Outcome of Relapsed or Refractory FLT3-Mutated Acute Myeloid Leukemia before Second-Generation FLT3 Tyrosine Kinase Inhibitors: A Toulouse–Bordeaux DATAML Registry Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Statistical Analysis
3. Results
3.1. Study Population
3.2. First-Line Treatment and Outcome
3.3. Characteristics of Relapsed or Refractory FLT3-Mutated AML
3.4. Treatment of Relapsed or Refractory FLT3-Mutated AML
3.5. Outcome of Relapsed or Refractory FLT3-Mutated AML
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Small, D.; Levenstein, M.; Kim, E.; Carow, C.; Amin, S.; Rockwell, P.; Witte, L.; Burrow, C.; Ratajczak, M.Z.; Gewirtz, A.M.; et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc. Natl. Acad. Sci. USA 1994, 91, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Yokota, S.; Iwai, T.; Kaneko, H.; Horiike, S.; Kashima, K.; Sonoda, Y.; Fujimoto, T.; Misawa, S. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996, 10, 1911–1918. [Google Scholar] [PubMed]
- Kiyoi, H.; Naoe, T.; Yokota, S.; Nakao, M.; Minami, S.; Kuriyama, K.; Takeshita, A.; Saito, K.; Hasegawa, S.; Shimodaira, S.; et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho). Leukemia 1997, 11, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Wang, Q.; Chin, C.S.; Salerno, S.; Damon, L.E.; Levis, M.J.; Perl, A.E.; Travers, K.J.; Wang, S.; Hunt, J.P.; et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 2012, 485, 260–263. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J. Crenolanib besylate, a type I pan-FLT3 inhibitor, to demonstrate clinical activity in multiply relapsed FLT3-ITD and D835 AML. In Oral Abstract #7008; AACR: Chicago, IL, USA, 2016. [Google Scholar]
- Cortes, J.E.; Khaled, S.; Martinelli, G.; Perl, A.E.; Ganguly, S.; Russell, N.; Krämer, A.; Dombret, H.; Hogge, D.; Jonas, B.A.; et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 984–997. [Google Scholar] [CrossRef]
