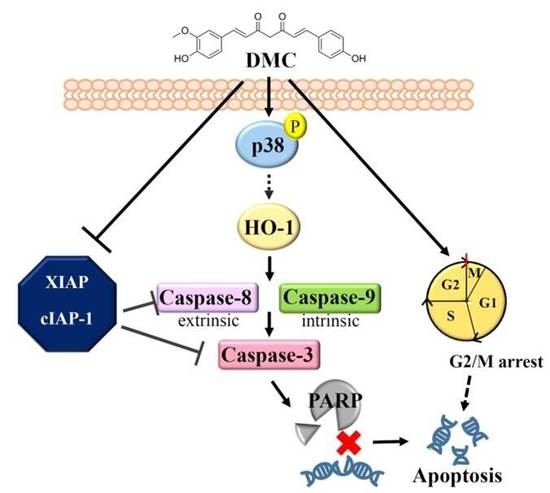
Dual Targeting of the p38 MAPK-HO-1 Axis and cIAP1/XIAP by Demethoxycurcumin Triggers Caspase-Mediated Apoptotic Cell Death in Oral Squamous Cell Carcinoma Cells
Abstract
1. Introduction
2. Results
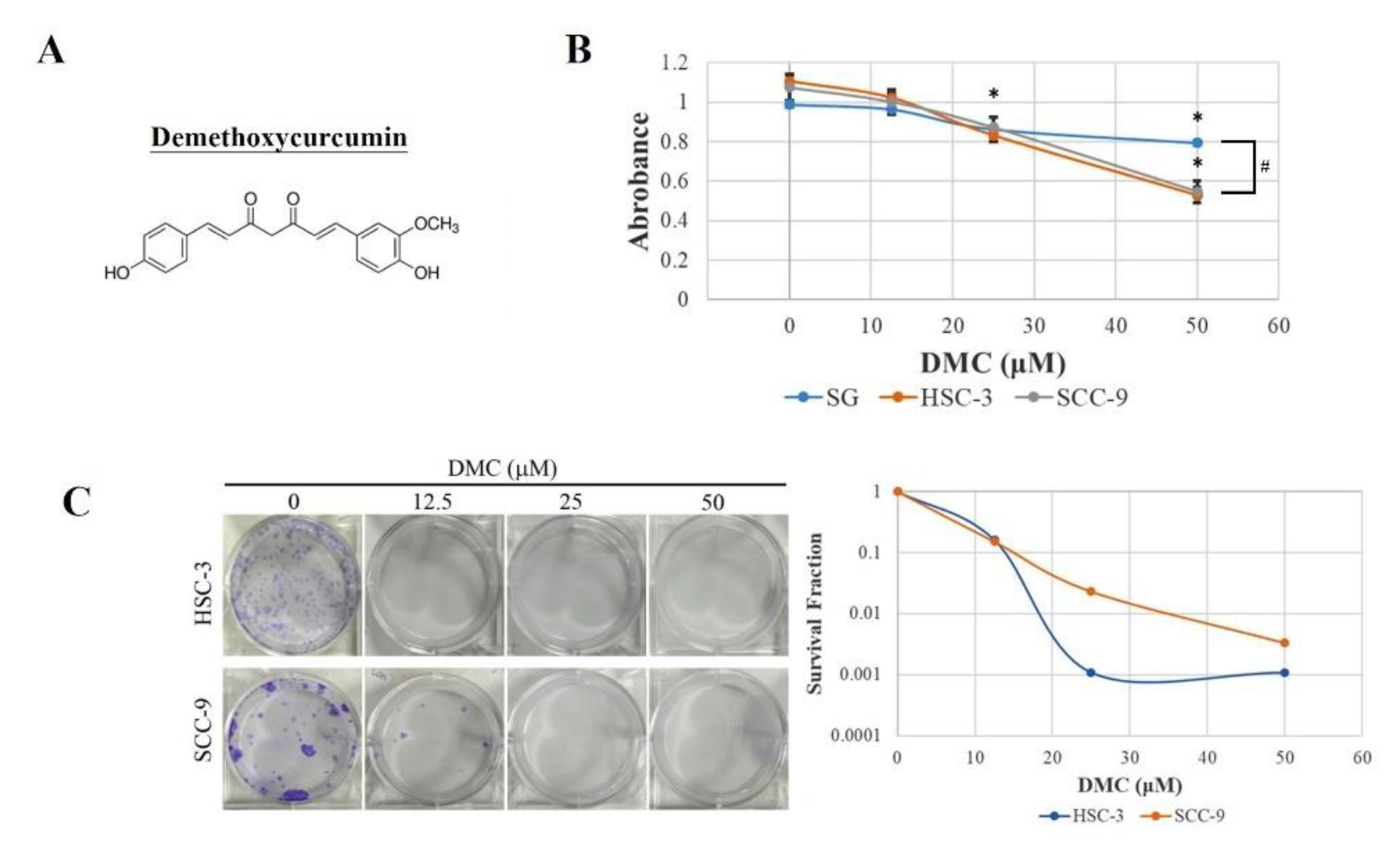
