Primary Resistance to PD-1-Based Immunotherapy—A Study in 319 Patients with Stage IV Melanoma
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients Cohort
2.2. Statistical Analysis
3. Results
3.1. Univariate and Multivariate Analysis
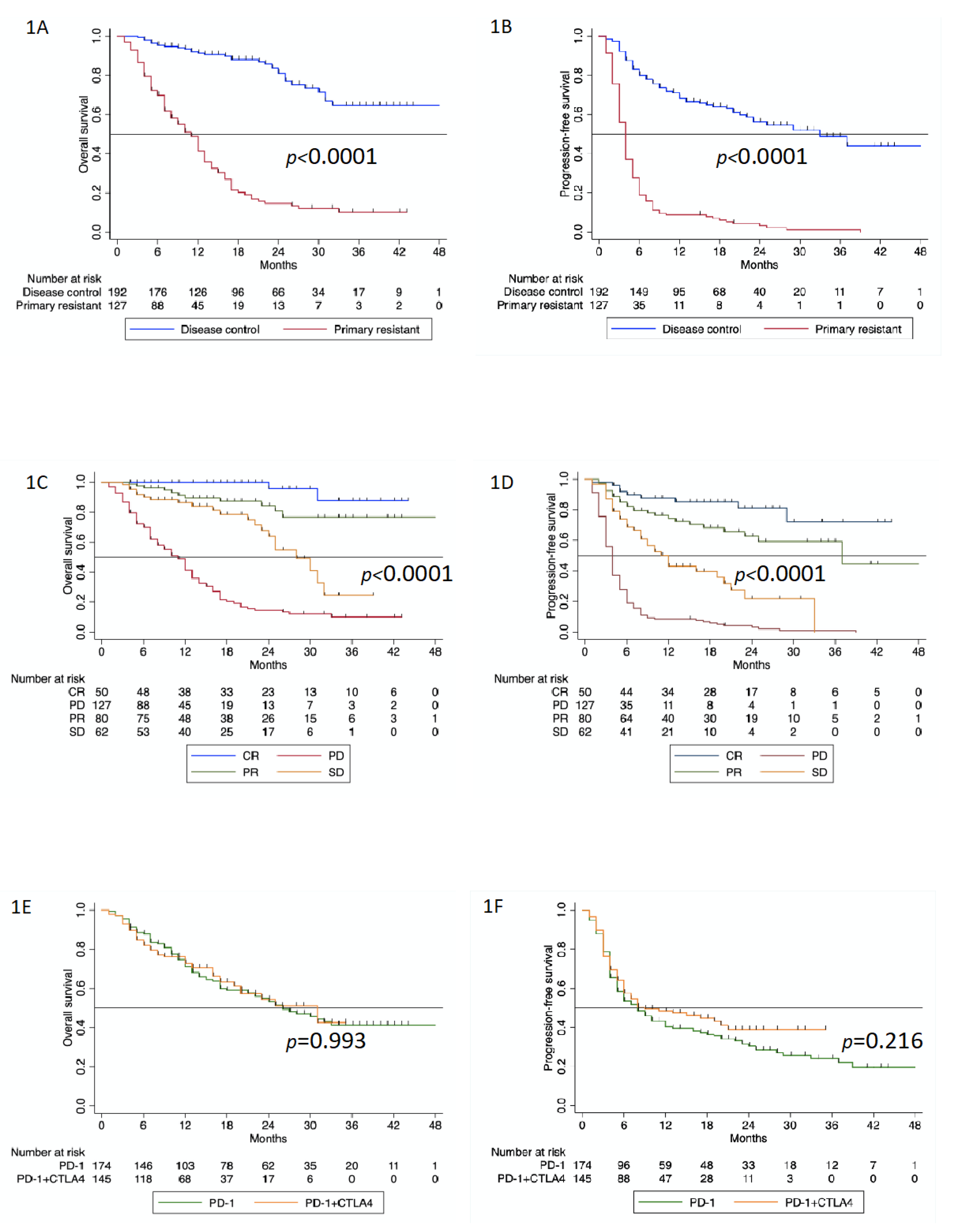
3.2. Survival Analysis
3.3. Second-Line Therapies and Outcomes
3.4. Pseudoprogression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Franken, M.G.; Leeneman, B.; Gheorghe, M.; Uyl-de Groot, C.A.; Haanen, J.B.A.G.; van Baal, P.H.M. A systematic literature review and network meta-analysis of effectiveness and safety outcomes in advanced melanoma. Eur. J. Cancer 2019, 123, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Maio, M.; Grob, J.-J.; Aamdal, S.; Bondarenko, I.; Robert, C.; Thomas, L.; Garbe, C.; Chiarion-Sileni, V.; Testori, A.; Chen, T.-T.; et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J. Clin. Oncol. 2015, 33, 1191–1196. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.-T.; Berman, D.M.; Wolchok, J.D. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Sam, D.; Pan, M. Mechanisms of primary and secondary resistance to immune checkpoint inhibitors in cancer. Med. Sci. 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Fuereder, T. Resistance to immune checkpoint inhibitors. Next steps and combinational approaches. Memo Mag. Eur. Med Oncol. 2019, 12, 123–127. [Google Scholar] [CrossRef]
- Veldman, J.; Visser, L.; Berg, A.v.d.; Diepstra, A. Primary and acquired resistance mechanisms to immune checkpoint inhibition in Hodgkin lymphoma. Cancer Treat. Rev. 2020, 82, 101931. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations associated with acquired resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Ladányi, A.; Kiss, J.; Somlai, B.; Gilde, K.; Fejos, Z.; Mohos, A.; Gaudi, I.; Tímár, J. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. CII 2007, 56, 1459–1469. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Wang, Y.; Li, W.; Yu, G.; Li, Z.; Fang, C.; Shen, Y.; Sun, Z.; Han, L.; et al. IL-37b suppresses T cell priming by modulating dendritic cell maturation and cytokine production via dampening ERK/NF-κB/S6K signalings. Acta Biochim. Et Biophys. Sin. 2015, 47, 597–603. [Google Scholar] [CrossRef]
