Primary Neuroendocrine Neoplasms of the Breast: Case Series and Literature Review
Abstract
1. Introduction
2. Case Reports
2.1. Case 1
2.2. Case 2
2.3. Case 3
2.4. Case 4
2.5. Case 5
3. Review of the Literature and Discussion
3.1. Summary of the Case Reports
3.2. Terminology, Frequency, Epidemiology
3.3. Clinical Presentation and Diagnostic Work-Up
3.4. Histology
3.5. Management
4. Patients and Methods
5. Conclusion and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NECB | Neuroendocrine carcinoma of the breast (NECB) |
| WHO | World Health Organization |
| NEN | Neuroendocrine neoplasia |
| NET | Neuroendocrine tumors |
| NEC | Neuroendocrine carcinoma |
| SSTR | Somatostatin receptors |
| SSA | Somatostatin analogs |
| CT | Computed tomography |
| PET/CT | Positron-emission tomography/computed tomography |
| MRI | Magnetic resonance imaging |
| SRS | Somatostatin receptor scintigraphy |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
References
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System, 4th ed.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Rinke, A.; Muller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Blaker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Yao, Y.; Sturdevant, D.E.; Otto, M. Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: Insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. J. Infect. Dis. 2005, 191, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Horsch, D.; Schmid, K.W.; Anlauf, M.; Darwiche, K.; Denecke, T.; Baum, R.P.; Spitzweg, C.; Grohe, C.; Presselt, N.; Stremmel, C.; et al. Neuroendocrine tumors of the bronchopulmonary system (typical and atypical carcinoid tumors): Current strategies in diagnosis and treatment. Conclusions of an expert meeting February 2011 in Weimar, Germany. Oncol. Res. Treat. 2014, 37, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, J.M.; Trama, A.; Otter, R.; Larranaga, N.; Tavilla, A.; Marcos-Gragera, R.; Dei Tos, A.P.; Baudin, E.; Poston, G.; Links, T. Rare neuroendocrine tumours: Results of the surveillance of rare cancers in Europe project. Eur. J. Cancer 2013, 49, 2565–2578. [Google Scholar] [CrossRef]
