lung, Pleura, Thymus and Heart; Travis, W.D., Brambilla, E., Müller-Hermelink, H.K., Harris, C.C., Eds.; IARC Press: Lyon, France, 2004. [Google Scholar]
- Lamberti, G.; Brighi, N.; Maggio, I.; Manuzzi, L.; Peterle, C.; Ambrosini, V.; Ricci, C.; Casadei, R.; Campana, D. The Role of mTOR in neuroendocrine tumors: Future cornerstone of a winning strategy? Int. J. Mol. Sci. 2018, 19, e747. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Albores-Saavedra, J.; Batich, K.; Chable-Montero, F.; Sagy, N.; Schwartz, A.M.; Henson, D.E. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population based study. J. Cutan. Pathol. 2010, 37, 20–27. [Google Scholar] [CrossRef]
- Tolstov, Y.L.; Pastrana, D.V.; Feng, H.; Becker, J.C.; Jenkins, F.J.; Moschos, S.; Chang, Y.; Buck, C.B.; Moore, P.S. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int. J. Cancer 2009, 125, 1250–1256. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab vs. docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab vs. docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus ipilimumab vs. sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab vs. ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.; Lao, C.D.; et al. Nivolumab vs. chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone vs. ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Durable tumor regression and overall survival in patients with advanced merkel cell carcinoma receiving pembrolizumab as first-line therapy. J. Clin. Oncol. 2019, 37, 693–702. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide vs. platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab vs. docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.H.; Grivas, P.; Van Der Heijden, M.S.; Dreicer, R.; Loriot, Y.; Retz, M.; Vogelzang, N.J.; Perez-Gracia, J.L.; Rezazadeh, A.; Bracarda, S.; et al. IMvigor 210, a phase II trial of atezolizumab (MPDL3280A) in platinum-treated locally advanced or metastatic urothelial carcinoma (mUC). J. Clin. Oncol. 2016, 34, 355-355. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab vs. chemotherapy for PD-L1-Positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab vs. docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab vs. everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Russell, J.S.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Milella, M.; Brownell, I.; et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J. Immunother. Cancer 2018, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, H.; Sanchez-Vega, F.; La, K.; Chatila, W.; Jonsson, P.; Halpenny, D.; Plodkowski, A.; Long, N.; Sauter, J.L.; Rekhtman, N.; et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol. 2018, 36, 633–641. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Lawson, K.; Butaney, M.; Satkunasivam, R.; Parikh, J.; Freedland, S.J.; Patel, S.P.; Hamid, O.; Pal, S.K.; Klaassen, Z. Association between PD-L1 status and immune checkpoint inhibitor response in advanced malignancies: A systematic review and meta-analysis of overall survival data. Jpn. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Sabari, J.K.; Lok, B.H.; Laird, J.H.; Poirier, J.T.; Rudin, C.M. Unravelling the biology of SCLC: Implications for therapy. Nat. Rev. Clin. Oncol. 2017, 14, 549–561. [Google Scholar] [CrossRef]
- Schultheis, A.M.; Scheel, A.H.; Ozretić, L.; George, J.; Thomas, R.K.; Hagemann, T.; Zander, T.; Wolf, J.; Buettner, R. PD-L1 expression in small cell neuroendocrine carcinomas. Eur. J. Cancer 2015, 51, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Samstein, R.M.; Valero, C.; Chan, T.A.; Morris, L.G.T. Tumor mutational burden as a predictive biomarker for checkpoint inhibitor immunotherapy. Hum. Vaccin. Immunother. 2020, 16, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Ha, S.Y.; Lee, S.; Ahn, S.; Lee, J.; Park, S.H.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Kim, K.-M.; et al. The impact of PD-L1 expression in patients with metastatic GEP-NETs. J. Cancer 2016, 7, 484–489. [Google Scholar] [CrossRef]