- Lindenberg, J.J.; van de Ven, R.; Lougheed, S.M.; Zomer, A.; Santegoets, S.J.A.M.; Griffioen, A.W.; Hooijberg, E.; van den Eertwegh, A.J.M.; Thijssen, V.L.; Scheper, R.J.; et al. Functional characterization of a STAT3-dependent dendritic cell-derived CD14+ cell population arising upon IL-10-driven maturation. Oncoimmunology 2013, 2, e23837. [Google Scholar] [CrossRef]
- Strauss, L.; Bergmann, C.; Szczepanski, M.; Gooding, W.; Johnson, J.T.; Whiteside, T.L. A Unique Subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-β1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 2007, 13, 4345. [Google Scholar] [CrossRef]
- Viguier, M.; Lemaître, F.; Verola, O.; Cho, M.-S.; Gorochov, G.; Dubertret, L.; Bachelez, H.; Kourilsky, P.; Ferradini, L. Foxp3 expressing CD4+CD25high regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004, 173, 1444. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression-implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and perspective for cancer immunotherapy—Blockade, knockdown, or inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Furness, A.J.S.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (N. Y.) 2016, 351, 1463. [Google Scholar] [CrossRef] [PubMed]
- del Campo, A.B.; Kyte, J.A.; Carretero, J.; Zinchencko, S.; Méndez, R.; González-Aseguinolaza, G.; Ruiz-Cabello, F.; Aamdal, S.; Gaudernack, G.; Garrido, F.; et al. Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. Int. J. Cancer 2014, 134, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Forschner, A.; Battke, F.; Hadaschik, D.; Schulze, M.; Weißgraeber, S.; Han, C.-T.; Kopp, M.; Frick, M.; Klumpp, B.; Tietze, N.; et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma–results of a prospective biomarker study. J. Immunother. Cancer 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA A Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Saccà, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Shabto, J.M.; Martini, D.J.; Liu, Y.; Lewis, C.; Collins, H.; Akce, M.; Kissick, H.; Carthon, B.C.; Shaib, W.L.; et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer 2019, 19, 857. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.L.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef]
- Weide, B.; Elsässer, M.; Büttner, P.; Pflugfelder, A.; Leiter, U.; Eigentler, T.K.; Bauer, J.; Witte, M.; Meier, F.; Garbe, C. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br. J. Cancer 2012, 107, 422–428. [Google Scholar] [CrossRef]
