Public Adverse Event Data Insights into the Safety of Pembrolizumab in Melanoma Patients
Abstract
1. Introduction
2. Materials and Methods
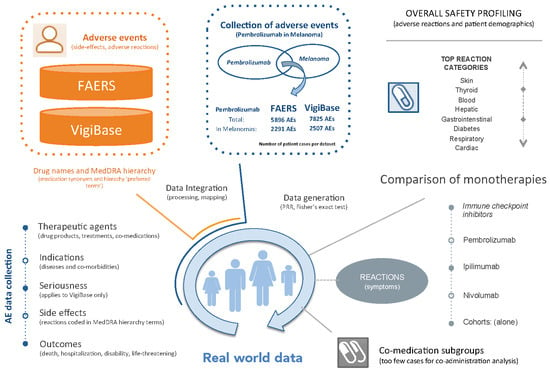
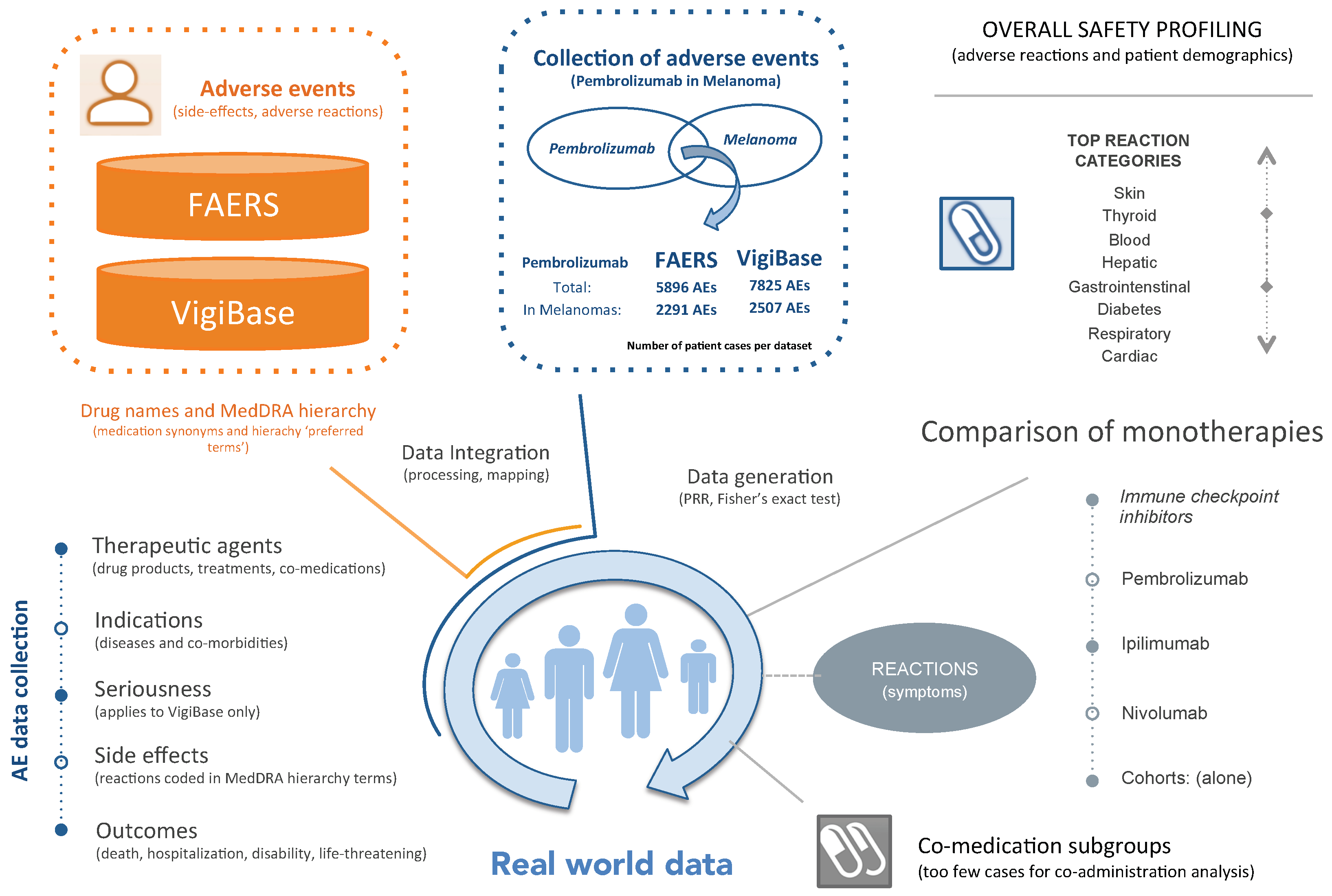
2.1. Adverse Event Data Integration
2.2. Definition of Cohorts
- FAERS PembroM: 2291 AE cases from FAERS of melanoma patients treated with Pembrolizumab.