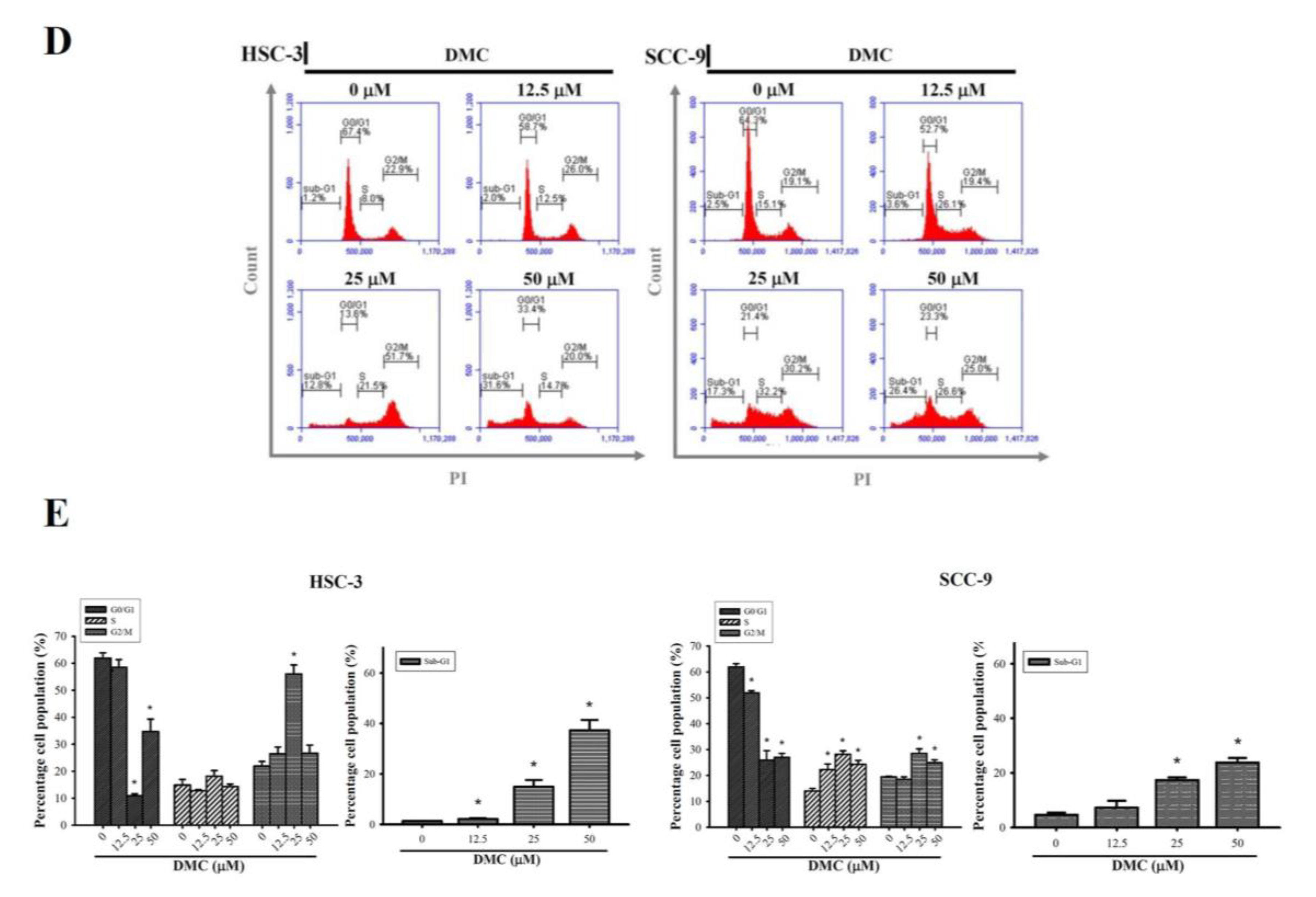
2.1. DMC Exerts Antiproliferative Activity and Causes G2/M Cell Cycle Arrest in OSCC Cells
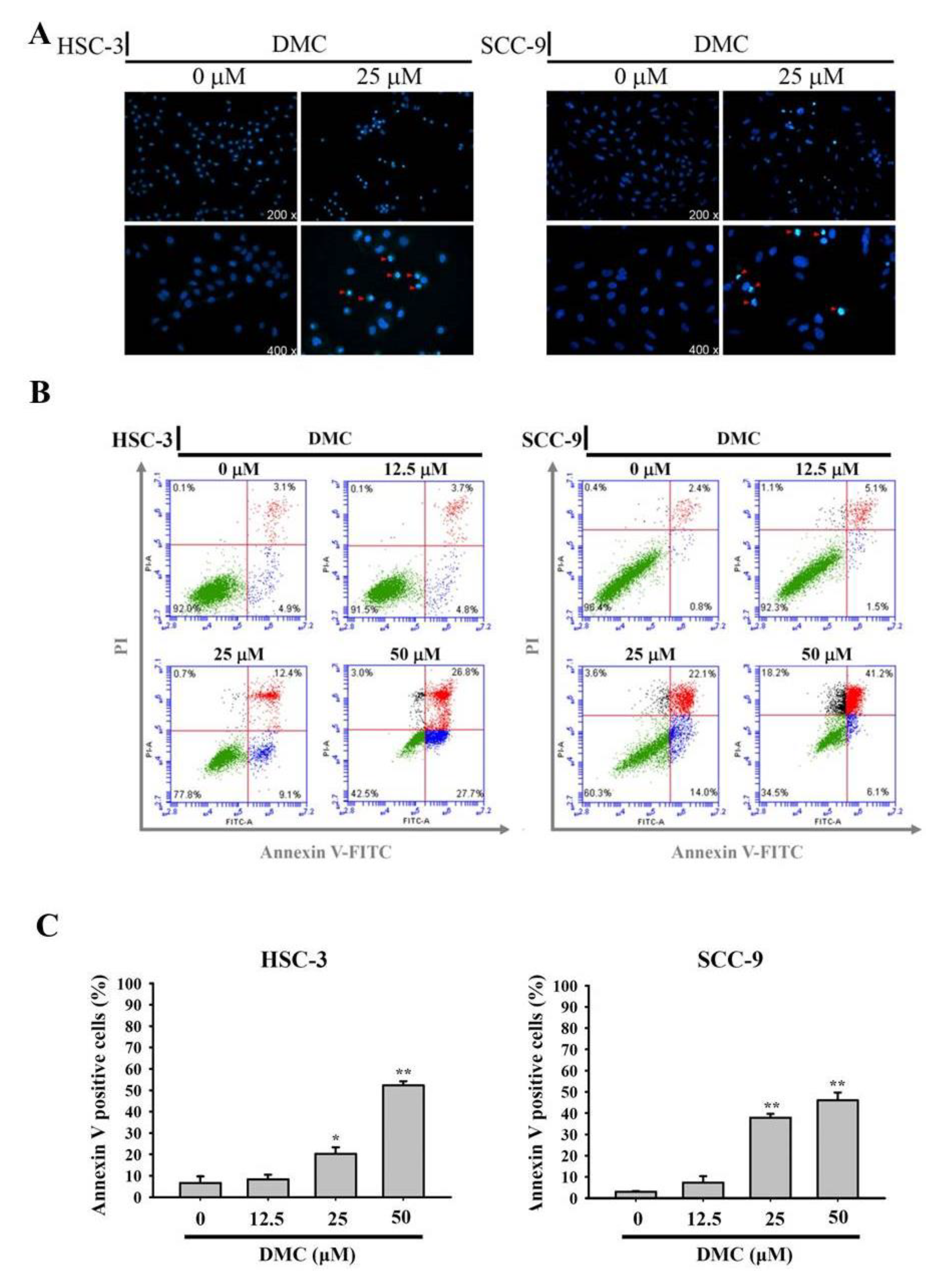