- Oktay, E.; Yalcin, G.D.; Ekmekci, S.; Kahraman, D.S.; Yalcin, A.; Degirmenci, M.; Dirican, A.; Altin, Z.; Ozdemir, O.; Surmeli, Z.; et al. Programmed cell death ligand-1 expression in gastroenteropancreatic neuroendocrine tumors. JBUON 2019, 24, 779–790. [Google Scholar] [PubMed]
- Yang, M.-W.; Fu, X.-L.; Jiang, Y.-S.; Chen, X.-J.; Tao, L.-Y.; Yang, J.-Y.; Huo, Y.-M.; Liu, W.; Zhang, J.-F.; Liu, P.-F.; et al. Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas. World J. Gastroenterol. 2019, 25, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Bösch, F.; Brüwer, K.; Altendorf-Hofmann, A.; Auernhammer, C.J.; Spitzweg, C.; Westphalen, C.B.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Heinemann, V.; et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr. Relat. Cancer 2019, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Vallipuram, A.; Evans, J.S.; Wong, C.; Zhang, H.; Brown, M.; Dina, R.E.; Trivedi, P.; Akarca, A.U.; Marafioti, T.; et al. Programmed cell death ligands expression drives immune tolerogenesis across the diverse subtypes of neuroendocrine tumours. Neuroendocrinology 2020. [Google Scholar] [CrossRef] [PubMed]
- Arnason, T.; Sapp, H.L.; Rayson, D.; Barnes, P.J.; Drewniak, M.; Nassar, B.A.; Huang, W.-Y. Loss of expression of DNA mismatch repair proteins is rare in pancreatic and small intestinal neuroendocrine tumors. Arch. Pathol. Lab. Med. 2011, 135, 1539–1544. [Google Scholar] [CrossRef]
- Sahnane, N.; Furlan, D.; Monti, M.; Romualdi, C.; Vanoli, A.; Vicari, E.; Solcia, E.; Capella, C.; Sessa, F.; La Rosa, S. Microsatellite unstable gastrointestinal neuroendocrine carcinomas: A new clinicopathologic entity. Endocr. Relat. Cancer 2015, 22, 35–45. [Google Scholar] [CrossRef]
- Salem, M.E.; Puccini, A.; Grothey, A.; Raghavan, D.; Goldberg, R.M.; Xiu, J.; Korn, W.M.; Weinberg, B.A.; Hwang, J.J.; Shields, A.F.; et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol. Cancer Res. 2018, 16, 805–812. [Google Scholar] [CrossRef]
- Cives, M.; Pelle’, E.; Quaresmini, D.; Rizzo, F.M.; Tucci, M.; Silvestris, F. The tumor microenvironment in neuroendocrine tumors: Biology and therapeutic implications. Neuroendocrinology 2019, 109, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.-W.; Akerley, W.; Pietanza, M.C.; Wu, Y.; Zielinski, C.; Thomas, M.; et al. Phase III randomized trial of ipilimumab plus etoposide and platinum vs. placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 2016, 34, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Kim, H.R.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodriguez-Cid, J.; Schenker, M.; et al. Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): Results from the double-blind, rando. Ann. Oncol. 2019, 30, ii77. [Google Scholar] [CrossRef]
- Horn, L.; Reck, M.; Gettinger, S.N.; Spigel, D.R.; Antonia, S.J.; Rupnow, B.A.; Pieters, A.; Selvaggi, G.; Fairchild, J.P.; Peters, S. CheckMate 331: An open-label, randomized phase III trial of nivolumab vs. chemotherapy in patients (pts) with relapsed small cell lung cancer (SCLC) after first-line platinum-based chemotherapy (PT-DC). J. Clin. Oncol. 2016, 34, TPS8578. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef]
- Chung, H.C.; Lopez-Martin, J.A.; Kao, S.C.-H.; Miller, W.H.; Ros, W.; Gao, B.; Marabelle, A.; Gottfried, M.; Zer, A.; Delord, J.-P.; et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J. Clin. Oncol. 2018, 36, 8506. [Google Scholar] [CrossRef]
- Pujol, J.-L.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Uwer, L.; Hureaux, J.; Guisier, F.; Carmier, D.; Madelaine, J.; Otto, J.; et al. A Randomized Non-Comparative Phase 2 Study of Anti-Programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients with Small Cell Lung Cancer: Results from the IFCT-1603 Trial. J. Thorac. Oncol. 2019, 14, 903–913. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Keam, B.; Ock, C.-Y.; Song, S.; Kim, M.; Kim, S.H.; Kim, K.H.; Kim, J.-S.; Kim, T.M.; Kim, D.-W.; et al. A phase II study of pembrolizumab and paclitaxel in patients with relapsed or refractory small-cell lung cancer. Lung Cancer 2019, 136, 122–128. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.-J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.-C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-Cell-Inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Ruszniewski, P.; Bergsland, E.; Li, D.; Tafuto, S.; Raj, N.; Campana, D.; et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progre. Ann. Oncol. 2018, 29, viii467–viii468. [Google Scholar]