- Dowd, K.; Rotenberry, C.; Russell, D.; Wachtel, M.; de Riese, W. Rare Occurrence of a Poorly Differentiated Neuroendocrine Tumor of the Bladder. Case Rep. Med. 2017, 2017, 4812453. [Google Scholar] [CrossRef]
- Van der Laan, T.P.; Plaat, B.E.; van der Laan, B.F.; Halmos, G.B. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: A meta-analysis of 436 reported cases. Head Neck 2015, 37, 707–715. [Google Scholar] [CrossRef]
- Modlin, I.M.; Shapiro, M.D.; Kidd, M. An analysis of rare carcinoid tumors: Clarifying these clinical conundrums. World J. Surg. 2005, 29, 92–101. [Google Scholar] [CrossRef]
- Hauso, O.; Gustafson, B.I.; Kidd, M.; Waldum, H.L.; Drozdov, I.; Chan, A.K.C.; Modlin, I.M. Neuroendocrine Tumor Epidemiology Contrasting Norway and North America. Cancer 2008, 113, 2655–2664. [Google Scholar] [CrossRef]
- Ogawa, H.; Nishio, A.; Satake, H.; Naganawa, S.; Imai, T.; Sawaki, M.; Yamamoto, E.; Miyata, T. Neuroendocrine tumor in the breast. Radiat. Med. 2008, 26, 28–32. [Google Scholar] [CrossRef]
- Wang, J.; Wei, B.; Albarracin, C.T.; Hu, J.; Abraham, S.C.; Wu, Y. Invasive neuroendocrine carcinoma of the breast: A population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer 2014, 14, 1471–2407. [Google Scholar] [CrossRef] [PubMed]
- Marinova, L.; Malinova, D.; Vicheva, S. Primary Neuroendocrine Carcinoma of the Breast: Histopathological Criteria, Prognostic Factors, and Review of the Literature. Case Rep. Pathol. 2016, 2016, 6762085. [Google Scholar] [CrossRef] [PubMed]
- Gunhan-Bilgen, I.; Zekioglu, O.; Ustun, E.E.; Memis, A.; Erhan, Y. Neuroendocrine differentiated breast carcinoma: Imaging features correlated with clinical and histopathological findings. Eur. Radiol. 