Carfilzomib Based Treatment Strategies in the Management of Relapsed/Refractory Multiple Myeloma with Extramedullary Disease
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Treatment, Response, and Outcome
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Treatment and Response to Therapy
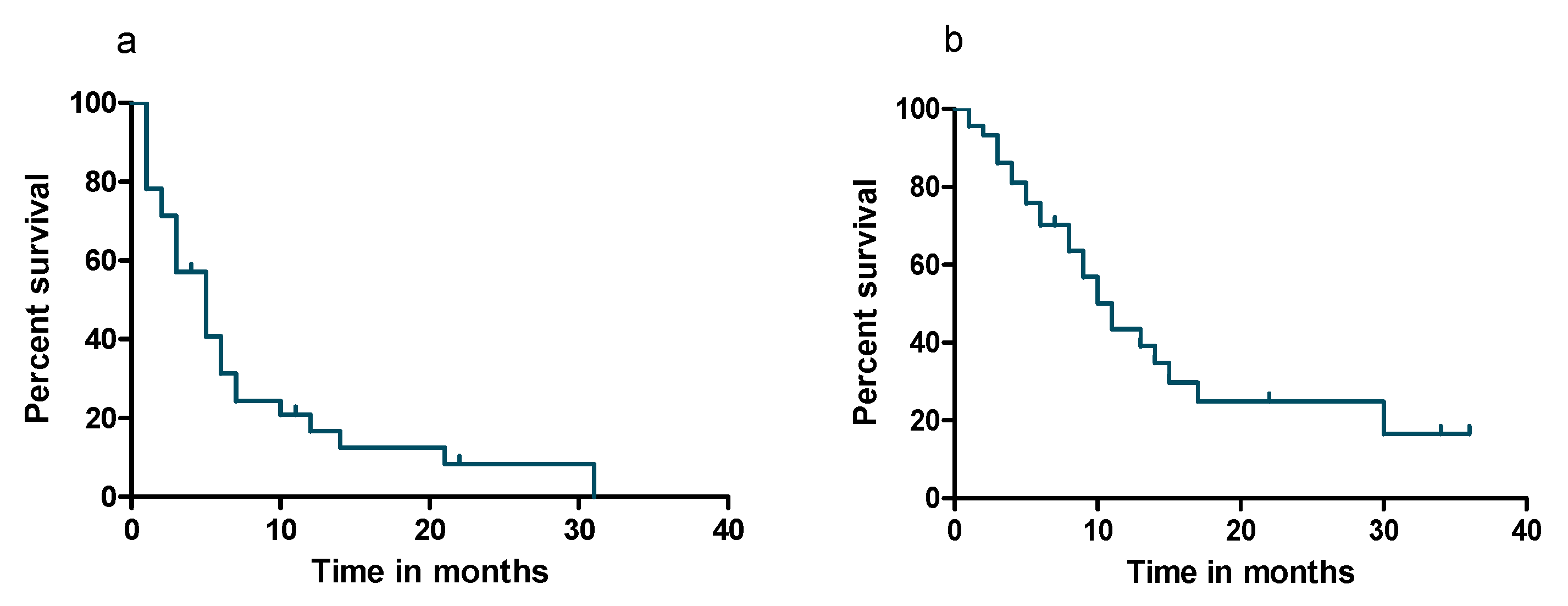
3.3. Survival Analyses
3.4. Adverse Events (AEs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bladé, J.; De Larrea, C.F.; Rosiñol, L.; Cibeira, M.T.; Jiménez, R.; Powles, R. Soft-Tissue Plasmacytomas in Multiple Myeloma: Incidence, Mechanisms of Extramedullary Spread, and Treatment Approach. J. Clin. Oncol. 2011, 29, 3805–3812. [Google Scholar] [CrossRef]
- Sevcikova, S.; Minarik, J.; Stork, M.; Jelinek, T.; Pour, L.; Hajek, R. Extramedullary disease in multiple myeloma—Controversies and future directions. Blood Rev. 2019, 36, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.; Ghobrial, I.M. Extramedullary multiple myeloma. Leuk. Lymphoma 2012, 54, 1135–1141. [Google Scholar] [CrossRef]
- Weinstock, M.; Aljawai, Y.; Morgan, E.A.; Laubach, J.; Gannon, M.; Roccaro, A.; Varga, C.; Mitsiades, C.S.; Paba-Prada, C.; Schlossman, R.; et al. Incidence and clinical features of extramedullary multiple myeloma in patients who underwent stem cell transplantation. Br. J. Haematol. 2015, 169, 851–858. [Google Scholar] [CrossRef]
- Hedvat, C.; Comenzo, R.L.; Teruya-Feldstein, J.; Olshen, A.B.; Ely, S.A.; Osman, K.; Zhang, Y.; Kalakonda, N.; Nimer, S.D. Insights into extramedullary tumour cell growth revealed by expression profiling of human plasmacytomas and multiple myeloma. Br. J. Haematol. 2003, 122, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.K.; Hu, J.; Quang, P.; Azab, F.; Pitsillides, C.; Awwad, R.; Thompson, B.; Maiso, P.; Sun, J.D.; Hart, C.; et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood 2012, 119, 5782–5794. [Google Scholar] [CrossRef]
- Roccaro, A.; Mishima, Y.; Sacco, A.; Moschetta, M.; Tai, Y.-T.; Shi, J.; Zhang, Y.; Reagan, M.R.; Huynh, D.; Kawano, Y.; et al. CXCR4 regulates extra-medullary myeloma through epithelial-mesenchymal transition-like transcriptional activation. Cell Rep. 2015, 12, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Bešše, L.; Sedlaříková, L.; Greslikova, H.; Kupska, R.; Almáši, M.; Penka, M.; Jelinek, T.; Pour, L.; Adam, Z.; Kuglik, P.; et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Eur. J. Haematol. 2015, 97, 93–100. [Google Scholar] [CrossRef]