- Lu, M.; Zhang, P.; Zhang, Y.; Li, Z.; Gong, J.; Li, J.; Li, J.; Li, Y.; Zhang, X.; Lu, Z.; et al. Efficacy, safety and biomarkers of toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms: A multiple-center phase Ib trial. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Al Baghdadi, T.; et al. A Phase II Basket trial of dual Anti-CTLA-4 and Anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with non-pancreatic neuroendocrine tumors. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Halperin, D.M.; Liu, S.; Dasari, A.; Fogelman, D.R.; Bhosale, P.; Mahvash, A.; Dervin, S.; Estrella, J.; Cortazar, P.; Maru, D.M.; et al. A phase II trial of atezolizumab and bevacizumab in patients with advanced, progressive neuroendocrine tumors (NETs). J. Clin. Oncol. 2020, 38, 619-619. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Russell, J.; Lebbé, C.; Chmielowski, B.; Gambichler, T.; Grob, J.-J.; Kiecker, F.; Rabinowits, G.; Terheyden, P.; Zwiener, I.; et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic merkel cell carcinoma. JAMA Oncol. 2018, 4, e180077. [Google Scholar] [CrossRef] [PubMed]
- Früh, M.; De Ruysscher, D.; Popat, S.; Crinò, L.; Peters, S.; Felip, E. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi99-vi105. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, F.; Lamberti, G.; Parisi, C.; Casolari, L.; Melotti, B.; Sperandi, F.; Ardizzoni, A. The evolving landscape of immunotherapy in small-cell lung cancer: A focus on predictive biomarkers. Cancer Treat. Rev. 2019, 79, 101887. [Google Scholar] [CrossRef] [PubMed]
- Ready, N.; Farago, A.F.; de Braud, F.; Atmaca, A.; Hellmann, M.D.; Schneider, J.G.; Spigel, D.R.; Moreno, V.; Chau, I.; Hann, C.L.; et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J. Thorac. Oncol. 2019, 14, 237–244. [Google Scholar] [CrossRef]
- Chung, H.C.; Piha-Paul, S.A.; Lopez-Martin, J.; Schellens, J.H.M.; Kao, S.; Miller, W.H.; Delord, J.-P.; Gao, B.; Planchard, D.; Gottfried, M.; et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: Results from the KEYNOTE-028 and KEYNOTE-158 studies. J. Thorac. Oncol. 2020, 15, 618–627. [Google Scholar] [CrossRef]
- Mauclet, C.; Duplaquet, F.; Pirard, L.; Rondelet, B.; Dupont, M.; Pop-Stanciu, C.; Vander Borght, T.; Remmelink, M.; D’Haene, N.; Lambin, S.; et al. Complete tumor response of a locally advanced lung large-cell neuroendocrine carcinoma after palliative thoracic radiotherapy and immunotherapy with nivolumab. Lung Cancer 2019, 128, 53–56. [Google Scholar] [CrossRef]
- Wang, V.E.; Urisman, A.; Albacker, L.; Ali, S.; Miller, V.; Aggarwal, R.; Jablons, D. Checkpoint inhibitor is active against large cell neuroendocrine carcinoma with high tumor mutation burden. J. Immunother. Cancer 2017, 5, 75. [Google Scholar] [CrossRef]
- Levra, M.G.; Mazieres, J.; Valette, C.A.; Molinier, O.; Planchard, D.; Frappat, V.; Ferrer, L.; Toffart, A.C.; Moro-Sibilot, D. P1.07-012 Efficacy of immune checkpoint inhibitors in large cell neuroendocrine lung cancer: Results from a french retrospective cohort. J. Thorac. Oncol. 2017, 12, S702–S703. [Google Scholar] [CrossRef]
- Colunga, A.; Pulliam, T.; Nghiem, P. Merkel cell carcinoma in the age of immunotherapy: Facts and hopes. Clin. Cancer Res. 2018, 24, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichakjian, C.K.; Wong, S.L. Analysis of prognostic factors from 9387 merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Liu, L.; Triplet, J.; Li, Z.; Mansur, D. The role of postoperative radiation and chemoradiation in merkel cell carcinoma: A systematic review of the literature. Front. Oncol. 2013, 3, e276. [Google Scholar] [CrossRef]