2003, 13, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bonet, E.; Alonso-Ruano, M.; Barraza, G.; Vazquez-Martin, A.; Bernadoo, L.; Menendez, J.A. Solid neuroendocrine breast carcinomas: Incidence, clinico-pathological features and immunohistochemical profiling. Oncol. Rep. 2008, 20, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R. WHO Classification of Tumours of the Breast, 4th ed.; Ellis, I.O., Ed.; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Kawasaki, T.; Kondo, T.; Nakazawa, T.; Mochizuki, K.; Yamane, T.; Murata, S.; Inoue, S.; Tsunoda, H.; Katoh, R. Is CD56 a specific and reliable neuroendocrine marker for discriminating between endocrine/neuroendocrine ductal carcinoma in situ and intraductal papilloma of the breast? Pathol. Int. 2011, 61, 49–51. [Google Scholar] [CrossRef]
- Moll, R.; Mitze, M.; Frixen, U.H.; Birchmeier, W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am. J. Pathol. 1993, 143, 1731–1742. [Google Scholar]
- Anlauf, M.; Neumann, M.; Bomberg, S.; Luczak, K.; Heikaus, S.; Gustmann, C.; Antke, C.; Ezziddin, S.; Fottner, C.; Pavel, M.; et al. [Neuroendocrine neoplasms of the breast]. Neuroendokrine Neoplasien der Mamma. Pathologe 2015, 36, 261–270. [Google Scholar] [CrossRef]
- Feyrter, F.; Hartmann, G. On the Carcinoid Growth Form of the Carcinoma Mammae, Especially the Carcinoma Solidum (Gelatinosum) Mammae. Frankf. Z. Pathol. 1963, 73, 24–39. [Google Scholar]
- Sapino, A.; Bussolati, G. Is detection of endocrine cells in breast adenocarcinoma of diagnostic and clinical significance? Histopathology 2002, 40, 211–214. [Google Scholar] [CrossRef]
- Li, Y.Q.; Du, F.; Zhu, W.J.; Xu, B.H. Neuroendocrine carcinoma of the breast: A review of 126 cases in China. Chin. J. Cancer 2017, 36, 45. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Mochizuki, K.; Yamauchi, H.; Yagata, H.; Kondo, T.; Tsunoda, H.; Nakamura, S.; Oishi, N.; Nakazawa, T.; Yamane, T.; et al. High prevalence of neuroendocrine carcinoma in breast lesions detected by the clinical symptom of bloody nipple discharge. Breast 2012, 21, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Kim, S.Y.; Lee, J.H.; Han, S.W. Primary neuroendocrine carcinoma of the breast: Radiologic and pathologic correlation. Clin. Imaging 2014, 38, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.D.; Kim, M.K.; Kim, J.S.; Whang, I.Y. Primary neuroendocrine tumor of the breast: Imaging features. Korean J. Radiol. 2013, 14, 395–399. [Google Scholar] [CrossRef]