- Deng, S.; Xu, Y.; An, G.; Sui, W.; Zou, D.; Zhao, Y.; Qi, J.; Li, F.; Hao, M.; Qiu, L. Features of Extramedullary Disease of Multiple Myeloma: High Frequency of P53 Deletion and Poor Survival: A Retrospective Single-Center Study of 834 Cases. Clin. Lymphoma Myeloma Leuk. 2015, 15, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Billecke, L.; Penas, E.M.M.; May, A.M.; Engelhardt, M.; Nagler, A.; Leiba, M.; Schiby, G.; Kröger, N.; Zustin, J.; Marx, A.H.; et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br. J. Haematol. 2013, 161, 87–94. [Google Scholar] [CrossRef]
- Rasche, L.; Bernard, C.; Topp, M.S.; Kapp, M.; Duell, J.; Wesemeier, C.; Haralambieva, E.; Maeder, U.; Einsele, H.; Knop, S. Features of extramedullary myeloma relapse: High proliferation, minimal marrow involvement, adverse cytogenetics: A retrospective single-center study of 24 cases. Ann. Hematol. 2012, 91, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Heuck, C.; Mitchell, A.; Szymonifka, J.; Nair, B.; Hoering, A.; Alsayed, Y.; Waheed, S.; Haider, S.; Restrepo, A.; et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica 2012, 97, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Corso, A.; Pica, G.; Mangiacavalli, S.; Pascutto, C.; Lazzarino, M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: A longitudinal study on 1003 consecutive patients. Ann. Oncol. 2010, 21, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Pour, L.; Ševčíková, S.; Greslikova, H.; Kupska, R.; Majkova, P.; Zahradova, L.; Sandecka, V.; Adam, Z.; Krejci, M.; Kuglik, P.; et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematol. 2013, 99, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Short, K.D.; Rajkumar, S.V.; Larson, D.; Buadi, F.; Hayman, S.; Dispenzieri, A.; Gertz, M.; Kumar, S.; Mikhael, J.; Roy, V.; et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia 2011, 25, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Mangiacavalli, S.; Pompa, A.; Ferretti, V.; Klersy, C.; Cocito, F.; Varettoni, M.; Cartia, C.S.; Cazzola, M.; Corso, A. The possible role of burden of therapy on the risk of myeloma extramedullary spread. Ann. Hematol. 2016, 96, 73–80. [Google Scholar] [CrossRef]
- Rasche, L.; Strifler, S.; Duell, J.; Rosenwald, A.; Buck, A.; Maeder, U.; Einsele, H.; Knop, S. The lymphoma-like polychemotherapy regimen “Dexa-BEAM” in advanced and extramedullary multiple myeloma. Ann. Hematol. 2014, 93, 1207–1214. [Google Scholar] [CrossRef]
- Lakshman, A.; Singh, P.P.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Dingli, D.; Hwa, Y.L.; Fonder, A.L.; et al. Efficacy of VDT PACE-like regimens in treatment of relapsed/refractory multiple myeloma. Am. J. Hematol. 2017, 93, 179–186. [Google Scholar] [CrossRef]
- Rasche, L.; Röllig, C.; Stuhler, G.; Danhof, S.; Mielke, S.; Grigoleit, G.U.; Dissen, L.; Schemmel, L.; Middeke, J.M.; Rücker, V.; et al. Allogeneic Hematopoietic Cell Transplantation in Multiple Myeloma: Focus on Longitudinal Assessment of Donor Chimerism, Extramedullary Disease, and High-Risk Cytogenetic Features. Boil. Blood Marrow Transplant. 2016, 22, 1988–1996. [Google Scholar] [CrossRef]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.-V.; et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef]