- Megías-Vericat, J.E.; Martínez-Cuadrón, D.; Sanz, M.A.; Montesinos, P. Salvage Regimens Using Conventional Chemotherapy Agents for Relapsed/Refractory Adult AML Patients: A Systematic Literature Review. Ann. Hematol. 2018, 97, 1115–1153. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.; Tavitian, S.; Huynh, A.; Borel, C.; Guenounou, S.; Luquet, I.; Delabesse, E.; Sarry, A.; Laurent, G.; Attal, M.; et al. Improved outcome for AML patients over the years 2000–2014. Blood Cancer J. 2017, 7, 635. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, D.; Walker, H.; Oliver, F.; Wheatley, K.; Harrison, C.; Harrison, G.; Rees, J.; Hann, I.; Stevens, R.; Burnett, A.; et al. The importance of diagnostic cytogenetics on outcome in AML: Analysis of 1612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood 1998, 92, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Metzelder, S.K.; Michel, C.; von Bonin, M.; Rehberger, M.; Hessmann, E.; Inselmann, S.; Solovey, M.; Wang, Y.; Sohlbach, K.; Brendel, C.; et al. NFATc1 as a therapeutic target in FLT3-ITD-positive AML. Leukemia 2015, 29, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.; Gray, R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Concato, J.; Feinstein, A.R.; Holford, T.R. The risk of determining risk with multivariable models. Ann. Int. Med. 1993, 118, 201–210. [Google Scholar] [CrossRef]
- Breems, D.A.; Van Putten, W.L.; Huijgens, P.C.; Ossenkoppele, G.J.; Verhoef, G.E.; Verdonck, L.F.; Vellenga, E.; De Greef, G.E.; Jacky, E.; Van der Lelie, J.; et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J. Clin. Oncol. 2005, 23, 1969–1978. [Google Scholar] [CrossRef]
- Chevallier, P.; Labopin, M.; Turlure, P.; Prebet, T.; Pigneux, A.; Hunault, M.; Filanovsky, K.; Cornillet-Lefebvre, P.; Luquet, I.; Lode, L.; et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: A GOELAMS study. Leukemia 2011, 25, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Daver, N.G.; Pratz, K.W.; Maly, J.; Hong, W.-J.; Bahceci, E.; Tong, B.; Tian, T.; Dilley, K. Venetoclax in Combination with Gilteritinib in Patients with Relapsed/Refractory Acute Myeloid Leukemia: A Phase 1b Study. Blood 2019, 134, 3910–3911. [Google Scholar] [CrossRef]