- Yildirim, Y.; Elagoz, S.; Koyuncu, A.; Aydin, C.; Karadayi, K. Management of neuroendocrine carcinomas of the breast: A rare entity. Oncol. Lett. 2011, 2, 887–890. [Google Scholar] [CrossRef]
- Ambrosini, V.; Nanni, C.; Fanti, S. The use of gallium-68 labeled somatostatin receptors in PET/CT imaging. PET Clin. 2014, 9, 323–329. [Google Scholar] [CrossRef]
- Inno, A.; Bogina, G.; Turazza, M.; Bortesi, L.; Duranti, S.; Massocco, A.; Zamboni, G.; Carbognin, G.; Alongi, F.; Salgarello, M.; et al. Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. Oncologist 2016, 21, 28–32. [Google Scholar] [CrossRef]
- Angarita, F.A.; Rodriguez, J.L.; Meek, E.; Sanchez, J.O.; Tawil, M.; Torregrosa, L. Locally-advanced primary neuroendocrine carcinoma of the breast: Case report and review of the literature. World J. Surg. Oncol. 2013, 11, 1477–7819. [Google Scholar] [CrossRef]
- Wei, B.; Ding, T.; Xing, Y.; Wei, W.; Tian, Z.; Tang, F.; Abraham, S.; Nayeemuddin, K.; Hunt, K.; Wu, Y. Invasive neuroendocrine carcinoma of the breast: A distinctive subtype of aggressive mammary carcinoma. Cancer 2010, 116, 4463–4473. [Google Scholar] [CrossRef]
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef]
- Righi, L.; Sapino, A.; Marchio, C.; Papotti, M.; Bussolati, G. Neuroendocrine differentiation in breast cancer: Established facts and unresolved problems. Semin. Diagn. Pathol. 2010, 27, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wachter, D.L.; Hartmann, A.; Beckmann, M.W.; Fasching, P.A.; Hein, A.; Bayer, C.M.; Agaimy, A. Expression of neuroendocrine markers in different molecular subtypes of breast carcinoma. BioMed Res. Int. 2014, 2014, 408459. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, C.; Porembka, J.; Fang, Y.; Sarode, V.; Syed, S. Primary neuroendocrine carcinoma of the breast. Breast J. 2019, 25, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Yagyu, R.; Murase, K.; Kawajiri, H.; Ohtani, H.; Arimoto, Y.; Yamamura, T.; Inoue, T.; Moritani, S. A case of solid neuroendocrine carcinoma of the breast in a 40-year-old woman. Breast Cancer 2007, 14, 250–253. [Google Scholar] [CrossRef]