- Hodgson, N.C. Merkel cell carcinoma: Changing incidence trends. J. Surg. Oncol. 2005, 89, 1–4. [Google Scholar] [CrossRef]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Peñas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef]
- Mott, R.T.; Smoller, B.R.; Morgan, M.B. Merkel cell carcinoma: A clinicopathologic study with prognostic implications. J. Cutan. Pathol. 2004, 31, 217–223. [Google Scholar] [CrossRef]
- Paulson, K.G.; Iyer, J.G.; Tegeder, A.R.; Thibodeau, R.; Schelter, J.; Koba, S.; Schrama, D.; Simonson, W.T.; Lemos, B.D.; Byrd, D.R.; et al. Transcriptome-Wide Studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J. Clin. Oncol. 2011, 29, 1539–1546. [Google Scholar] [CrossRef]
- Chan, I.S.; Bhatia, S.; Kaufman, H.L.; Lipson, E.J. Immunotherapy for Merkel cell carcinoma: A turning point in patient care. J. Immunother. Cancer 2018, 6, 23. [Google Scholar] [CrossRef]
- Chen, K.T.; Papavasiliou, P.; Edwards, K.; Zhu, F.; Perlis, C.; Wu, H.; Turaka, A.; Berger, A.; Farma, J.M. A better prognosis for Merkel cell carcinoma of unknown primary origin. Am. J. Surg. 2013, 206, 752–757. [Google Scholar] [CrossRef]
- Tarantola, T.I.; Vallow, L.A.; Halyard, M.Y.; Weenig, R.H.; Warschaw, K.E.; Weaver, A.L.; Roenigk, R.K.; Brewer, J.D.; Otley, C.C. Unknown primary Merkel cell carcinoma: 23 new cases and a review. J. Am. Acad. Dermatol. 2013, 68, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.C.; Busam, K.J.; Chou, J.F.; Panageas, K.S.; Pulitzer, M.P.; Allen, P.J.; Kraus, D.H.; Brady, M.S.; Coit, D.G. Five Hundred Patients With Merkel Cell Carcinoma Evaluated at a Single Institution. Ann. Surg. 2011, 254, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Nghiem, P.; Bhatia, S.; Hauschild, A.; Saiag, P.; Mahnke, L.; Hariharan, S.; Kaufman, H.L. Immune evasion mechanisms and immune checkpoint inhibition in advanced merkel cell carcinoma. Oncoimmunology 2017, 6, e1338237. [Google Scholar] [CrossRef]
- Goh, G.; Walradt, T.; Markarov, V.; Blom, A.; Riaz, N.; Doumani, R.; Stafstrom, K.; Moshiri, A.; Yelistratova, L.; Levinsohn, J.; et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 2016, 7, e3403. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Bhatia, S.; Hollebecque, A.; Awada, A.; De Boer, J.P.; Kudchadkar, R.R.; Goncalves, A.; Delord, J.-P.; Martens, U.M.; Picazo, J.M.L.; et al. Abstract CT074: Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma (MCC). In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017; p. CT074. [Google Scholar]
- Stüven, A.K.; Wiedenmann, B. Sustained partial remission of a metastatic NEN using off-label immunotherapy with pembrolizumab. Oncotarget 2019, 10, e3302. [Google Scholar] [CrossRef]
- Ugwu, J.K.; Nwanyanwu, C.; Shelke, A.R. Dramatic response of a metastatic primary small-cell carcinoma of the pancreas to a trial of immunotherapy with nivolumab: A Case Report. Case Rep. Oncol. 2017, 10, 720–725. [Google Scholar] [CrossRef]
- Schmidt, D.; Wiedenmann, B. Extremely long survival under combined immunotherapy in a metastatic functional neuroendocrine neoplasia patient. Neuroendocrinology 2018, 106, 381–388. [Google Scholar] [CrossRef]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Della Corte, C.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA damage response promotes anti-tumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef]
- Krebs, M.; Ross, K.; Kim, S.; De Jonge, M.; Barlesi, F.; Postel-Vinay, S.; Domchek, S.; Lee, J.; Angell, H.; Bui, K.; et al. P1.15-004 An open-label, multitumor phase ii basket study of olaparib and durvalumab (MEDIOLA): Results in patients with relapsed SCLC. J. Thorac. Oncol. 2017, 12, S2044–S2045. [Google Scholar] [CrossRef]
- Crabtree, J.S.; Singleton, C.S.; Miele, L. Notch signaling in neuroendocrine tumors. Front. Oncol. 2016, 6, e94. [Google Scholar] [CrossRef] [PubMed]
- AbbVie Discontinues Rovalpituzumab Tesirine (Rova-T) Research and Development Program. Available online: https://news.abbvie.com/news/press-releases/abbvie-discontinues-rovalpituzumab-tesirine-rova-t-research-and-development-program.htm (accessed on 29 August 2019).