| Patients’ Characteristics | N = 347 |
|---|---|
| Age (years) | |
| Median (IQR) | 57.3 (47.8–67.6) |
| Range | 18.6–81.4 |
| Gender: n (%) | |
| Female | 176 (50.7) |
| Male | 171 (49.3) |
| ECOG performance status: n (%) | |
| 0–1 | 226 (73.9) |
| ≥2 | 80 (26.1) |
| WBC (× 109/L) | |
| Median (IQR) | 52.6 (20.6–117.8) |
| Range | 0.4–433.0 |
| Tumor burden: n (%) | |
| Extramedullary involvement | |
| Yes | 137 (42.7) |
| No | 184 (57.3) |
| Leukostasis | |
| Yes | 55 (15.5) |
| No | 289 (84.5) |
| LDH | |
| >normal | 311 (93.4) |
| normal | 22 (6.6) |
| Biochemistry: median (IQR) | |
| Creatinine (µmol/L) | 80.0 (64.0–101.0) |
| Albumin (g/L) | 36.0 (32.0–39.5) |
| Fibrinogen (g/L) | 4.0 (2.8–5.3) |
| AML status: n (%) | |
| De novo | 306 (88.4) |
| Secondary AML | 40 (11.6) |
| Cytogenetic risk: n (%) | |
| Favorable | 13 (3.7) |
| Intermediate | 318 (91.6) |
| Normal | 255/311 (82.0) |
| Intermediate-abnormal | 56/311 (18.0) |
| Adverse | 16 (4.6) |
| ELN 2010 classification: n (%) | |
| Favorable | 27 (8.2) |
| Intermediate-1 | 232 (70.1) |
| Intermediate-2 | 56 (16.9) |
| Adverse | 16 (4.8) |
| FLT3 mutation: n (%) | |
| ITD | 317/342 (92.7) |
| TKD | 39/141 (27.7) |
| FLT3 ratio ITD/wt: n (%) | |
| 0.03–0.25 | 34 (24.1) |
| 0.26–0.50 | 40 (28.4) |
| 0.51–0.78 | 43 (30.5) |
| >0.78 | 24 (17.0) |
| NPM1: n (%) | |
| Mutation | 214 (65.6) |
| No mutation | 112 (34.4) |
| IDH1/2 mutations: n (%) | |
| IDH1R132 | 13 (7.6) |
| IDH2R140 | 9 (5.3) |
| IDH2R172 | 0 (0.0) |
| No mutation | 148 (87.1) |
| Induction chemotherapy | |
| Daunorubicin–cytarabine | 127 (36.6) |
| Idarubicin–cytarabine | 101 (29.1) |
| Idarubicin–cytarabine–lomustine | 103 (29.7) |
| Daunorubicin–cytarabine–gemtuzumab ozogamicin | 8 (2.3) |
| Other | 8 (2.3) |