- Groen, K.; Van De Donk, N.W.C.J.; Stege, C.; Zweegman, S.; Nijhof, I. Carfilzomib for relapsed and refractory multiple myeloma. Cancer Manag. Res. 2019, 11, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.-J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1327–1337. [Google Scholar] [CrossRef]
- Chng, W.-J.; Goldschmidt, H.; Dimopoulos, M.A.; Moreau, P.; Joshua, D.; Palumbo, A.; Facon, T.; Ludwig, H.; Pour, L.; Niesvizky, R.; et al. Carfilzomib–dexamethasone vs bortezomib–dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia 2017, 31, 1368–1374. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Spicka, I.; Oriol, A.; Hajek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Lopez, J.M.; Mateos, M.-V.; Bladé, J.; Benboubker, L.; Oriol, A.; Arnulf, B.; Rodriguez-Otero, P.; Pineiro, L.; Jakubowiak, A.; et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 421–431. [Google Scholar] [CrossRef]
- Shah, J.J.; Stadtmauer, E.A.; Abonour, R.; Cohen, A.D.; Bensinger, W.I.; Gasparetto, C.; Kaufman, J.L.; Lentzsch, S.; Vogl, D.T.; Gomes, C.L.; et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood 2015, 126, 2284–2290. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Harousseau, J.L.; Durie, B.; Anderson, K.C.; Dimopoulos, M.; Kyle, R.; Blade, J.; Richardson, P.; Orlowski, R.; Siegel, D.; et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 2011, 117, 4691–4695. [Google Scholar] [CrossRef]
- Fonseca, R.; Blood, E.; Rué, M.; Harrington, D.; Oken, M.; Kyle, R.A.; Dewald, G.W.; Van Ness, B.; Van Wier, S.A.; Henderson, K.J.; et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 2003, 101, 4569–4575. [Google Scholar] [CrossRef]
- Mikhael, J.; Dingli, D.; Roy, V.; Reeder, C.B.; Buadi, F.K.; Hayman, S.R.; Dispenzieri, A.; Fonseca, R.; Sher, T.; Kyle, R.A.; et al. Management of Newly Diagnosed Symptomatic Multiple Myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines 2013. Mayo Clin. Proc. 2013, 88, 360–376. [Google Scholar] [CrossRef]
- Chng, W.J.; Dispenzieri, A.; Chim, C.-S.; Fonseca, R.; Goldschmidt, H.; Lentzsch, S.; Munshi, N.; Palumbo, A.; San-Miguel, J.F.; Sonneveld, P.; et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2013, 28, 269–277. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Harousseau, J.-L.; Miguel, J.S.; Blade, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Paiva, B.D.L.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.C.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Smith, T.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef] [PubMed]