- Shin, S.J.; DeLellis, R.A.; Ying, L.; Rosen, P.P. Small cell carcinoma of the breast: A clinicopathologic and immunohistochemical study of nine patients. Am. J. Surg. Pathol. 2000, 24, 1231–1238. [Google Scholar] [CrossRef]
- Zimmermann, N.; Lazar-Karsten, P.; Keck, T.; Billmann, F.; Schmid, S.; Brabant, G.; Thorns, C. Expression Pattern of CDX2, Estrogen and Progesterone Receptors in Primary Gastroenteropancreatic Neuroendocrine Tumors and Metastases. Anticancer Res. 2016, 36, 921–924. [Google Scholar]
- Arnason, T.; Sapp, H.L.; Barnes, P.J.; Drewniak, M.; Abdolell, M.; Rayson, D. Immunohistochemical expression and prognostic value of ER, PR and HER2/neu in pancreatic and small intestinal neuroendocrine tumors. Neuroendocrinology 2011, 93, 249–258. [Google Scholar] [CrossRef]
- Viale, G.; Doglioni, C.; Gambacorta, M.; Zamboni, G.; Coggi, G.; Bordi, C. Progesterone receptor immunoreactivity in pancreatic endocrine tumors. An immunocytochemical study of 156 neuroendocrine tumors of the pancreas, gastrointestinal and respiratory tracts, and skin. Cancer 1992, 70, 2268–2277. [Google Scholar] [CrossRef]
- Curioni-Fontecedro, A. A Comprehensive Analysis of Markers for Neuroendocrine Tumors of the Lungs Demonstrates Estrogen Receptor Beta to be a Prognostic Markers in SCLC Male Patients. J. Cytol. Histol. 2014. [Google Scholar] [CrossRef]
- Barros, M.; Felismino, T.C.; de Jesus, V.H.F.; Mello, C.A.; Silva, V.S.; Camandaroba, M.; Rodrigues, N.; Donadio, M.D.; Nobrega, E.; Chinen, L.; et al. 1404TiP HORMONET: Study of tamoxifen in well differentiated neuroendocrine tumours and hormone receptor positive expression. Ann. Oncol. 2019, 30, 573. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Kim, S.A.; DeLair, D.F.; Bose, S.; Laury, A.R.; Chopra, S.; Mertens, R.B.; Dhall, D. Comparison of metastatic neuroendocrine neoplasms to the breast and primary invasive mammary carcinomas with neuroendocrine differentiation. Mod. Pathol. 