| Allogeneic stem cell transplantation in first CR: n (%) | 100/271 (36.9) |
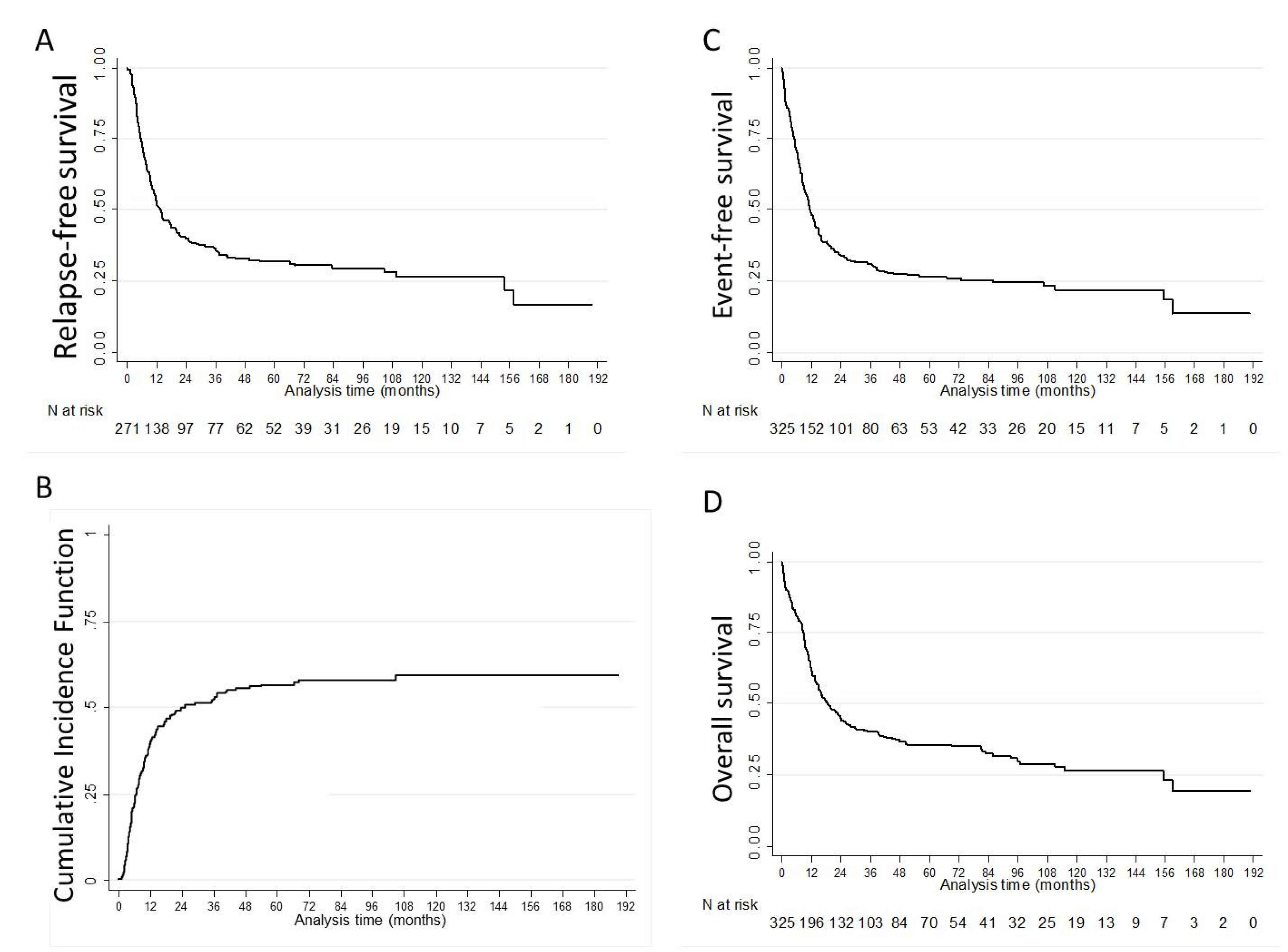
| Endpoint | N | Median (Months, (IQR)) | 1-Year % (95%CI) | 3-Year % (95%CI) | 5-Year % (95%CI) |
|---|---|---|---|---|---|
| RFS | 271 | 13.6 (5.7–154.0) | 52.7 (46.5–58.5) | 36.3 (30.4–42.1) | 31.8 (26.1–37.7) |
| CIR | 271 | 39.0 (34.0–45.0) | 52.0 (46.0–58.0) | 57.0 (50.0–63.0) | |
| EFS | 325 | 11.3 (5.1–85.8) | 48.0 (42.4–53.3) | 31.0 (25.9–36.2) | 26.5 (21.6–31.6) |
| OS | 325 | 17.5 (8.2–115.2) | 62.0 (56.4–67.0) | 40.1 (34.6–45.5) | 35.5 (30.0–41.0) |
| Patients’ Characteristics | R/R FLT3-Mutated AML N = 174 |
|---|---|
| Age (years): | |
| Refractory: Median (IQR) | 57.8 (43.1–67.3) |
| Relapse: Median (IQR) | 59.9 (47.2–70.7) |
| Gender: n (%) | |
| Female | 88 (50.6) |
| Male | 86 (49.4) |
| ECOG performance status: n (%) | |
| 0–1 | 106 (79.7) |
| ≥2 | 27 (20.3) |
| Status: n (%) | |
| Refractory | 48 (27.6) |
| One induction course | 12 (6.9) |
| Two induction courses | 36 (20.7) |
| Relapse | 126 (72.4) |
| <6 months | 48 (27.6) |
| ≥6 months | 78 (44.8) |
| Duration of CR/CRi before relapse (months): | |
| Median (IQR) | 7.7 (4.7–12.6) |
| Previous allogeneic HSCT in first CR: n (%) | 29 (23.0) |
| FLT3 ITD/wt ratio (N = 65) (%): | |
| Median (IQR) | 50.0 (28.0–68.0) |
| Co-mutations: n (%) | |
| NPM1 mutations | |
| Yes | 94 (56.6) |
| No | 72 (43.4) |
| DNMT3A mutations | |
| Yes | 20 (29.9) |
| No | 47 (70.1) |
| CEBPA mutations | |
| Yes | 6 (7.4) |
| No | 75 (92.6) |
| IDH1/2 mutations | |
| Yes | 8 (9.4) |
| No | 77 (90.6) |
| N/K RAS mutations | |
| Yes | 3 (8.6) |
| No | 32 (91.4) |
| WBC (×109/L): | |
| At diagnosis (refractory) | |
| Median (IQR) | 72.8 (18.3–149.8) |
| Range | 0.6–317.0 |
| At relapse | |
| Median (IQR) | 7.4 (3.4–26.9) |
| Range | 0.1–436.0 |
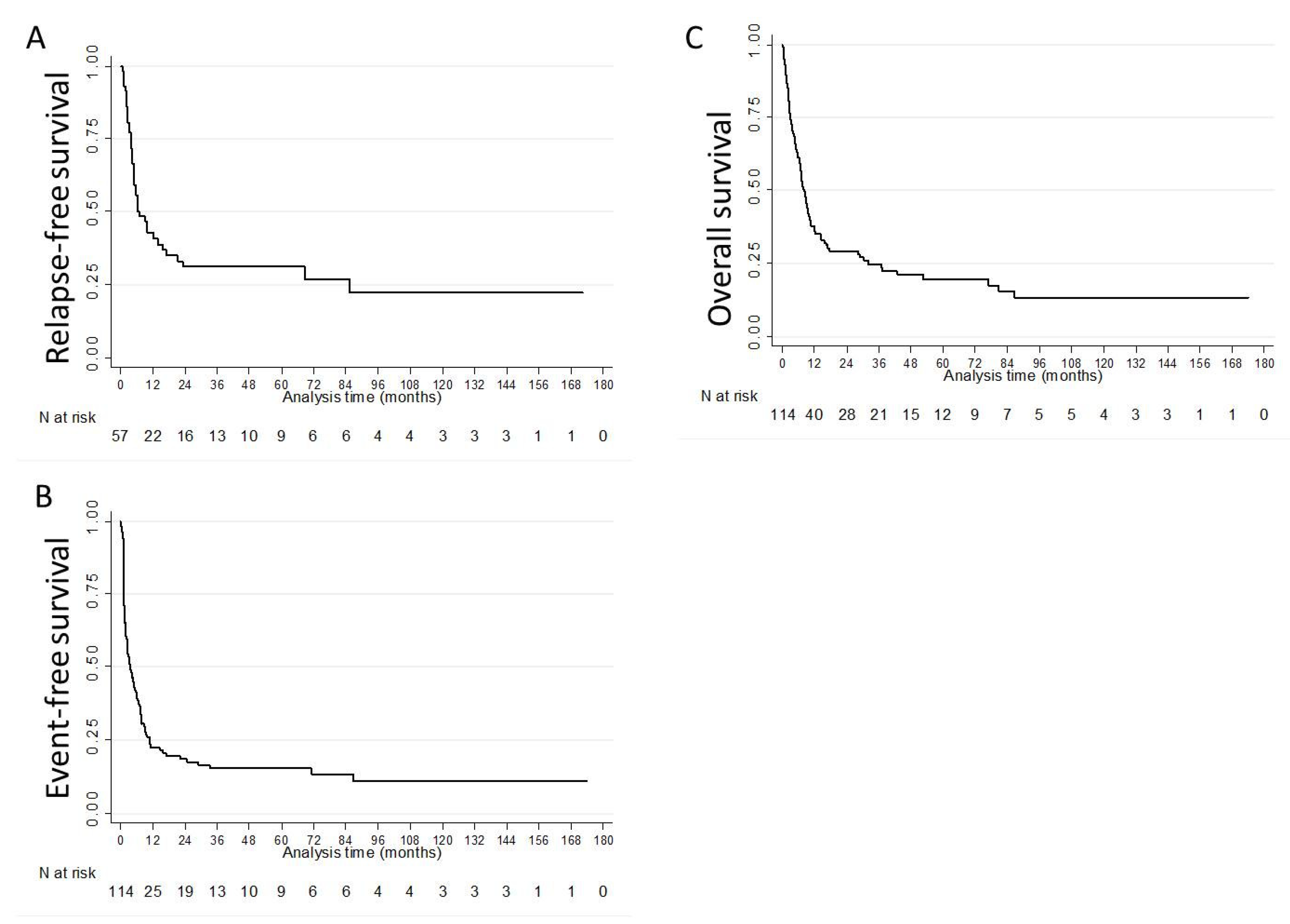
| N | Median (Months, (IQR)) | 1-Year % (95%CI) | 3-Year % (95%CI) | 5-Year % (95%CI) | |
|---|---|---|---|---|---|