- VigiBase PembroM: 2507 AE cases from VigiBase of melanoma patients treated with Pembrolizumab.
2.3. Statistical Characterization
| AE Cases | Event (E) | Not E | Totals |
|---|---|---|---|
| Cohort | a | b | a + b |
| Not Cohort | c | d | c + d |
| Totals | a + c | b + d | N = a + b + c + d |
2.4. Data Availability
3. Results
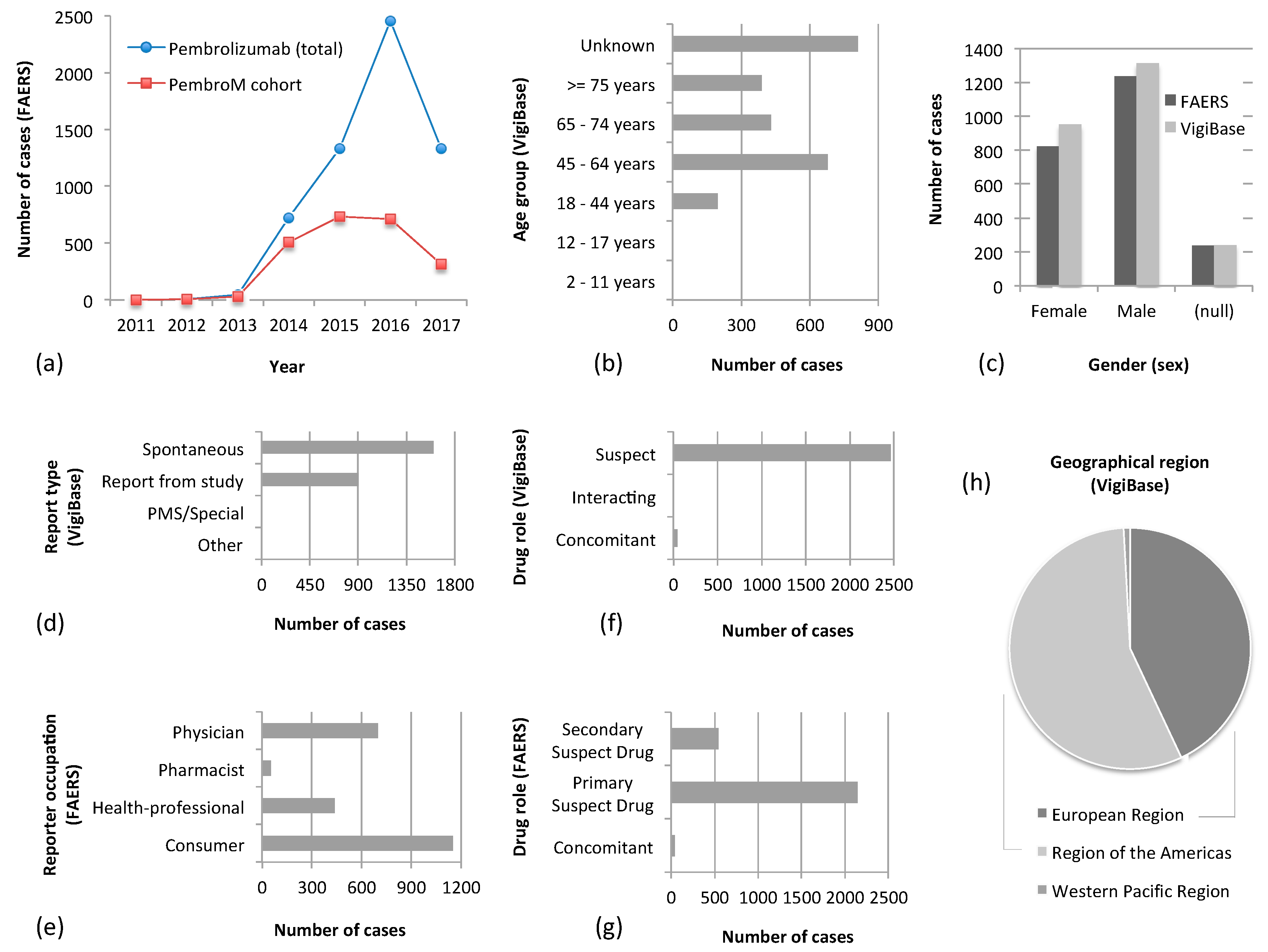
3.1. Overview of the PemboM Cohorts
3.2. Adverse Event Profiling
- ‘Hypophysitis’ (PRRs: FAERS = 267.82; VigiBase = 308.83) and ‘Adrenal insufficiency’ (PRR: FAERS = 28.10; VigiBase = 47.47)
- ‘Vitiligo’ (PRRs: FAERS = 166.64; VigiBase = 373.83), ‘Pemphigoid’ (PRRs: FAERS = 24.48; VigiBase = 53.54), and ‘Rash maculo-papular’ (PRR: FAERS = 9.53)
- ‘Autoimmune colitis’ (PRR: VigiBase = 565.03) and ‘Colitis’ (PRRs: FAERS = 35.8; VigiBase = 22.1)
- ‘Thyroiditis’ (PRRs: FAERS = 42.42; VigiBase = 81.45), ‘Hypothyroidism’ (PRRs: FAERS = 11.22; VigiBase = 30.35), ‘Hyperthyroidism’ (PRRs: FAERS = 7.16; VigiBase = 23.94), and ‘Thyroid disorder’ (PRRs: FAERS = 5.38; VigiBase = 9.46)
- ‘Autoimmune hepatitis’ (PRRs: FAERS = 35.9; VigiBase = 60.3), ‘Hepatitis’ (PRRs: FAERS = 9.76; VigiBase = 2.9), and ‘Hepatotoxicity’ (PRRs: FAERS = 4.65; VigiBase = 7.73)
- ‘Pneumonitis’ (PRRs: FAERS = 21.44; VigiBase = 37.14)
- ‘Myasthenia gravis’ (PRR: FAERS = 21.46)
- ‘Type 1 diabetes mellitus’ (PRRs: FAERS = 18.63; VigiBase = 34.90) and ‘Diabetic ketoacidosis’ (PRRs: FAERS = 4.3; VigiBase = 9.4)
- ‘Uveitis’ (PRRs: FAERS = 15.51; VigiBase = 35.22)
- ‘Myocarditis’ (PRR: FAERS = 12.54)
- ‘Myositis’ (PRR: FAERS = 10.23)
- ‘Tubulointerstitial nephritis’ (PRRs: FAERS = 5.66; VigiBase = 7.69)
3.3. Comparison of Pembrolizumab, Ipilimumab and Nivolumab Monotherapies in Melanoma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Wherry, E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Korman, A.J.; Peggs, K.S.; Allison, J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006, 90, 297–339. [Google Scholar] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Hazarika, M.; Chuk, M.K.; Theoret, M.R.; Mushti, S.; He, K.; Weis, S.L.; Putman, A.H.; Helms, W.S.; Cao, X.; Li, H.; et al. U.S. FDA Approval Summary: Nivolumab for Treatment of Unresectable or Metastatic Melanoma Following Progression on Ipilimumab. Clin. Cancer Res. 2017, 23, 3484–3488. [Google Scholar] [CrossRef]