2016, 29, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Kouros-Mehr, H.; Slorach, E.M.; Sternlicht, M.D.; Werb, Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 2006, 127, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Epstein, J.I. GATA3 expression in paragangliomas: A pitfall potentially leading to misdiagnosis of urothelial carcinoma. Mod. Pathol. 2013, 26, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Shetty, M.R. Neuroendocrine primary small cell carcinoma of the breast. Am. J. Clin. Oncol. 1996, 19, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Richter-Ehrenstein, C.; Arndt, J.; Buckendahl, A.C.; Eucker, J.; Weichert, W.; Kasajima, A.; Schneider, A.; Noske, A. Solid neuroendocrine carcinomas of the breast: Metastases or primary tumors? Breast Cancer Res. Treat. 2010, 124, 413–417. [Google Scholar] [CrossRef]
- Perry, K.D.; Reynolds, C.; Rosen, D.G.; Edgerton, M.E.T.; Albarracin, C.; Gilcrease, M.Z.; Sahin, A.A.; Abraham, S.C.; Wu, Y. Metastatic neuroendocrine tumour in the breast: A potential mimic of in-situ and invasive mammary carcinoma. Histopathology 2011, 59, 619–630. [Google Scholar] [CrossRef]
- Morrow, M.; Goldstein, L. Surgery of the primary tumor in metastatic breast cancer: Closing the barn door after the horse has bolted? J. Clin. Oncol. 2006, 24, 2694–2696. [Google Scholar] [CrossRef]
- Jablon, L.K.; Somers, R.G.; Kim, P.Y. Carcinoid tumor of the breast: Treatment with breast conservation in three patients. Ann. Surg. Oncol. 1998, 5, 261–264. [Google Scholar] [CrossRef]
- Wei, X.; Chen, C.; Xi, D.; Bai, J.; Huang, W.; Rong, L.; Wu, M.; Zhang, G. A case of primary neuroendocrine breast carcinoma that responded to neo-adjuvant chemotherapy. Front. Med. 2015, 9, 112–116. [Google Scholar] [CrossRef]