- Morgensztern, D.; Besse, B.; Greillier, L.; Santana-Davila, R.; Ready, N.; Hann, C.L.; Glisson, B.S.; Farago, A.F.; Dowlati, A.; Rudin, C.M.; et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: Results from the phase II TRINITY study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Nikolinakos, P.; Leal, T.; Lehman, J.; Morgensztern, D.; Patel, J.D.; Wrangle, J.M.; Curigliano, G.; Dansin, E.; Greillier, L.; et al. Ph1/2 study of Rova-T in combination with nivolumab (Nivo) ± ipilimumab (Ipi) for patients (pts) with 2L+ extensive-stage (ED) SCLC. J. Clin. Oncol. 2019, 37, 8516-8516. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus axitinib vs. sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus axitinib vs. sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Q.; Li, K.; Shi, J.; Liu, Y.; Wu, L.; Han, B.; Chen, G.; He, J.; Wang, J.; et al. Overall survival (OS) update in ALTER 1202: Anlotinib as third-line or further-line treatment in relapsed small-cell lung cancer (SCLC). Ann. Oncol. 2019, 30, v711. [Google Scholar] [CrossRef]
- Siva, S.; MacManus, M.P.; Martin, R.F.; Martin, O.A. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015, 356, 82–90. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Wersäll, P.J.; Blomgren, H.; Pisa, P.; Lax, I.; Kälkner, K.-M.; Svedman, C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006, 45, 493–497. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177 lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Gardair, C.; Samimi, M.; Touze, A.; Coursaget, P.; Lorette, G.; Caille, A.; Wierzbicka, E.; Croue, A.; Avenel-Audran, M.; Aubin, F.; et al. Somatostatin receptors 2A and 5 are expressed in merkel cell carcinoma with no association with disease severity. Neuroendocrinology 2015, 101, 223–235. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Ohashi, P.S. Clinical blockade of PD1 and LAG3—Potential mechanisms of action. Nat. Rev. Immunol. 2015, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Huang, K.; Zhang, Y.; Kupfer, G.; Zhao, Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018, 11, e22. [Google Scholar] [CrossRef]
| NEN | Trial Name and Reference | Experimental Treatment/Control | Line of Therapy | Phase | OS | PFS | ORR |
|---|---|---|---|---|---|---|---|
| Small cell lung cancer | IMpower-133, 2019 [30] | Exp: atezolizumab + carboplatin/etoposide Control: carboplatin/etoposide | I line | III | mOS Exp: 12.3 months Control: 10.3 months (HR 0.70; 95% CI: 0.54–0.91) | mPFS Exp: 5.2 months Control: 4.3 months (HR 0.77; 95% CI: 0.62–0.96) | Exp: 60.2% Control: 64.4% |
| Small cell lung cancer | CASPIAN, 2019 [31] | Exp: durvalumab + carboplatin/etoposide Control: carboplatin/etoposide | I line | III | mOS Exp: 13.0 months Control: 10.3 months (HR 0.73; 95% CI: 0.59–0.91) | mPFS Exp: 5.1 months Control: 5.4 months (HR 0.78; 95% CI: 0.65–0.94) | Exp: 68% Control: 58% (HR 1.56; 95% CI: 1.10–2.22) |
| Small cell lung cancer | CA184-156, 2016 [54] | Exp: Ipilimumab + carboplatin/etoposide Control: carboplatin/etoposide | I line | III | mOS Exp: 11.0 months Control: 10.9 months (HR 0.94; 95% CI: 0.81–1.09) | mPFS Exp: 4.6 months Control: 4.4 months (HR 0.85; 95% CI: 0.75–0.97) | Exp: 58% Control: 58% |
| Small cell lung cancer | CheckMate-451, 2019 [55] | Exp: nivolumab Exp: nivolumab + ipilimumab Control: placebo | I line maintenance | III | mOS Exp: 9.2 months in nivolumab + ipilimumab arm Control: 9.6 months (HR 0.92; 95% CI: 0.75–1.12) Exp: 10.4 months in nivo arm Control: 9.6 months (HR 0.84; 95% CI: 0.69–1.02) | mPFS Exp: 1.7 months in nivolumab + ipilimumab arm Control: 1.4 months (HR 0.72; 95% CI: 0.60–0.87) Exp: 1.9 months in nivo arm Control: 1.4 months (HR 0.67; 95% CI: 0.56–0.81) | |