- Wagner, N.B.; Forschner, A.; Leiter, U.; Garbe, C.; Eigentler, T.K. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br. J. Cancer 2018, 119, 339–346. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Berneburg, M.; Gutzmer, R.; Meier, F.; Mohr, P.; Hauschild, A.; et al. Adjuvant immunotherapy with nivolumab (nivo) alone or in combination with ipilimumab (ipi) versus placebo in stage iv melanoma patients with no evidence of disease (ned): A randomized, double-blind phase 2 trial (immuned). Ann. Oncol. 2019, 30 (Suppl. 5), v851–v934. [Google Scholar] [CrossRef]
- Kugel, C.H.; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Teterycz, P.; Mariuk-Jarema, A.; Lugowska, I.; Rogala, P.; Dudzisz-Sledz, M.; Switaj, T.; Rutkowski, P. Treatment Sequencing and Clinical Outcomes in BRAF-Positive and BRAF-Negative Unresectable and Metastatic Melanoma Patients Treated with new systemic therapies in routine practice. Target. Oncol. 2019, 14, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Pectasides, E.; Feld, E.; Ye, F.; Zhao, S.; Johnpulle, R.; Merritt, R.; McDermott, D.F.; Puzanov, I.; Lawrence, D.; et al. Sequencing Treatment in BRAFV600 Mutant Melanoma: Anti-PD-1 Before and After BRAF Inhibition. J. Immunother. (Hagerstown Md. 1997) 2017, 40, 31–35. [Google Scholar] [CrossRef]
- Schilling, B.; Martens, A.; Geukes Foppen, M.H.; Gebhardt, C.; Hassel, J.C.; Rozeman, E.A.; Gesierich, A.; Gutzmer, R.; Kähler, K.C.; Livingstone, E.; et al. First-line therapy-stratified survival in BRAF-mutant melanoma: A retrospective multicenter analysis. Cancer Immunol. Immunother. CII 2019, 68, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.C.; Chen, D.; Hu-Lieskovan, S.; Grossmann, K.F.; Patel, S.; Colonna, S.V.; Ying, J.; Hyngstrom, J.R. Real-world survival of patients with advanced BRAF V600 mutated melanoma treated with front-line BRAF/MEK inhibitors, anti-PD-1 antibodies, or nivolumab/ipilimumab. Cancer Med. 2019, 8, 7637–7643. [Google Scholar] [CrossRef]
| Characteristics | ICI Cohort n = 319 n (%) | n (%) | Univariate Analysis Test  | Multivariate Logistic Regression Analysis | |
|---|---|---|---|---|---|
| Primary Resistance n = 127 (40) | DC (CR, PR, SD) n = 192 (60) | ||||
| Age Distribution | 0.732 | ||||
| Median (years [IQR]) | 68 (56–77) | 65 (55–78) | 68 (56–77) | ||