2.2. DMC Treatment Results in the Apoptosis of OSCC Cells
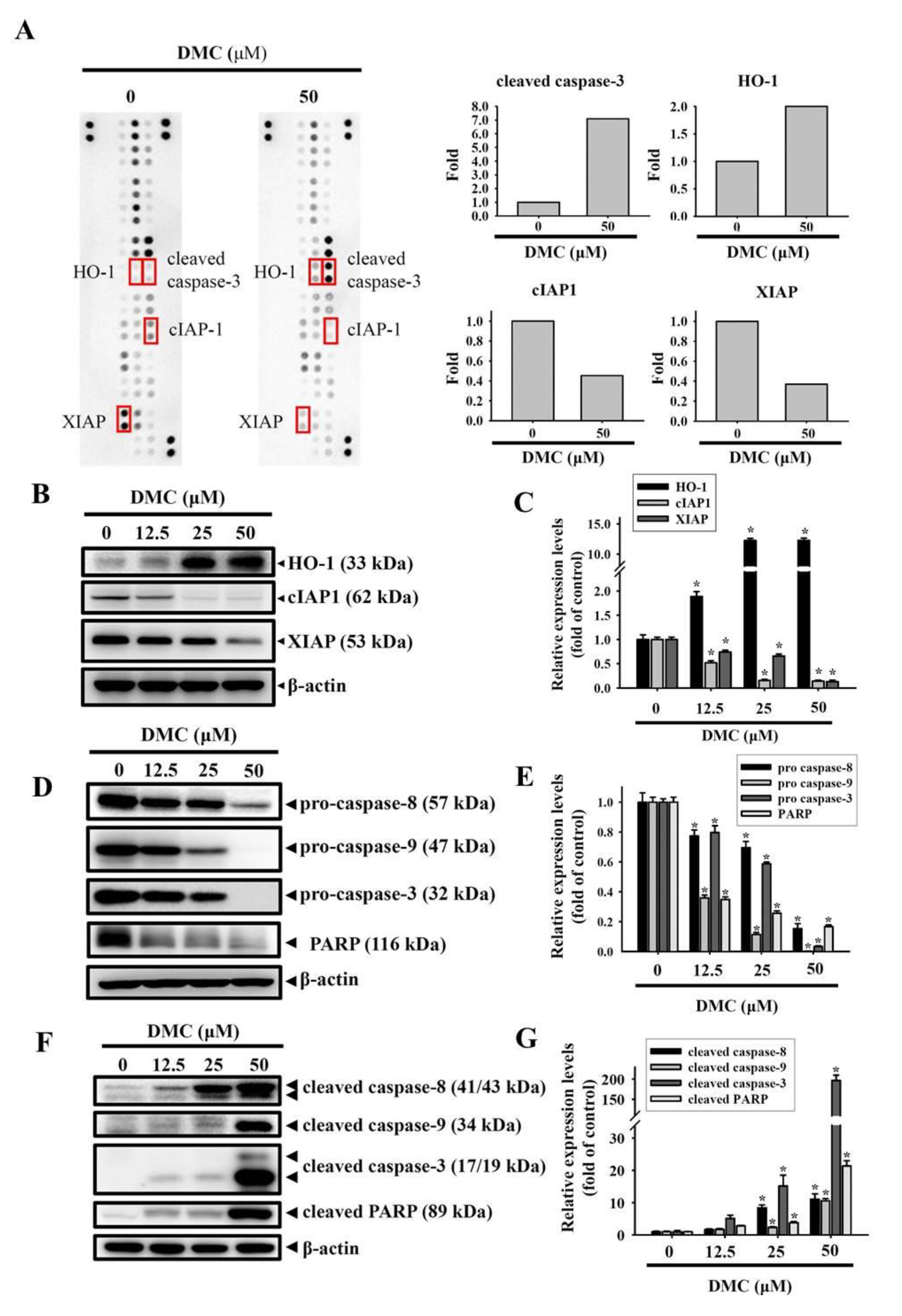
2.3. Targeting of cIAP1 and