- Chuk, M.K.; Chang, J.T.; Theoret, M.R.; Sampene, E.; He, K.; Weis, S.L.; Helms, W.S.; Jin, R.; Li, H.; Yu, J.; et al. FDA Approval Summary: Accelerated Approval of Pembrolizumab for Second-Line Treatment of Metastatic Melanoma. Clin. Cancer Res. 2017, 23, 5666–5670. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.J.; Atkins, M.B.; Kirkwood, J.M.; Agarwala, S.S.; Clark, J.I.; Ernstoff, M.S.; Fecher, L.; Gajewski, T.F.; Gastman, B.; Lawson, D.H.; et al. An update on the Society for Immunotherapy of Cancer consensus statement on tumor immunotherapy for the treatment of cutaneous melanoma: Version 2.0. J. Immunother. Cancer 2018, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2017, 8, 49. [Google Scholar] [CrossRef]
- Weber, J.S.; Postow, M.; Lao, C.D.; Schadendorf, D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist 2016, 21, 1230–1240. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Day, D.; Hansen, A.R. Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. BioDrugs 2016, 30, 571–584. [Google Scholar] [CrossRef]
- Ji, H.-H.; Tang, X.-W.; Dong, Z.; Song, L.; Jia, Y.-T. Adverse Event Profiles of Anti-CTLA-4 and Anti-PD-1 Monoclonal Antibodies Alone or in Combination: Analysis of Spontaneous Reports Submitted to FAERS. Clin. Drug Investig. 2019, 39, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y. Biomarkers for Immune Checkpoint Inhibitor-Mediated Tumor Response and Adverse Events. Front. Med. (Lausanne) 2019, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.E.; Burke, K.P.; Van Allen, E.M. Genomic correlates of response to immune checkpoint blockade. Nat. Med. 2019, 25, 389–402. [Google Scholar] [CrossRef] [PubMed]
- So, A.C.; Board, R.E. Real-world experience with pembrolizumab toxicities in advanced melanoma patients: A single-center experience in the UK. Melanoma Manag. 2018, 5, MMT05. [Google Scholar] [CrossRef] [PubMed]
- Buder-Bakhaya, K.; Hassel, J.C. Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment-A Review From the Melanoma Perspective and Beyond. Front. Immunol. 2018, 9, 1474. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, S.; Deng, Y.; Wang, P.; Hou, Q.; Xu, H. Prognostic Factors for Checkpoint Inhibitor Based Immunotherapy: An Update With New Evidences. Front. Pharmacol. 2018, 9, 1050. [Google Scholar] [CrossRef]
- Soldatos, T.G.; Perdigão, N.; Brown, N.P.; Sabir, K.S.; O’Donoghue, S.I. How to learn about gene function: Text-mining or ontologies? Methods 2015, 74, 3–15. [Google Scholar] [CrossRef]