- Lancet Haematol. Updates on blood transfusion guidelines. Lancet Haematol. 2016, 3, e547. [Google Scholar] [CrossRef]
- Mele, G.; Pastore, D. Efficacy of Carfilzomib, Lenalidomide, and Dexamethasone for Extramedullary Intracranial Localization of Multiple Myeloma. Case Rep. Hematol. 2018, 2018, 1–3. [Google Scholar] [CrossRef]
- Español, I.; Romera, M.; Gutiérrez-Meca, M.D.; García, M.D.C.; Tejedor, A.; Martínez, A.; Ibáñez, J.; De Arriba, F.; Minguela, A.; Iturbe, T.; et al. Carfilzomib and dexamethasone for extramedullary myeloma with pleuropericardial involvement. Clin. Case Rep. 2017, 5, 1258–1260. [Google Scholar] [CrossRef]
- Parnell, K.; Ahmed, M.; Smalligan, R.D.; Nadesan, S. Extramedullary plasmacytoma mimicking colon carcinoma: An unusual presentation and review of the literature. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef]
- Muchtar, E.; Gatt, M.E.; Rouvio, O.; Ganzel, C.; Chubar, E.; Suriu, C.; Tadmor, T.; Shevetz, O.; Lavi, N.; Shochat, T.; et al. Efficacy and safety of salvage therapy using Carfilzomib for relapsed or refractory multiple myeloma patients: A multicentre retrospective observational study. Br. J. Haematol. 2015, 172, 89–96. [Google Scholar] [CrossRef]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.A.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef]
- López-Anglada, L.; Gutiérrez, N.C.; García, J.L.; Mateos, M.V.; Flores, T.; San-Miguel, J.F. P53deletion may drive the clinical evolution and treatment response in multiple myeloma. Eur. J. Haematol. 2010, 84, 359–361. [Google Scholar] [CrossRef]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.-V.; Lonial, S.; Joao, C.; Anderson, K.C.; García-Sanz, R.; Riva, E.; et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Lapa, C.; Herrmann, K.; Schirbel, A.; Hänscheid, H.; Lückerath, K.; Schottelius, M.; Kircher, M.; Werner, R.A.; Schreder, M.; Samnick, S.; et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed Multiple Myeloma. Theranostics 2017, 7, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Beksac, M.; Seval, G.C.; Kanellias, N.; Coriu, D.; Rosiñol, L.; Ozet, G.; Goranova-Marinova, V.; Unal, A.; Bila, J.; Ozsan, H.; et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: Analysis of parameters that improve outcome. Haematologica 2019, 105, 201–208. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Iida, S.; Huang, S.-Y.; Takezako, N.; Chng, W.J.; Zahlten-Kumeli, A.; Sersch, M.A.; Li, J.; Huang, M.; et al. Outcomes for Asian patients with multiple myeloma receiving once- or twice-weekly carfilzomib-based therapy: A subgroup analysis of the randomized phase 3 ENDEAVOR and A.R.R.O.W. Trials. Int. J. Hematol. 