| <60y | 101 (32) | 37 (29) | 64 (33) | ||
| 60y–75y | 114 (36) | 47 (37) | 67 (35) | ||
| >75y | 104 (32) | 43 (34) | 61 (32) | ||
| Sex | 0.049 | 0.822 | |||
| Male | 192 (60) | 68 (54) | 124 (65) | ||
| Female | 127 (40) | 59 (46) | 68 (35) | ||
| Tumor localization * | 0.000 | 0.001 | |||
| Head and neck | 54 (22) | 12 (13) | 42 (27) | ||
| Trunk | 73 (29) | 18 (20) | 55 (34) | ||
| Extremity | 109 (43) | 54 (59) | 55 (34) | ||
| Other | 15 (6) | 7 (8) | 8 (5) | ||
| Histological subtype * | 0.007 | 0.452 | |||
| SSM | 76 (32) | 31(37) | 45 (29) | ||
| NM | 72 (30) | 18(21) | 54 (35) | ||
| LMM | 13 (6) | 0 | 13 (9) | ||
| ALM | 30 (12) | 15 (18) | 15 (10) | ||
| Mucosal | 15 (6) | 7 (8) | 8 (5) | ||
| Other | 32 (14) | 14 (16) | 18 (12) | ||
| Stage at initial diagnosis * | 0.114 | ||||
| I | 48 (17) | 19 (18) | 29 (17) | ||
| II | 84 (31) | 25 (23) | 59 (35) | ||
| III | 95 (35) | 38 (37) | 57 (34) | ||
| IV | 47 (17) | 24 (22) | 23 (14) | ||
| Number of organs with metastases | 0.098 | 0.470 | |||
| 1-3 | 285 (89) | 109 (86) | 176 (92) | ||
| >3 | 34 (11) | 18 (14) | 16 (8) | ||
| Brain metastases | 0.618 | ||||
| No | 258 (81) | 101 (79) | 157 (82) | ||
| Yes | 61 (19) | 26 (21) | 35 (18) | ||
| Liver metastases | 0.139 | ||||
| No | 204 (64) | 75 (59) | 129 (67) | ||
| Yes | 115 (36) | 52 (41) | 63 (33) | ||
| BRAF mutation * | 0.844 | ||||
| BRAFmut | 88 (45) | 32 (44) | 56 (46) | ||
| BRAFwt | 106 (56) | 40 (56) | 66 (54) | ||
| LDH level * | 0.029 | 0.532 | |||
| Normal | 190 (68) | 67 (60) | 123 (73) | ||
| Elevated | 90 (32) | 44 (40) | 46 (27) | ||
| S100B level * | 0.000 | 0.008 | |||
| Normal | 157 (56) | 44 (40) | 113 (65) | ||
| Elevated | 125 (44) | 65 (60) | 60 (35) | ||
 Chi-square test performed between the two groups—primary resistance and disease control; ICI = immune-checkpoint inhibitors cohort—145 patients received first-line treatment with nivolumab plus ipilimumab and 174 received antiPD-1 antibodies monotherapy (nivolumab n = 46 and pembrolizumab n = 128).; y = years; SSM = superficial spreading melanoma; NM = nodular melanoma; LMM = lentigo malignant melanoma; ALM = acral lentiginous melanoma; BRAFmut = presence of BRAFV600E/K mutation; BRAFwt = BRAF wild-type; LDH = lactate dehydrogenase; S100B = tumor marker protein S100B. p-values that are statistically significant are noted in bold.