- Soldatos, T.G.; Taglang, G.; Jackson, D.B. In Silico Profiling of Clinical Phenotypes for Human Targets Using Adverse Event Data. High-Throughput 2018, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, A.C.G. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, T.G.; Dimitrakopoulou-Strauss, A.; Larribere, L.; Hassel, J.C.; Sachpekidis, C. Retrospective Side Effect Profiling of the Metastatic Melanoma Combination Therapy Ipilimumab-Nivolumab Using Adverse Event Data. Diagnostics (Basel) 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, F.F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma—An Update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Perez-Ruiz, E.; Minute, L.; Otano, I.; Alvarez, M.; Ochoa, M.C.; Belsue, V.; de Andrea, C.; Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Marquez-Rodas, I.; et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019, 569, 428–432. [Google Scholar] [CrossRef]
- Downey, S.G.; Klapper, J.A.; Smith, F.O.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Kammula, U.S.; Hughes, M.S.; Allen, T.E.; Levy, C.L.; et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res. 2007, 13, 6681–6688. [Google Scholar] [CrossRef]
- Attia, P.; Phan, G.Q.; Maker, A.V.; Robinson, M.R.; Quezado, M.M.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Kammula, U.S.; Royal, R.E.; et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J. Clin. Oncol. 2005, 23, 6043–6053. [Google Scholar] [CrossRef]
- Kaehler, K.C.; Piel, S.; Livingstone, E.; Schilling, B.; Hauschild, A.; Schadendorf, D. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: Identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin. Oncol. 2010, 37, 485–498. [Google Scholar] [CrossRef]
- Sato, K.; Mano, T.; Iwata, A.; Toda, T. Neurological and related adverse events in immune checkpoint inhibitors: A pharmacovigilance study from the Japanese Adverse Drug Event Report database. J. Neurooncol. 2019, 145, 1–9. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K.S. A Review of Immune-Mediated Adverse Events in Melanoma. Oncol Ther. 2019, 7, 101–120. [Google Scholar] [CrossRef]
- Hsiehchen, D.; Watters, M.K.; Lu, R.; Xie, Y.; Gerber, D.E. Variation in the Assessment of Immune-Related Adverse Event Occurrence, Grade, and Timing in Patients Receiving Immune Checkpoint Inhibitors. JAMA Netw. Open 2019, 2, e1911519. [Google Scholar] [CrossRef]