2019, 110, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Mateos, M.-V.; Berenson, J.R.; Weisel, K.; Lazzaro, A.; Song, K.; Dimopoulos, M.A.; Huang, M.; Zahlten-Kumeli, A.; Stewart, A.K. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): Interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018, 19, 953–964. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Goldschmidt, H.; San-Miguel, J.F.; Mikhael, J.; DeCosta, L.; Zhou, L.; Obreja, M.; Blaedel, J.; Szabo, Z.; Leleu, X. Carfilzomib in relapsed or refractory multiple myeloma patients with early or late relapse following prior therapy: A subgroup analysis of the randomized phase 3 ASPIRE and ENDEAVOR trials. Hematol. Oncol. 2018, 36, 463–470. [Google Scholar] [CrossRef]

| Patients, n | 45 |
| Gender, n (%) | |
| Male | 33 (73) |
| Female | 12 (27) |
| Subtype, n (%) | |
| IgG | 26 (58) |
| IgA | 14 (31) |
| LC | 5 (11) |
| ISS Stage, n (%) | |
| I | 22 (49) |
| II | 6 (13) |
| III | 8 (18) |
| NA | 9 (20) |
| Cytogenetics, n (%) | |
| High-risk | 22 (49) |
| Standard-risk | 18 (40) |
| NA | 5 (11) |
| Age at Start of Carfilzomib due to EMD Relapse, Median, Years (Range) | 64 (40–80) |
| Bone Marrow Involvement at Start of Carfilzomib due to EMD Relapse | |
| Yes | 21 (47) |
| No | 4 (9) |
| NA | 20 (44) |
| Serological MM Activity at Start of Carfilzomib due to EMD Relapse, n (%) | |
| With Secretory Activity | 42 (93) |
| Non-Secretory | 3 (7) |
| Serum LDH at Start of Carfilzomib due to EMD Relapse, n (%) | |
| Elevated | 22 (49) |
| Normal | 23 (51) |
| Prior Lines of Therapy, n (%) | |
| 1–2 | 15 (33) |
| 3–5 | 16 (36) |
| ≥ 6 | 14 (31) |
| Response Status to The Last Therapy Line, n (%) | |
| Refractory to The Last Line of Therapy | 33 (73) |
| Progression from Remission | 12 (27) |
| Characteristics of EMD at Start of Carfilzomib, n (%) | |
| EMD Adjacent to Bone | 20 (44) |
| EMD without Adjacency to Bone | 25 (56) |
| Presentation/Localization of EMD, n (%) | |
| Muscle, Skin, and Soft Tissue | 38 (84) |
| Spinal Cord and Paravertebral Lesion | 25 (56) |
| Lymph Node | 20 (44) |
| Pleural Effusion | 13 (29) |
| Parenchymal Organ | 11 (24) |
| Gastrointestinal Tract | 2 (4) |
| Prior Treatment, n (%) | |
| PIs | |
| Bortezomib | 43 (96) |
| Carfilzomib | 8 (18) |
| IMiDs | |
| Lenalidomide | 35 (78) |
| Pomalidomide | 22 (49) |
| Thalidomide | 10 (22) |
| Monoclonal Antibodies | |
| Daratumumab | 18 (40) |
| Elotuzumab | 3 (7) |
| SCT | |
| Prior Autologous SCT | 44 (98) |
| Prior Allogenic SCT | 7 (15) |
| Pat | Regimen | Number of Cycles | Maximal Dose of Carf | Dosing of IMiD, Alkylating Agents and Monoclonal Antibodies | Best Response | |
|---|---|---|---|---|---|---|
| Serology | EMD | |||||
| 1 | Kd | 11 | 56 mg/m2 | N/A | PR | PR |