| RFS | 57 | 6.8 (3.6–85.6) | 42.9 (29.8–55.3) | 31.2 (19.4–43.7) | 31.2 (19.4–43.7) |
| EFS | 114 | 3.4 (1.3–10.6) | 22.4 (15.2–30.4) | 15.4 (9.3–22.9) | 15.4 (9.3–22.9) |
| OS | 114 | 8.2 (3.0–32.0) | 36.0 (27.2–44.8) | 24.7 (17.0–33.3) | 19.7 (12.5–28.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoli, S.; Dumas, P.-Y.; Bérard, E.; Largeaud, L.; Bidet, A.; Delabesse, E.; Tavitian, S.; Gadaud, N.; Leguay, T.; Leroy, H.; et al. Outcome of Relapsed or Refractory FLT3-Mutated Acute Myeloid Leukemia before Second-Generation FLT3 Tyrosine Kinase Inhibitors: A Toulouse–Bordeaux DATAML Registry Study. Cancers 2020, 12, 773. https://doi.org/10.3390/cancers12040773
Bertoli S, Dumas P-Y, Bérard E, Largeaud L, Bidet A, Delabesse E, Tavitian S, Gadaud N, Leguay T, Leroy H, et al. Outcome of Relapsed or Refractory FLT3-Mutated Acute Myeloid Leukemia before Second-Generation FLT3 Tyrosine Kinase Inhibitors: A Toulouse–Bordeaux DATAML Registry Study. Cancers. 2020; 12(4):773. https://doi.org/10.3390/cancers12040773
Chicago/Turabian StyleBertoli, Sarah, Pierre-Yves Dumas, Emilie Bérard, Laetitia Largeaud, Audrey Bidet, Eric Delabesse, Suzanne Tavitian, Noémie Gadaud, Thibaut Leguay, Harmony Leroy, and et al. 2020. "Outcome of Relapsed or Refractory FLT3-Mutated Acute Myeloid Leukemia before Second-Generation FLT3 Tyrosine Kinase Inhibitors: A Toulouse–Bordeaux DATAML Registry Study" Cancers 12, no. 4: 773. https://doi.org/10.3390/cancers12040773
APA StyleBertoli, S., Dumas, P.-Y., Bérard, E., Largeaud, L., Bidet, A., Delabesse, E., Tavitian, S., Gadaud, N., Leguay, T., Leroy, H., Rieu, J.-B., Vial, J.-P., Vergez, F., Lechevalier, N., Luquet, I., Klein, E., Sarry, A., De Grande, A.-C., Récher, C., & Pigneux, A. (2020). Outcome of Relapsed or Refractory FLT3-Mutated Acute Myeloid Leukemia before Second-Generation FLT3 Tyrosine Kinase Inhibitors: A Toulouse–Bordeaux DATAML Registry Study. Cancers, 12(4), 773. https://doi.org/10.3390/cancers12040773