XIAP by DMC Triggers Caspase-Mediated Apoptotic Cell Death in OSCC
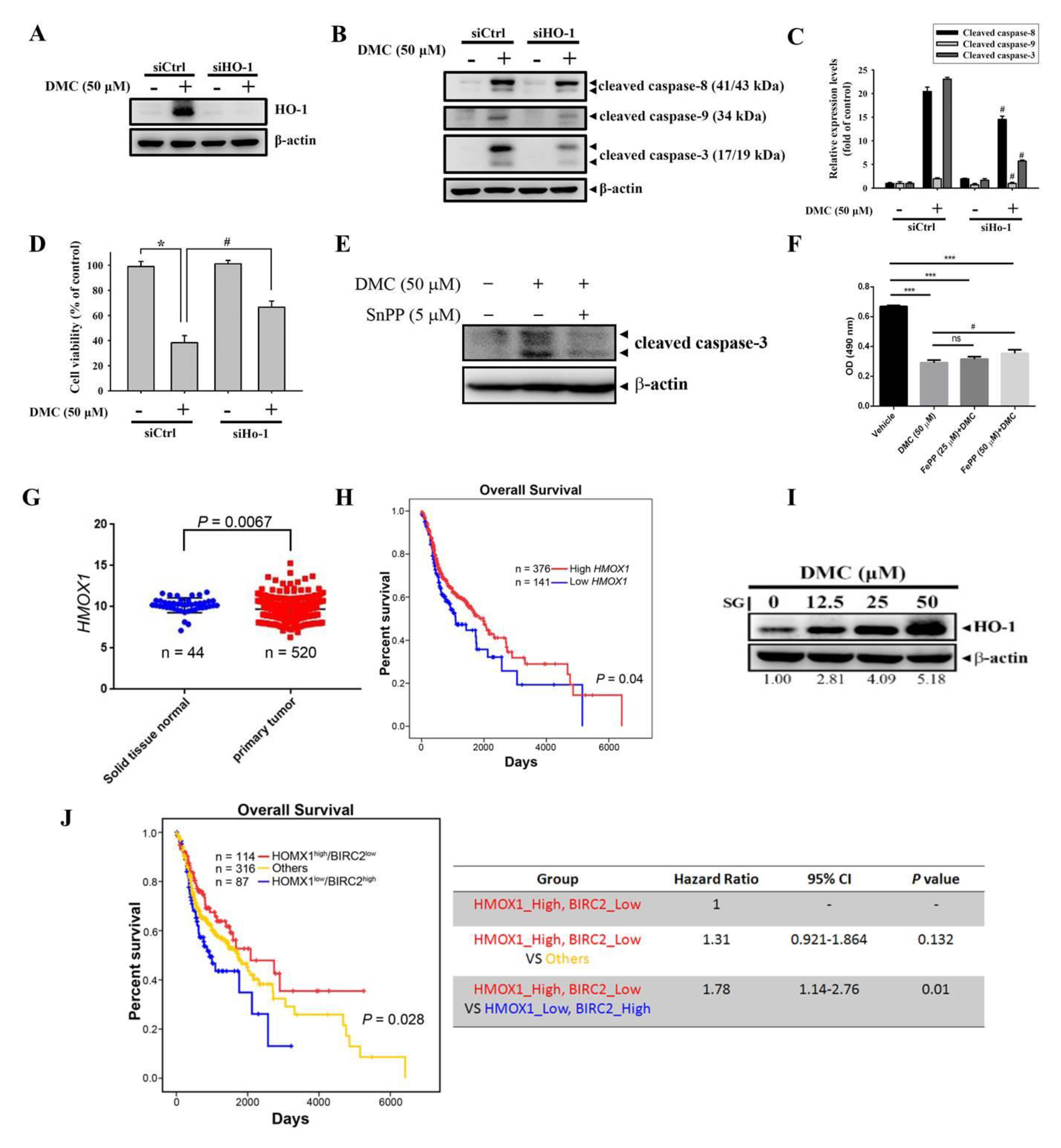
2.4. HO-1 Is a Critical Upstream