- Soldatos, T.G.; Iakovou, I.; Sachpekidis, C. Retrospective Toxicological Profiling of Radium-223 Dichloride for the Treatment of Bone Metastases in Prostate Cancer Using Adverse Event Data. Medicina (Kaunas) 2019, 55, 149. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Jackson, D.B.; Soldatos, T.G. Radioimmunotherapy in Non-Hodgkin’s Lymphoma: Retrospective Adverse Event Profiling of Zevalin and Bexxar. Pharmaceuticals (Basel) 2019, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, T.G.; Jackson, D.B. Adverse Event Circumstances and the Case of Drug Interactions. Healthcare (Basel) 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Soldatou, V.; Soldatos, A.; Soldatos, T. Examining Socioeconomic and Computational Aspects of Vaccine Pharmacovigilance. Biomed. Res. Int. 2019, 2019, 6576483. [Google Scholar] [CrossRef]
- Racz, R.; Soldatos, T.G.; Jackson, D.; Burkhart, K. Association between Serotonin Syndrome and Second-Generation Antipsychotics via Pharmacological Target-Adverse Event Analysis. Clin. Transl. Sci. 2018, 11, 322–329. [Google Scholar] [CrossRef]
| Reaction PT Name 1 | FAERS (PembroM Cohort) | VigiBase (PembroM Cohort) | ||||
|---|---|---|---|---|---|---|
| AEs | % Cohort | PRR | AEs | % Cohort | PRR | |
| Alanine aminotransferase increased | 26 | 1.13 | 2.59p | 23 | 0.92 | 2.58p |
| Anaemia 2 | 52 | 2.27 | 1.97p | 47 | 1.87 | 2.18p |
| Arthralgia 2 | 75 | 3.27 | 1.69p | 81 | 3.23 | 2.18p |
| Arthritis | 16 | 0.69 | 1.75 | 19 | 0.76 | 2.76 |
| Aspartate aminotransferase increased | 27 | 1.18 | 2.99p | 22 | 0.88 | 3.01p |
| Autoimmune hepatitis 2 + | 27 | 1.18 | 35.83p | 23 | 0.92 | 60.29p |
| Blood bilirubin increased | 12 | 0.52 | 2.61 | 11 | 0.44 | 3.96 |
| Colitis + | 47 | 2.05 | 13.09p | 60 | 2.39 | 22.12p |
| Constipation | 37 | 1.62 | 1.59 | 44 | 1.76 | 1.95p |
| Decreased appetite 2 | 53 | 2.3 | 1.83p | 70 | 2.79 | 2.71p |
| Diabetes mellitus | 22 | 0.96 | 1.87 | 16 | 0.64 | 2.35 |
| Diabetic ketoacidosis + | 11 | 0.48 | 4.29p | 14 | 0.56 | 9.41p |
| Diarrhoea 2 | 104 | 4.54 | 1.53p | 129 | 5.15 | 1.78p |
| Dry mouth | 18 | 0.79 | 1.71 | 22 | 0.88 | 1.56 |
| Dry skin | 13 | 0.57 | 1.98 | 15 | 0.59 | 2.97 |
| Dyspnoea exertional + | 11 | 0.48 | 3.01 | 17 | 0.68 | 8.82p |
| Fatigue 2 | 202 | 8.82 | 2.42p | 248 | 9.89 | 3.80p |
| General physical health deterioration + | 25 | 1.09 | 2.09 | 26 | 1.04 | 5.05p |
| Hepatic enzyme increased | 15 | 0.65 | 2.02 | 19 | 0.76 | 2.39 |
| Hepatitis + | 33 | 1.44 | 9.76p | 20 | 0.79 | 2.89p |
| Hepatotoxicity + | 10 | 0.44 | 4.65p | 10 | 0.39 | 7.73p |
| Hyperglycaemia | 16 | 0.69 | 3.11p | 18 | 0.72 | 3.20p |
| Hyperthyroidism + | 11 | 0.48 | 7.16p | 38 | 1.52 | 23.94p |
| Hyponatraemia | 31 | 1.35 | 4.20p | 27 | 1.08 | 3.92p |
| Hypophysitis 2 + | 34 | 1.48 | 267.83p | 26 | 1.04 | 308.83p |
| Hypothyroidism 2 + | 41 | 1.79 | 11.22p | 74 | 2.95 | 30.35p |