| 2 | Kd | 1 | 15 mg/m2 | N/A | SD | n.a. |
| 3 | Kd | 1 | 56 mg/m2 | N/A | PR | SD |
| 4 | KBd | 1 | 27 mg/m2 | Benda 70 mg/m2 qw | N/A | PD |
| 5 | KBd | 1 | 27 mg/m2 | Benda 70 mg/m2 qw | PD | PD |
| 6 | KCyd | 5 | 27 mg/m2 | Cyclo 200 mg qw | PR | SD |
| 7 | KCyd | 2 | 56 mg/m2 | Cyclo 300 mg qw | PR | n.a. |
| 8 | KCyd | 1 | 27 mg/m2 | Cyclo 750 mg qw | PR | PR |
| 9 | KCyd | 1 | 20 mg/m2 | Cyclo 300 mg qw | PR | mixed response |
| 10 | KCyd | 3 | 27 mg/m2 | Cyclo 300 mg qw | SD | SD |
| 11 | KRd | 11 | 27 mg/m2 | Rev 25 mg qd | PR | PR |
| 12 | KRd | 7 | 20 mg/m2 | Rev 15 mg qod | VGPR | PR |
| 13 | KRd | 6 | 36 mg/m2 | Rev 5 mg qd | VGPR | n.a. |
| 14 | KRd | 2 | 20 mg/m2 | Rev 5 mg qod | SD | n.a. |
| 15 | KRd | 6 | 27 mg/m2 | Rev 10 mg qd | VGPR | mixed response |
| 16 | KRd | 3 | 27 mg/m2 | Rev 10 mg qd | SD | PD |
| 17 | KRd | 2 | 27 mg/m2 | Rev 25 mg qd | PD | PD |
| 18 | KRd | 2 | 27 mg/m2 | Rev 25 mg qd | VGPR | n.a. |
| 19 | KRd | 18 | 27 mg/m2 | Rev 25 mg qd | VGPR | PR |
| 20 | KRd | 6 | 27 mg/m2 | Rev 25 mg qd | PR | mixed response |
| 21 | KRd | 3 | 27 mg/m2 | Rev 25 mg qd | PR | PR |
| 22 | KRd | 5 | 27 mg/m2 | Rev 25 mg qd | VGPR | PR |
| 23 | KRd | 3 | 27 mg/m2 | Rev 20 mg qd | PR | mixed response |
| 24 | KRd | 9 | 27 mg/m2 | Rev 25 mg qd | VGPR | n.a. |
| 25 | KRd | 6 | 27 mg/m2 | Rev 15 mg qd | PR | n.a. |
| 26 | KRd | 3 | 27 mg/m2 | Rev 10 mg qd | VGPR | SD |
| 27 | KRd | 1 | 27 mg/m2 | Rev 10 mg qd | PD | n.a. |
| 28 | KPd | 3 | 27 mg/m2 | Pom 4 mg qd | SD | mixed response |
| 29 | KTd | 4 | 56 mg/m2 | Thal 50 mg qd | SD | PD |
| 30 | KRCyd | 1 | 20 mg/m2 | Cyclo 300 mg qw, Rev 15 mg qd | PD | PD |
| 31 | KRCyd | 3 | 56 mg/m2 | Cyclo 300 mg qw, Rev 10 mg qd | PD | PD |
| 32 | KRCyd | 3 | 27 mg/m2 | Cyclo 300 mg qw, Rev 10 mg qd | SD | n.a. |
| 33 | KTCyd | 4 | 36 mg/m2 | Cyclo 300 mg qw, Thal 100 mg qd | PR | SD |
| 34 | Dara-Kd | 2 | 56 mg/m2 | Dara 16 mg/kg qw | PR | SD |
| 35 | Dara-Kd | 1 | 20 mg/m2 | Dara 16 mg/kg qw | PR | SD |
| 36 | Dara-KCyd | 2 | 27 mg/m2 | Dara 16 mg/kg qw, Cyclo 300 mg qw | SD | SD |
| 37 | Dara-KCyd | 2 | 27 mg/m2 | Dara 16 mg/kg qw, Cyclo 200 mg qw | N/A | n.a. |
| 38 | Dara-KCyd | 3 | 15 mg/m2 | Dara 16 mg/kg qw, Cyclo 200 mg qw | PR | PR |
| 39 | Dara-KCyd | 5 | 56 mg/m2 | Dara 16 mg/kg qw, Cyclo 300 mg qw | PR | SD |
| 40 | Dara-KPd | 3 | 27 mg/m2 | Dara 16 mg/kg qw, Pom 3 mg qd | N/A | mixed response |
| 41 | Dara-KPd | 2 | 20 mg/m2 | Dara 16 mg/kg qw, Pom 2 mg qod | PD | PD |
| 42 | Dara-KPCyd | 1 | 20 mg/m2 | Dara 16 mg/kg qw, Pom 2 mg qd, Cyclo 250 mg qw | PD | n.a. |
| 43 | Dara-KPCyd | 4 | 27 mg/m2 | Dara 16 mg/kg qw, Pom 2 mg qd, Cyclo 200 mg qw | SD | PD |
| 44 | Dara-KPCyd | 1 | 15 mg/m2 | Dara 16 mg/kg qw, Pom 2 mg qd, Cyclo 200 mg qw | SD | n.a. |