Regulator Involved in DMC-Induced Caspase-Mediated Apoptotic Cell Death in OSCC Cells
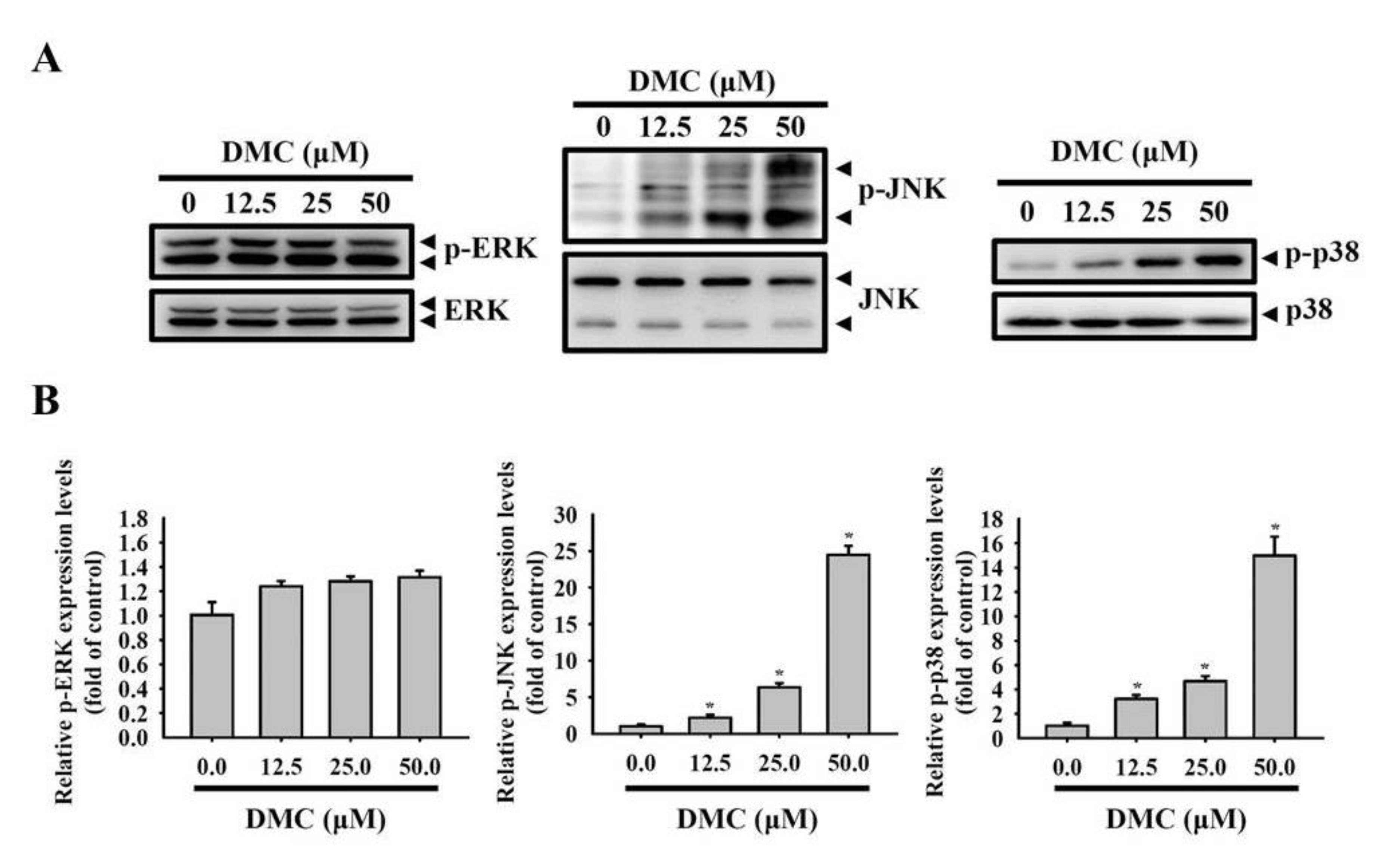