| Interstitial lung disease | 20 | 0.87 | 3.74p | 16 | 0.64 | 4.61p |
| Lung disorder + | 15 | 0.65 | 2.61 | 14 | 0.56 | 5.13p |
| Lymphadenopathy | 12 | 0.52 | 2.58 | 15 | 0.59 | 2.54 |
| Muscular weakness | 30 | 1.31 | 1.95 | 30 | 1.19 | 2.51p |
| Myalgia | 33 | 1.44 | 1.47 | 43 | 1.72 | 1.41 |
| Pancreatitis | 18 | 0.79 | 2.14 | 16 | 0.64 | 2.59 |
| Pemphigoid 2 + | 14 | 0.61 | 25.48p | 23 | 0.92 | 53.54p |
| Pleural effusion | 17 | 0.74 | 1.95 | 19 | 0.76 | 4.29p |
| Pneumonitis 2 + | 54 | 2.36 | 21.44p | 68 | 2.71 | 37.14p |
| Rhabdomyolysis | 12 | 0.52 | 1.99 | 10 | 0.39 | 2.21 |
| Thyroid disorder + | 10 | 0.44 | 5.38p | 10 | 0.39 | 9.46p |
| Thyroiditis 2 + | 10 | 0.44 | 42.42p | 17 | 0.68 | 81.45p |
| Tubulointerstitial nephritis + | 10 | 0.44 | 5.66p | 10 | 0.39 | 7.69p |
| Type 1 diabetes mellitus + | 16 | 0.69 | 18.63p | 17 | 0.68 | 34.90p |
| Uveitis + | 15 | 0.65 | 15.51p | 25 | 0.99 | 35.22p |
| Vitiligo 2 + | 26 | 1.13 | 166.64p | 84 | 3.35 | 373.84p |
| Organ/System Class 1 | Reaction PT Name (whether FAERS and/or VigiBase PembroM cohort) 2 |
|---|---|
| Brain, neurologic | ‘Cerebral haemorrhage’ F, ‘Myasthenia gravis’ F |
| Cardiac | ‘Myocarditis’ F |
| Endocrine | ‘Adrenal insufficiency’ FV, ‘Hyperglycaemia’ FV, ‘Hyperthyroidism’ FV, ‘Hypophysitis’ FV, ‘Hypopituitarism’ F, ‘Hypothyroidism’ FV, ‘Thyroid disorder’ FV, ‘Thyroiditis’ FV, ‘Diabetes mellitus’ FV, ‘Diabetic ketoacidosis’ FV, ‘Type 1 diabetes mellitus’ FV |
| Gastrointestinal | ‘Autoimmune colitis’ V, ‘Colitis’ FV, ‘Constipation’ FV, ‘Diarrhoea’ FV, ‘Dry mouth’ FV |
| Hematologic, vascular | ‘Anaemia’ FV, ‘Eosinophilia’ F, ‘Peripheral swelling’ V |
| Kidney, renal | ‘Acute kidney injury’ V, ‘Blood creatinine increased’ V, ‘Renal failure acute’ F, ‘Tubulointerstitial nephritis’ FV |
| Liver, hepatic | ‘Alanine aminotransferase increased’ FV, ‘Aspartate aminotransferase increased’ FV, ‘Autoimmune hepatitis’ FV, ‘Hepatic enzyme increased’ FV, ‘Hepatitis’ FV, ‘Hepatocellular injury’ V, ‘Hepatotoxicity’ FV, ‘Transaminases increased’ V |
| Lymphadenopathies | ‘Lymphadenopathy’ FV |
| Musculoskeletal | ‘Arthralgia’ FV, ‘Arthritis’ FV, ‘Muscular weakness’ FV, ‘Myalgia’ FV, ‘Myositis’ F, ‘Rhabdomyolysis’ FV |
| Opthalmologic | ‘Uveitis’ FV |
| Pancreatic | ‘Lipase increased’ V, ‘Pancreatitis’ FV |
| Respiratory, pulmonary | ‘Cough’ V, ‘Dyspnoea exertional’ FV, ‘Interstitial lung disease’ FV, ‘Lung disorder’ FV, ‘Pleural effusion’ FV, ‘Pneumonitis’ FV, ‘Pulmonary embolism’ V |