| 45 | Elo-KPd | 1 | 36 mg/m2 | Elo 10 mg/kg q2w, Pom 2 mg qd | PR | PR |
| Adverse Events | Any Grade ≥ 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Hematological Events, n (%) | |||
| Anemia | 37 (82) | 25 (56) | |
| White Blood Cell Decreased | 32 (71) | 19 (42) | 5 (11) |
| Neutrophil Count Decreased | 30 (67) | 7 (16) | 6 (13) |
| Platelet Count Decreased | 29 (64) | 9 (20) | 13 (29) |
| Febrile Neutropenia | 1 (2) | 1 (2) | |
| Non-Hematological Events, n (%) | |||
| Pneumonia | 6 (13) | 4 (9) | |
| Heart Failure | 6 (13) | 5 (11) | 2 (4) |
| Influenza | 4 (9) | 4 (9) | |
| Upper Respiratory Infection | 3 (7) | 3 (7) | |
| Liver Enzyme Increased | 2 (4) | 1 (2) | |
| Urinary Tract Infection | 2 (4) | 2 (4) | |
| Cytokine Release Syndrome | 1 (2) | 1 (2) | |
| Gastrointestinal Infection | 1 (2) | 1 (2) | |
| Catheter Related Infection | 1 (2) | 1 (2) | |
| Peripheral Polyneuropathy | 1 (2) | 1 (2) | |
| Convulsion | 1 (2) | 1 (2) | |
| Renal Failure | 1 (2) | 1 (2) | |
| Oral Hemorrhage | 1 (2) | 1 (2) | |
| Bacterial Meningitis | 1 (2) | 1 (2) | |
| Skin Infection | 1 (2) | 1 (2) | |
| Sinusitis | 1 (2) | 1 (2) | |
| Atrial Fibrillation | 1 (2) | ||
| Thromboembolic Events | 2 (4) | ||
| Death | 2 (4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Flüchter, P.; Nickel, K.; Meckel, K.; Messerschmidt, J.; Böckle, D.; Knorz, S.; Steinhardt, M.J.; Krummenast, F.; Danhof, S.; et al. Carfilzomib Based Treatment Strategies in the Management of Relapsed/Refractory Multiple Myeloma with Extramedullary Disease. Cancers 2020, 12, 1035. https://doi.org/10.3390/cancers12041035
Zhou X, Flüchter P, Nickel K, Meckel K, Messerschmidt J, Böckle D, Knorz S, Steinhardt MJ, Krummenast F, Danhof S, et al. Carfilzomib Based Treatment Strategies in the Management of Relapsed/Refractory Multiple Myeloma with Extramedullary Disease. Cancers. 2020; 12(4):1035. https://doi.org/10.3390/cancers12041035
Chicago/Turabian StyleZhou, Xiang, Patricia Flüchter, Katharina Nickel, Katharina Meckel, Janin Messerschmidt, David Böckle, Sebastian Knorz, Maximilian Johannes Steinhardt, Franziska Krummenast, Sophia Danhof, and et al. 2020. "Carfilzomib Based Treatment Strategies in the Management of Relapsed/Refractory Multiple Myeloma with Extramedullary Disease" Cancers 12, no. 4: 1035. https://doi.org/10.3390/cancers12041035
APA StyleZhou, X., Flüchter, P., Nickel, K., Meckel, K., Messerschmidt, J., Böckle, D., Knorz, S., Steinhardt, M. J., Krummenast, F., Danhof, S., Einsele, H., Kortüm, K. M., & Rasche, L. (2020). Carfilzomib Based Treatment Strategies in the Management of Relapsed/Refractory Multiple Myeloma with Extramedullary Disease. Cancers, 12(4), 1035. https://doi.org/10.3390/cancers12041035