| Small cell lung cancer | CheckMate-331, 2018 [56] | Exp: nivolumab Control: topotecan/amrubicin | II line | III | mOS Exp: 7.5 months Control: 8.4 months (HR 0.86; 95% CI: 0.72–1.04) | mPFS Exp: 1.5 months Control: 3.8 months (HR 1.41; 95% CI: 1.18–1.69) | |
| Small cell lung cancer | CheckMate-032, 2016 [57] | Exp: nivolumab ± ipilimumab | ≥II line | I/II | mOS nivolumab 3 mg/kg: 4.4 months nivolumab 1 mg/kg + ipilimumab 3 mg/kg: 7.7 months nivolumab 3 mg/kg + ipilimumab 1 mg/kg: 6 months | mPFS nivolumab 3 mg/kg: 1.4 months nivolumab 1 mg/kg + ipilimumab 3 mg/kg: 2.6 months nivolumab 3 mg/kg + ipilimumab 1 mg/kg: 1.4 months | nivolumab 3 mg/kg: 10% nivolumab 1 mg/kg + ipilimumab 3 mg/kg: 23% nivolumab 3 mg/kg + ipilimumab 1 mg/kg: 19% |
| Small cell lung cancer | KEYNOTE-158, 2018 [58] basket trial | Exp: pembrolizumab | ≥II line | II | mOS 14.6 month in PDL1+ and 7.7 month in PDL1- | mPFS 2.1 month in PDL1+ and 1.9 month in PDL1- | 35.7% in PDL1+ 6% in PDL1- |
| Small cell lung cancer | IFCT-1603, 2019 [59] | Exp: atezolizumab Control: chemotherapy | II line | II | mOS Exp: 9.5 months Control: 8.7 months (HR 0.84; 95% CI: 0.45–1.58) | mPFS Exp: 1.4 months Control: 4.3 months | Exp: 2.3% Control: 10% |
| Small cell lung cancer | MISP-MK3475, 2019 [60] | Exp: pembrolizumab + paclitaxel | II line | II | mOS: 9.1 months | mPFS: 5.0 months | 23.1% |
| SCLC Low grade lung NEN Pancreatic NEC | KEYNOTE 028, 2019 [61] | Exp: pembrolizumab | ≥II line | Ib | mOS: 9.7 months mOS: 21.1 months mOS:21.0 months | mPFS: 1.9 months mPFS: 5.7 months mPFS: 4.5 months | 33% 12% 6% |
| Low grade GEP and lung NEN GEP NEC | CPDR001E2201, 2019 [62] | Exp: spartalizumab | ≥II line | II | ORR overall 7.4% ORR in GEP NEC 4,8% ORR in thoracic NET 20% | ||
| NEN with Ki67 >10% | NCT03167853, 2020 [63] | Exp: toripalimab | ≥II line | Ib | mOS: 9.1 months in PD-L1 ≥10% mOS: 7.2 months in PD-L1 <10% (HR 0.55; 95% CI: 0.24–1.23) | mPFS: 3.8 months in PD-L1 ≥10% mPFS: 2.2 months in PD-L1 <10% (HR 0.50; 95% CI: 0.24–1.06) | ORR was 42.9% (in PD-L1 expression ≥10%: 50.0%; in high TMB: 75.0%) ORR was 8.3% (in PD-L1 expression <10%) |
| NEN (no p-NEN) | DART/SWOG 1609, 2020 [64] | Exp: ipilimumab plus nivolumab | Any line (median II previous lines) | II | mOS: 11 months | mPFS: 4 months | 25% (45% in high-grade and 0% in low-intermediete grade) |
| NET and NEC (any site) | NCT03074513, 2020 [65] | Exp: atezolizumab plus bevacizumab | ≥II line | II | mPFS: 19.6 months in pNET mPFS: 14.9 months in extra-pNET | ORR: 20% in pNET ORR: 15% in extra-pNET | |
| Merkel cell carcinoma | (CITN)09/KEYNOTE 017, 2019 [28] | Exp: pembrolizumab | I line | II | PFS rate at 6 months: 67% | 56% | |
| Merkel cell carcinoma | JAVELIN Merkel 200, 2018 [66] | Exp: avelumab | I line | II | 62.1% | ||
| Merkel cell carcinoma | JAVELIN Merkel 200 2016 [29] | Exp: avelumab | ≥II line | II | mOS: 12.9 months | 1-year PFS: 30% | 33% |
| Merkel cell carcinoma | CheckMate 358, 2017 [37] | Exp: nivolumab | I–III line | I/II | 3-months OS rate: 92% | 3-months OS rate: 82% | 64%, I line: 71% II-III line: 63% |
| Clinicaltrials.gov Identifier Name | N | Phase | Arm/Arms | Primary Outcome Measure | Estimated Primary Completion Date |
|---|---|---|---|---|---|
| Lung NENs | |||||
| NCT02554812 a Phase 1b/2 dose-optimization study to evaluate safety, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity of avelumab (MSB0010718C) in combination with other cancer immunotherapies in patients with locally advanced or metastatic solid tumors. | 620 | Ib/II | Experimental:
| ORR | December 2022 |
| NCT03126110 Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of INCAGN01876 Combined With Immune Therapies in Advanced or Metastatic Malignancies | 285 | I/II | Experimental:
| ORR | January 2020 |
| NCT03241173 A Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of INCAGN01949 in Combination With Immune Therapies in Subjects With Advanced or Metastatic Malignancies | 52 | I/II | Experimental:
| ORR | November 2019 |
| NCT03958045 Phase II Study of Combination Rucaparib With Nivolumab in Platinum-Sensitive Small Cell Lung Carcinoma Patients as Maintenance After Induction Therapy With Platinum Doublet | 36 | II | Experimental:
| PFS | July 2023 |
| NCT03575793 A Phase I/II Study of Nivolumab, Ipilimumab, and Plinabulin in Patients With Recurrent Small Cell Lung Cancer: Big Ten Cancer Research Consortium. | 55 | I–II | Experimental:
| MTD PFS | September 2022 |
| NCT03406715 Combination Immunotherapy–Ipilimumab–Nivolumab–Dendritic Cell p53 Vac—Patients With Small Cell Lung Cancer (SCLC) | 41 | II | Experimental:
| DCR | April 2021 |
| NCT04192682 Anlotinib Combined With Sintilimab as Second-Line Treatment or Beyond in Patients With Small Cell Lung Cancer | 40 | II | Experimental:
| PFS | July 2021 |
| NCT03728361 A phase II trial Nivolumab and Temozolomide in Treating Patients With Recurrent or Refractory Small-Cell Lung Cancer or Advanced Neuroendocrine Cancer | 53 | II | Experimental:
| ORR | December 2021 |
| Lung and GEP NENs | |||||
| NCT03901378 A Phase II Trial of Pembrolizumab in Combination With Cisplatin or Carboplatin and Etoposide in Chemotherapy naïve Patients With Metastatic or Unresectable High-Grade Gastroenteropancreatic or Lung (Excluding Small Cell) Neuroendocrine Carcinoma | 36 | II | Experimental:
| PFS | April 2021 |
| NCT03591731 A GCO Trial Exploring the Efficacy and Safety of Nivolumab Monotherapy or Nivolumab Plus Ipilimumab in Pre-treated Patients With Advanced, Refractory Pulmonary or Gastroenteropancreatic Poorly Differentiated Neuroendocrine Tumors (NECs) | 180 | II | Experimental:
| ORR | September 2023 |
| NCT04079712 A phase 2 study of XL184 (Cabozantinib) in combination with Nivolumab and Ipilimumab for the treatment of poorly differentiated neuroendocrine carcinomas | 30 | II | Experimental:
| ORR | October 2021 |
| NCT03095274 Durvalumab (MEDI4736) Plus Tremelimumab for Advanced Neuroendocrine Neoplasms of Gastroenteropancreatic or Lung Origin (DUNE) | 126 | II | Experimental:
| CBR | April 2020 |
| NCT03074513 A Phase II, Single-Arm Open-Label Study of the Combination of Atezolizumab and Bevacizumab in Rare Solid Tumors | 160 | II | Experimental:
| ORR | March 2021 |
| Merkel cell carcinoma | |||||
| NCT02196961 Prospective Randomized Trial of an Adjuvant Therapy of Completely Resected Merkel Cell Carcinoma (MCC) With Immune Checkpoint Blocking Antibodies (Nivolumab, Opdivo®; Ipilimumab (Yervoy®) Every 3 Weeks for 12 Weeks Vs. Observation | 177 | II | Experimental:
Control:
| DFS-12 | March 2022 |
| NCT03271372 A Multicenter, Randomized, Double-Blinded, Placebo-Controlled, Phase 3 Trial of Adjuvant Avelumab (Anti-PDL-1 Antibody) in Merkel Cell Carcinoma Patients With Clinically Detected Lymph Node Metastases | 100 | III | Experimental:
Control:
| RFS | September 2024 |
| NCT02584829 Localized Radiation Therapy or Recombinant Interferon Beta and Avelumab With or Without Cellular Adoptive Immunotherapy in Treating Patients With Metastatic Merkel Cell Carcinoma | 8 | I–II | Experimental:
| Time to new metastasis | June 2022 |
| NCT03071406 Randomized Study of Nivolumab+Ipilimumab+/- SBRT for Metastatic Merkel Cell Carcinoma | 50 | II | Experimental:
| ORR | July 2023 |
| NCT02819843 A Study of T-VEC (Talimogene Laherparepvec) With or Without Radiotherapy for Melanoma, Merkel Cell Carcinoma, or Other Solid Tumors | 34 | II | Experimental:
| ORR | June 2020 |