| Skin | ‘Dry skin’ FV, ‘Eczema’ V,’Pemphigoid’ FV, ‘Pruritus’ F, ‘Psoriasis’ V, ‘Rash’ F, ‘Rash erythematous’ F, ‘Rash generalised’ V, ‘Rash maculo-papular’ F, ‘Vitiligo’ FV |
| Category/Class | Pembrolizumab (alone) 1 | Ipilimumab (alone) 2 | Nivolumab (alone) 2 |
|---|---|---|---|
| Adrenal | (+) | Adrenal insufficiency (42.38) | Adrenal insufficiency (27.29) |
| Blood 3 | Anaemia Hyponatremia (3.54) | Anaemia (1.48) Hyponatremia (4.02) | (+) |
| Febrile | Pyrexia | Pyrexia (2.03) | (+) |
| Gastrointestinal 3 | Colitis (10.45) Diarrhoea | Colitis (72.05) Diarrhoea (4.85) Intestinal Perforation (10.74) | Colitis (12.88) Diarrhoea (1.44) |
| Hepatic | (+) | Hepatitis (9.02) | Hepatic function abnormal (8.45) Liver disorder (3.93) |
| Hypothalamic | Hypophysitis (210.73) | Hypophysitis (1051.12) | (+) |
| Opthalmologic 3 | (+) | (+) | Uveitis (26.59) |
| Renal | (+) | Renal Failure Acute (1.97) | Acute Kidney Injury (2.27) |
| Respiratory | Pneumonitis (18.11) | Pneumonitis (11.07) | Pneumonitis (16.28) |
| Skin 3 | Pruritus (1.77) Rash (1.69) | Pruritus (1.85) Rash (3.29) | Leukoderma (2439.24) Pruritus (2.07) |
| Thyroid 3 | Hypothyroidism (8.48) | Hypothyroidism (7.88) | Hypothyroidism (29.59) |
| Other | Arthralgia (1.51) Decreased Appetite (1.58) Constipation Myalgia | Decreased Appetite (2.49) Dehydration (3.43) Sepsis (2.22) | Decreased Appetite (2.15) Infusion Related Reaction (4.41) Sepsis (1.95) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaefer, A.; Sachpekidis, C.; Diella, F.; Doerks, A.; Kratz, A.-S.; Meisel, C.; Jackson, D.B.; Soldatos, T.G. Public Adverse Event Data Insights into the Safety of Pembrolizumab in Melanoma Patients. Cancers 2020, 12, 1008. https://doi.org/10.3390/cancers12041008
Schaefer A, Sachpekidis C, Diella F, Doerks A, Kratz A-S, Meisel C, Jackson DB, Soldatos TG. Public Adverse Event Data Insights into the Safety of Pembrolizumab in Melanoma Patients. Cancers. 2020; 12(4):1008. https://doi.org/10.3390/cancers12041008
Chicago/Turabian StyleSchaefer, Anne, Christos Sachpekidis, Francesca Diella, Anja Doerks, Anne-Sophie Kratz, Christian Meisel, David B. Jackson, and Theodoros G. Soldatos. 2020. "Public Adverse Event Data Insights into the Safety of Pembrolizumab in Melanoma Patients" Cancers 12, no. 4: 1008. https://doi.org/10.3390/cancers12041008
APA StyleSchaefer, A., Sachpekidis, C., Diella, F., Doerks, A., Kratz, A.-S., Meisel, C., Jackson, D. B., & Soldatos, T. G. (2020). Public Adverse Event Data Insights into the Safety of Pembrolizumab in Melanoma Patients. Cancers, 12(4), 1008. https://doi.org/10.3390/cancers12041008