- Oberg, K. Management of neuroendocrine tumours. Ann. Oncol. 2004, 15, iv293–iv298. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, W.A.; Bodei, L.; Mueller-Brand, J.; de Herder, W.W.; Kvols, L.K.; Kwekkeboom, D.J. GEPNETs update: Radionuclide therapy in neuroendocrine tumors. Eur. J. Endocrinol. 2015, 172, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Savelli, G.; Zaniboni, A.; Bertagna, F.; Bosio, G.; Nisa, L.; Rodella, C.; Biasiotto, G.; Bettinsoli, G.; Migliorati, E.; Peli, A.; et al. Peptide Receptor Radionuclide Therapy (PRRT) in a Patient Affected by Metastatic Breast Cancer with Neuroendocrine Differentiation. Breast Care 2012, 7, 408–410. [Google Scholar] [CrossRef] [PubMed]
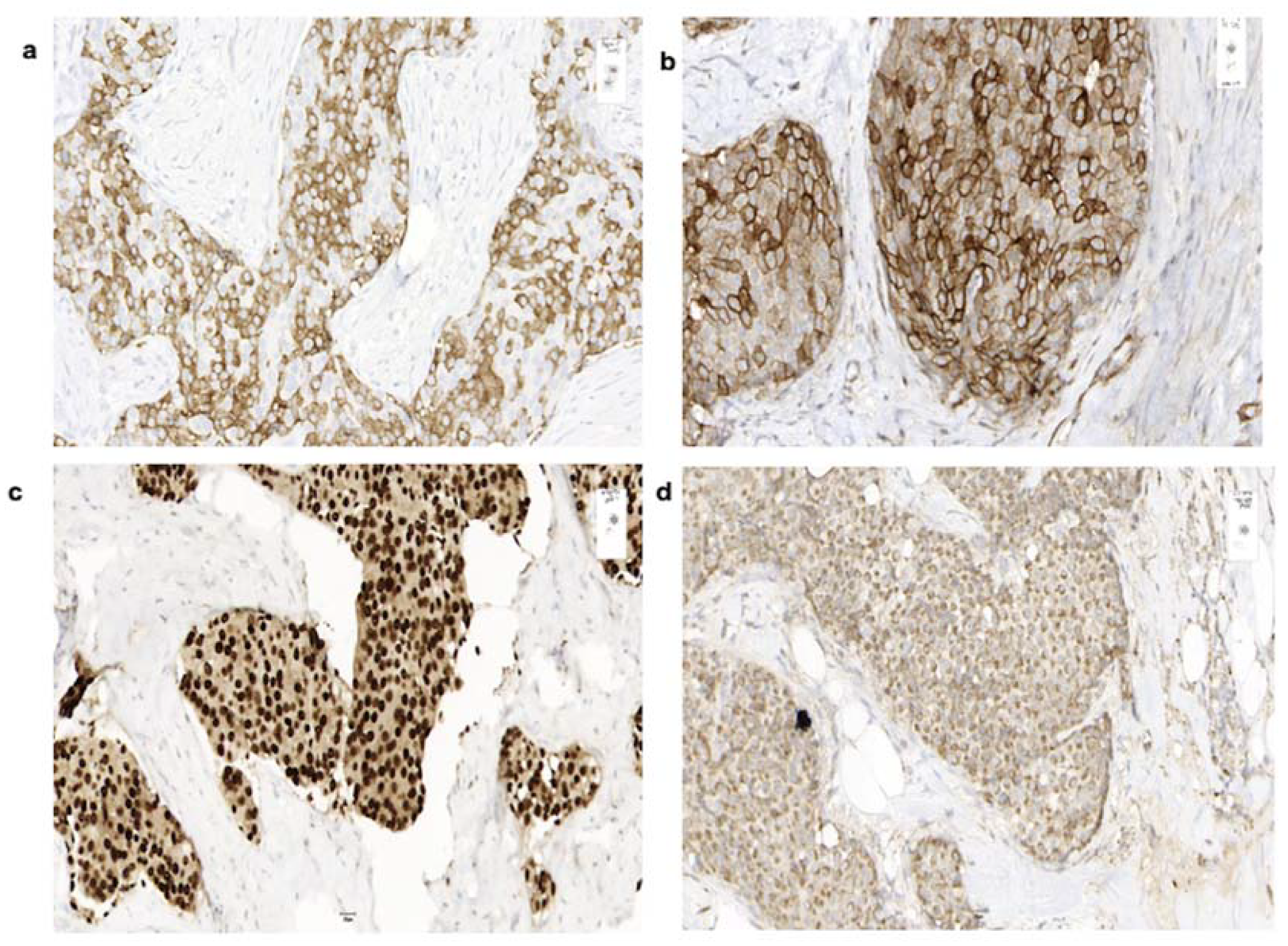
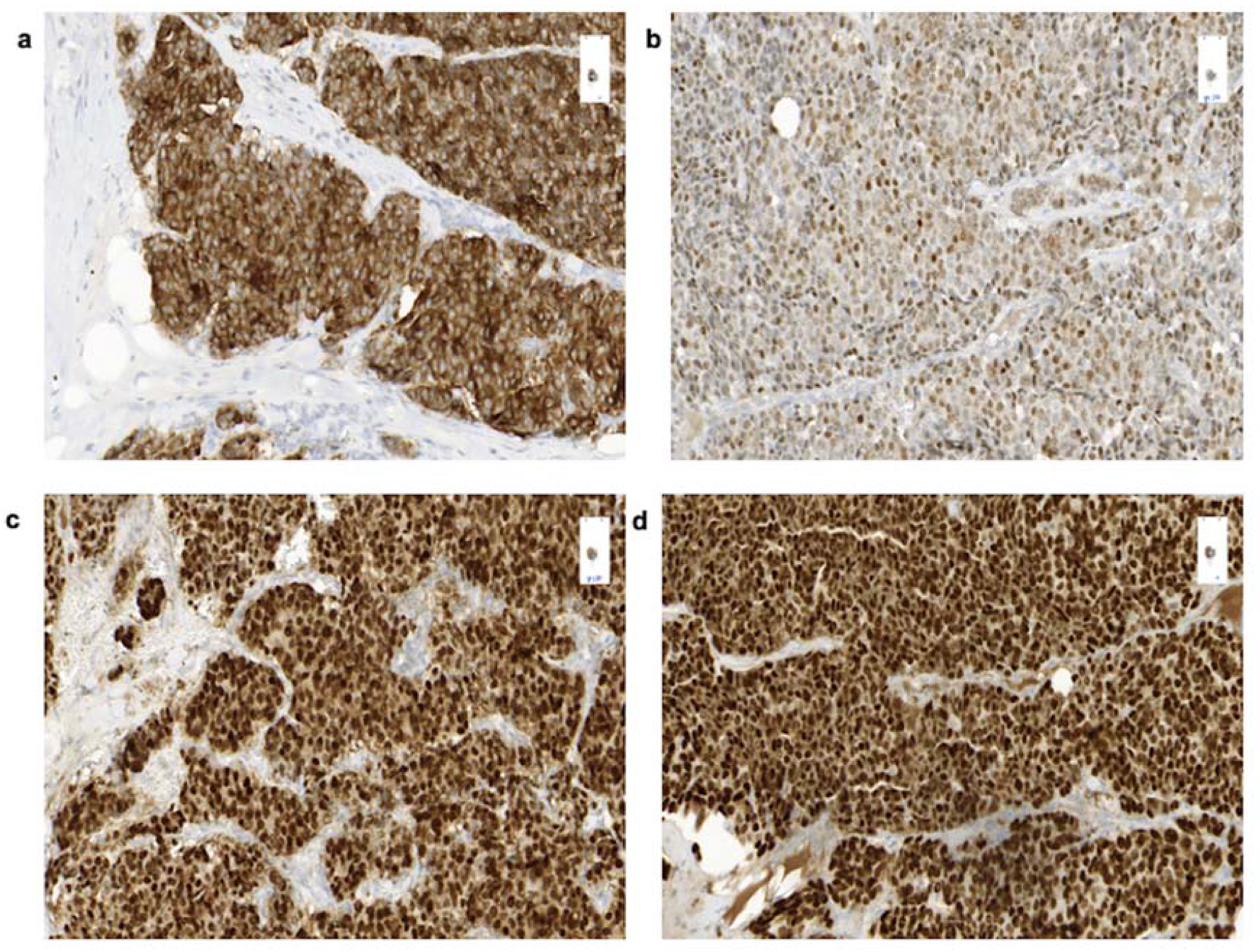
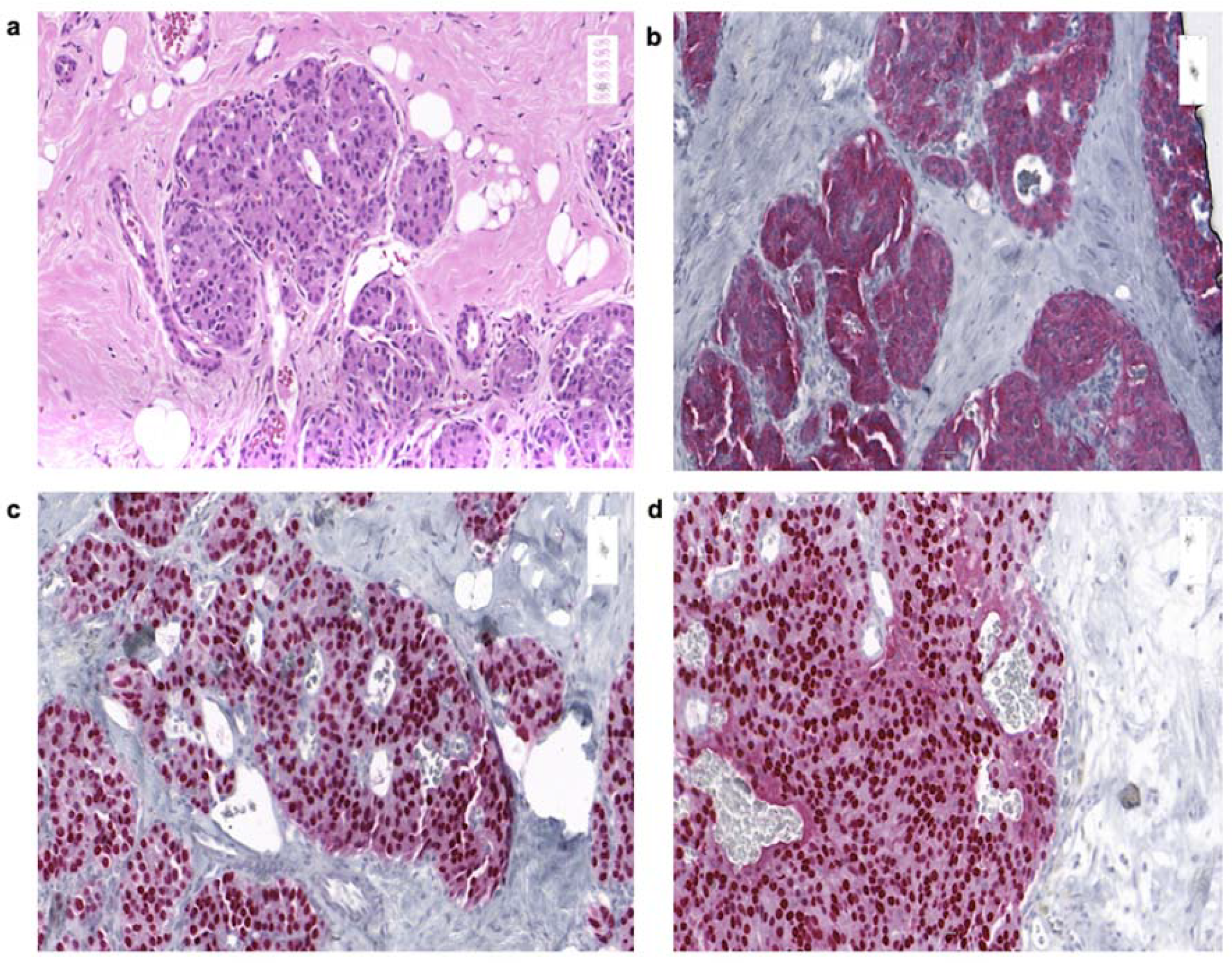


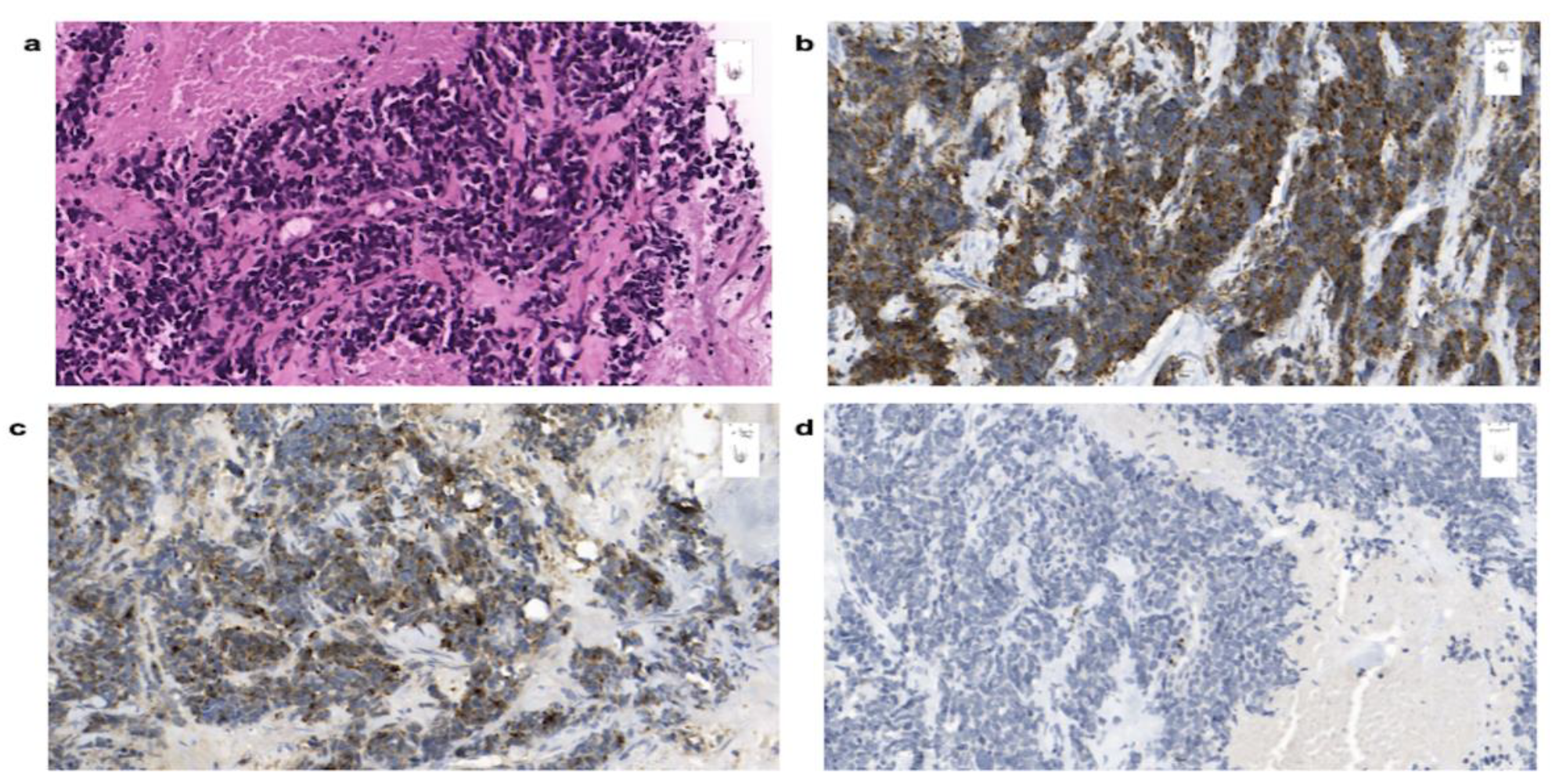
| Patient no. | Sex | Age | Date of Diagnosis | Diagnosis | Breast Localization | Family History | Primary Tumor Size (Diameter) | Axillary Node Status | Distant Metastases |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 73 | Feb-19 | Well-differentiated NET of the breast | Inferior-external, left | Yes (breast cancer) | 10 mm | 1/8 | None |
| 2 | F | 53 | Nov-17 | Well-/moderately-differentiated NET of the breast | Inferior, right | No | 12 mm | 0/3 | None |
| 3 | F | 49 | Mar-09 | Moderately-differentiated NET | Upper external, right | Yes (breast cancer) | 15 mm | 1/0 | None |
| 4 | F | 78 | May-18 | Poorly differentiated small cell neuroendocrine carcinoma | Upper-external, right, upper external left | Yes (small cell lung cancer) | Multiple lesions up to 9 mm | N.a. | Lymph nodes, bones, liver, pleura, peritoneum |
| 5 | F | 67 | Sep-16 | Poorly differentiated small cell neuroendocrine carcinoma | Upper, right | No | 53 mm | N.a. | Lymph nodes, liver |
| Patient no. | Grading | Ki67 | Intrinsic Subtype | Immunohistochemistry | Transcription Factors |
|---|---|---|---|---|---|
| 1 | G2 | 6% | 100% ER, 40% PR, Her2neu- | Synaptophysin+, chromogranin slightly+, CK-MNF116+, CK18+, SSTR2A+ | GATA3+, TTF-1-, CDX-2-, Islet1- |
| 2 | G2 | <5% | >80% ER, >90% PR, Her2neu- | Synaptophysin+, chromogranin slightly + CK18+, SSTR2A+ | GATA3+, TTF-1-, CDX-2-, Islet1- |
| 3 | G2 | 5% | 100% ER, 100% PR, Her2neu +1 | Synaptophysin+, chromogranin+, CK5/6-CK14- | N.a. |