Chi-square test performed between the two groups—primary resistance and disease control; ICI = immune-checkpoint inhibitors cohort—145 patients received first-line treatment with nivolumab plus ipilimumab and 174 received antiPD-1 antibodies monotherapy (nivolumab n = 46 and pembrolizumab n = 128).; y = years; SSM = superficial spreading melanoma; NM = nodular melanoma; LMM = lentigo malignant melanoma; ALM = acral lentiginous melanoma; BRAFmut = presence of BRAFV600E/K mutation; BRAFwt = BRAF wild-type; LDH = lactate dehydrogenase; S100B = tumor marker protein S100B. p-values that are statistically significant are noted in bold.| Best Response | Median OS (Months; 95% CI) | OS (%; 95% CI) | ||
|---|---|---|---|---|
| 1-Year | 2-Year | 3-Year | ||
| CR n = 50 (15.7%) | not reached | 100% | 95.7 (87.3–100) | 87.7 (70.8–100) |
| PR n = 80 (25.1%) | not reached | 89.5 (82.1–96.9) | 84.4 (74.4–94.4) | 84.4 (74.4–94.4) |
| SD n = 62 (19.4%) | 28 (22.9–33.1) | 86.3 (77.5–95.1) | 63.8 (47.7–79.9) | 24.6 (2.6–46.5) |
| PD n = 127 (39.8%) | 11 (9.0–13.0) | 41.3 (31.9–50.7) | 14.7 (7.4–22.0) | 10.1 (3.4–16.8) |
| DC n = 192 (60.2%) | not reached | 91.3 (87.0–95.6) | 81.0 (73.7–88.3) | 64.6 (53.2–76) |
| PD-1 monotherapy n = 174 (66.2%) | 26 (19.7–32.3) | 71.1 (64.0–78.2) | 53.3 (45.1–61.5) | 41.3 (32.1–50.5) |
| PD-1 + CTLA4 n = 145 (54.6%) | 31 (17.2–44.8) | 72.8 (65–80.6) | 54.5 (42.9–66.1) | 42.5 (24.1–60.9) |
| Best Response | Median PFS (Months; 95% CI) | PFS (%; 95% CI) | ||
|---|---|---|---|---|
| 1-Year | 2-Year | 3-Year | ||
| CR n = 50 (15.7%) | Not reached | 87.6 (78.4–96.8) | 81.2 (68.9–93.5) | 72.2 (52.2–92.2) |
| PR n = 80 (25.1%) | 37 (14.97–59.03) | 74.4 (64.2–85.0) | 62.7 (50.0–75.4) | 62.7 (50.0–75.4) |
| SD n = 62 (19.4%) | 12 (8.97–15.03) | 43.0 (29.3–56.7) | 21.8 (6.3–37.3) | - |
| PD n = 127 (39.8%) | 4 (3.56–4.44) | 8.7 (3.8–13.6) | 3.2 (0–6.5) | 1.1 (0–3.1) |
| DC n = 192(60.2%) | 33 (20.4–45.6) | 68.1 (61.0–75.2) | 56.2 (51.8–64.8) | 48.7 (37.7–59.7) |
| PD-1 monotherapy n = 174 (66.2%) | 8 (5.5–10.5) | 40.3 (32.7–47.9) | 30.5 (23.1–37.9) | 24.1 (16.3–31.9) |
| PD-1 + CTLA4 n = 145 (54.6%) | 9 (1.8–16.2) | 48.5 (40.1–56.9) | 39 (29.2–78.8) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, T.; Seeber, O.; Mersi, E.; Sanchez, S.; Thomas, I.; Meiwes, A.; Forschner, A.; Leiter, U.; Eigentler, T.; Keim, U.; et al. Primary Resistance to PD-1-Based Immunotherapy—A Study in 319 Patients with Stage IV Melanoma. Cancers 2020, 12, 1027. https://doi.org/10.3390/cancers12041027
Amaral T, Seeber O, Mersi E, Sanchez S, Thomas I, Meiwes A, Forschner A, Leiter U, Eigentler T, Keim U, et al. Primary Resistance to PD-1-Based Immunotherapy—A Study in 319 Patients with Stage IV Melanoma. Cancers. 2020; 12(4):1027. https://doi.org/10.3390/cancers12041027
Chicago/Turabian StyleAmaral, Teresa, Olivia Seeber, Edgar Mersi, Stephanie Sanchez, Ioannis Thomas, Andreas Meiwes, Andrea Forschner, Ulrike Leiter, Thomas Eigentler, Ulrike Keim, and et al. 2020. "Primary Resistance to PD-1-Based Immunotherapy—A Study in 319 Patients with Stage IV Melanoma" Cancers 12, no. 4: 1027. https://doi.org/10.3390/cancers12041027
APA StyleAmaral, T., Seeber, O., Mersi, E., Sanchez, S., Thomas, I., Meiwes, A., Forschner, A., Leiter, U., Eigentler, T., Keim, U., & Garbe, C. (2020). Primary Resistance to PD-1-Based Immunotherapy—A Study in 319 Patients with Stage IV Melanoma. Cancers, 12(4), 1027. https://doi.org/10.3390/cancers12041027