| NCT02978625 Talimogene Laherparepvec and Nivolumab in Treating Patients With Refractory Lymphomas or Advanced or Refractory Non-melanoma Skin Cancers | 68 | II | Experimental:
| ORR | January 2020 |
| NCT02488759 An Investigational Immuno-therapy Study to Investigate the Safety and Effectiveness of Nivolumab, and Nivolumab Combination Therapy in Virus-associated Tumors (CheckMate358) | 1100 | I–II | Experimental:
| Safety; ORR; Surgery delay | May 2022 |
| NCT02643303 A Phase 1/2 Study of In Situ Vaccination With Tremelimumab and IV Durvalumab Plus PolyICLC in Subjects With Advanced, Measurable, Biopsy-Accessible Cancers | 102 | I–II | Experimental:
| PFS-24 | August 2022 |
| NCT02035657 A Proof-of-Concept Trial of GLA—SE in Patients With Merkel Cell Carcinoma | 10 | I | Experimental:
| Safety | March 2018 |
| NCT02890368 Trial of Intratumoral Injections of TTI-621 in Subjects With Relapsed and Refractory Solid Tumors and Mycosis Fungoides | 240 | I | Experimental:
| MTD/RP2D | December 2019 |
| NCT02465957 QUILT-3.009: Patients With Stage III (IIIB) or Stage (IV) Merkel Cell Carcinoma (MCC) | 24 | II | Experimental:
| PFS | April 2019 |
| NCT04291885 Immunotherapy Merkel Adjuvant Trial (I-MAT) | 132 | II | Experimental:
| RFS | December 2028 |
| NCT03798639 Nivolumab and Radiation Therapy or Ipilimumab as Adjuvant Therapy in Treating Patients With Merkel Cell Cancer | 43 | I | Experimental:
| % completing 12 months of treatment | 31 December 2021 |
| NCT03988647 Palliative RT and Anti-PD-1/PD-L1 Checkpoint Blockade in Metastatic Merkel Cell Carcinoma | 30 | II | Experimental:
| ORR | June 2026 |
| NCT03304639 Pembrolizumab With or Without Stereotactic Body Radiation Therapy in Treating Patients With Advanced or Metastatic Merkel Cell Cancer | 100 | II | Experimental:
| PFS | 7 February 2022 |
| NCT04160065 Immunotherapy With IFx-Hu2.0 Vaccine for Advanced MCC or cSCC | 20 | I | Experimental:
| Safety | September 2021 |
| NCT03712605 STAMP: Surgically Treated Adjuvant Merkel Cell Carcinoma With Pembrolizumab, a Phase III Trial | 500 | III | Experimental:
Control:
| RFS, OS | 31 October 2023 |
| NCT04261855 Targeted Therapy and Avelumab in Merkel Cell Carcinoma (GoTHAM) | 65 | I/ II | Experimental:
| PFS-12 | January 2024 |
| NCT03589339 NBTXR3 Activated by Radiotherapy for Patients With Advanced Cancers Treated With An Anti-PD-1 Therapy | 60 | I | Experimental:
| RP2D | 30 March 2023 |
| Merkel cell carcinoma and SCLC | |||||
| NCT04272034 Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of INCB099318 in Participants With Advanced Solid Tumors | 140 | I | Experimental:
| Safety | 30 October 2023 |
| NCT03841110 FT500 as Monotherapy and in Combination With Immune Checkpoint Inhibitors in Subjects With Advanced Solid Tumors | 76 | I | Experimental:
| Safety | June 2022 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, I.; Manuzzi, L.; Lamberti, G.; Ricci, A.D.; Tober, N.; Campana, D. Landscape and Future Perspectives of Immunotherapy in Neuroendocrine Neoplasia. Cancers 2020, 12, 832. https://doi.org/10.3390/cancers12040832
Maggio I, Manuzzi L, Lamberti G, Ricci AD, Tober N, Campana D. Landscape and Future Perspectives of Immunotherapy in Neuroendocrine Neoplasia. Cancers. 2020; 12(4):832. https://doi.org/10.3390/cancers12040832
Chicago/Turabian StyleMaggio, Ilaria, Lisa Manuzzi, Giuseppe Lamberti, Angela Dalia Ricci, Nastassja Tober, and Davide Campana. 2020. "Landscape and Future Perspectives of Immunotherapy in Neuroendocrine Neoplasia" Cancers 12, no. 4: 832. https://doi.org/10.3390/cancers12040832
APA StyleMaggio, I., Manuzzi, L., Lamberti, G., Ricci, A. D., Tober, N., & Campana, D. (2020). Landscape and Future Perspectives of Immunotherapy in Neuroendocrine Neoplasia. Cancers, 12(4), 832. https://doi.org/10.3390/cancers12040832