| 4 | G3 | 47% | 0%ER, 15% PR, Her2neu- | Synaptophysin+, chromogranin-, MNF116+, CD 56 +, AE1/3+, E-cadherin+, CD3-, CD20- BCL2- | N.a. |
| 5 | G3 | 80% | 0%ER, 0%PR, Her2neu- | Synaptophysin+, chromogranin+, MNF116+ | GATA3+, TTF-1-, CDX2- |
| Patient no. | Surgery | Radiotherapy | Antihormone Therapy | Chemotherapy | Follow-Up 01/2020 |
|---|---|---|---|---|---|
| 1 | Partial mastectomy, lymphadenectomy, R0 | No | No | No | Alive, CR |
| 2 | Partial mastectomy, sentinel lymph node resection, R0 | Yes (15cycles) | Yes | No | Alive, CR |
| 3 | Partial mastectomy, sentinel lymph node resection, R0 | Yes | No | No | Alive, CR |
| 4 | No | No | No | 06/2018–02/2019 Carboplatin (300/m2)/Etoposide(120/m2) | Death Feb-2019 |
| 5 | No | No | No | 09/16–12/16: 5 cycles Cisplatin (80/m2) Etoposide (120/m2) 03/17 2nd line with Doxorubicin (16 mg/m2), Cyclophosphamide (750 mg/m2), Vincristine (0.4 mg/m2) in a 80% reduced dose | Death Jul-2017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdirik, B.; Kayser, A.; Ullrich, A.; Savic, L.J.; Reiss, M.; Tacke, F.; Wiedenmann, B.; Jann, H.; Roderburg, C. Primary Neuroendocrine Neoplasms of the Breast: Case Series and Literature Review. Cancers 2020, 12, 733. https://doi.org/10.3390/cancers12030733
Özdirik B, Kayser A, Ullrich A, Savic LJ, Reiss M, Tacke F, Wiedenmann B, Jann H, Roderburg C. Primary Neuroendocrine Neoplasms of the Breast: Case Series and Literature Review. Cancers. 2020; 12(3):733. https://doi.org/10.3390/cancers12030733
Chicago/Turabian StyleÖzdirik, Burcin, Antonin Kayser, Andrea Ullrich, Lynn J. Savic, Markus Reiss, Frank Tacke, Bertram Wiedenmann, Henning Jann, and Christoph Roderburg. 2020. "Primary Neuroendocrine Neoplasms of the Breast: Case Series and Literature Review" Cancers 12, no. 3: 733. https://doi.org/10.3390/cancers12030733
APA StyleÖzdirik, B., Kayser, A., Ullrich, A., Savic, L. J., Reiss, M., Tacke, F., Wiedenmann, B., Jann, H., & Roderburg, C. (2020). Primary Neuroendocrine Neoplasms of the Breast: Case Series and Literature Review. Cancers, 12(3), 733. https://doi.org/10.3390/cancers12030733