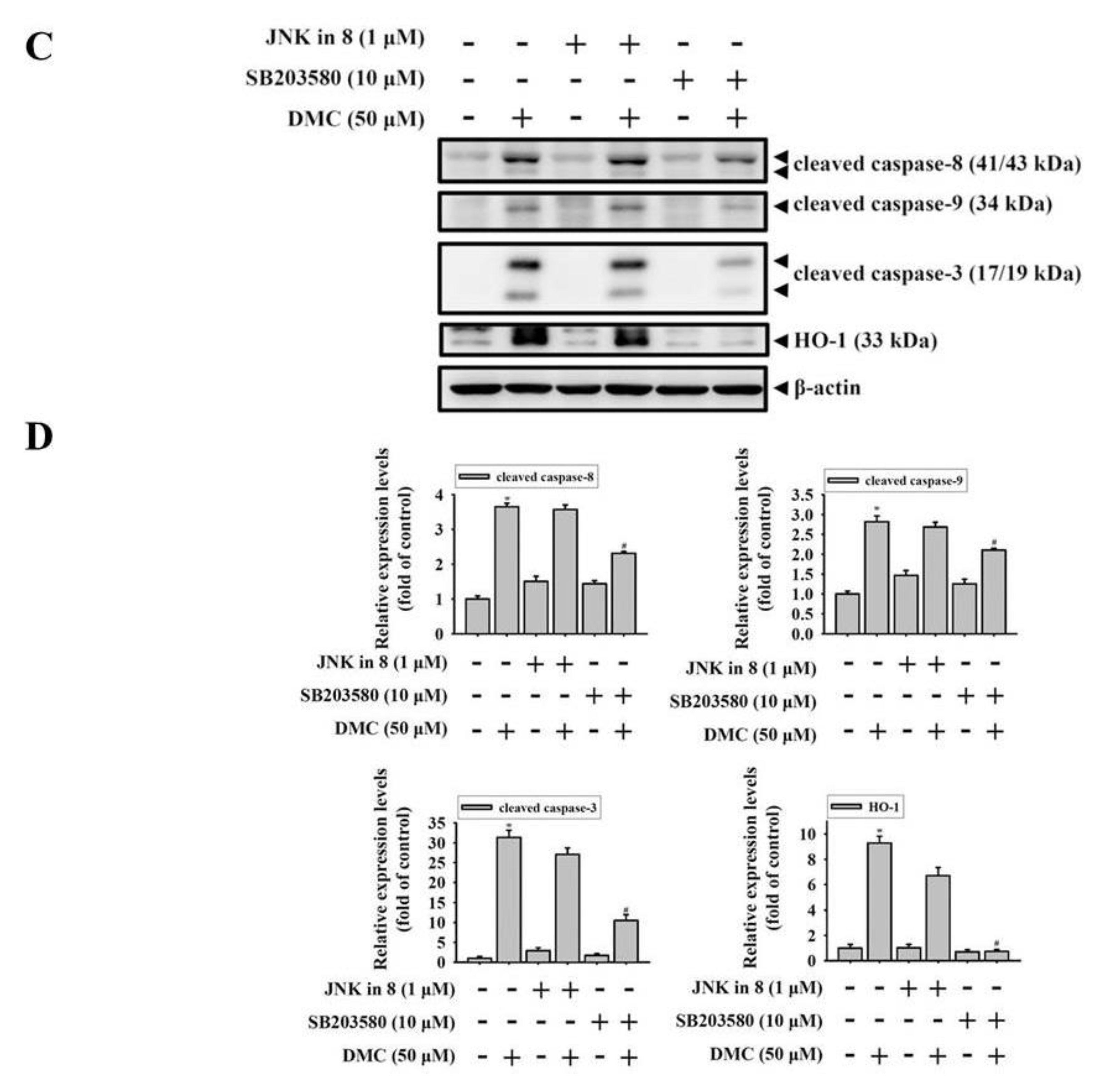
2.5. Activation of the p38 MAPK-HO-1 Signaling Cascade by DMC Triggers Caspase-Mediated Apoptotic Cell Death in OSCC Cells
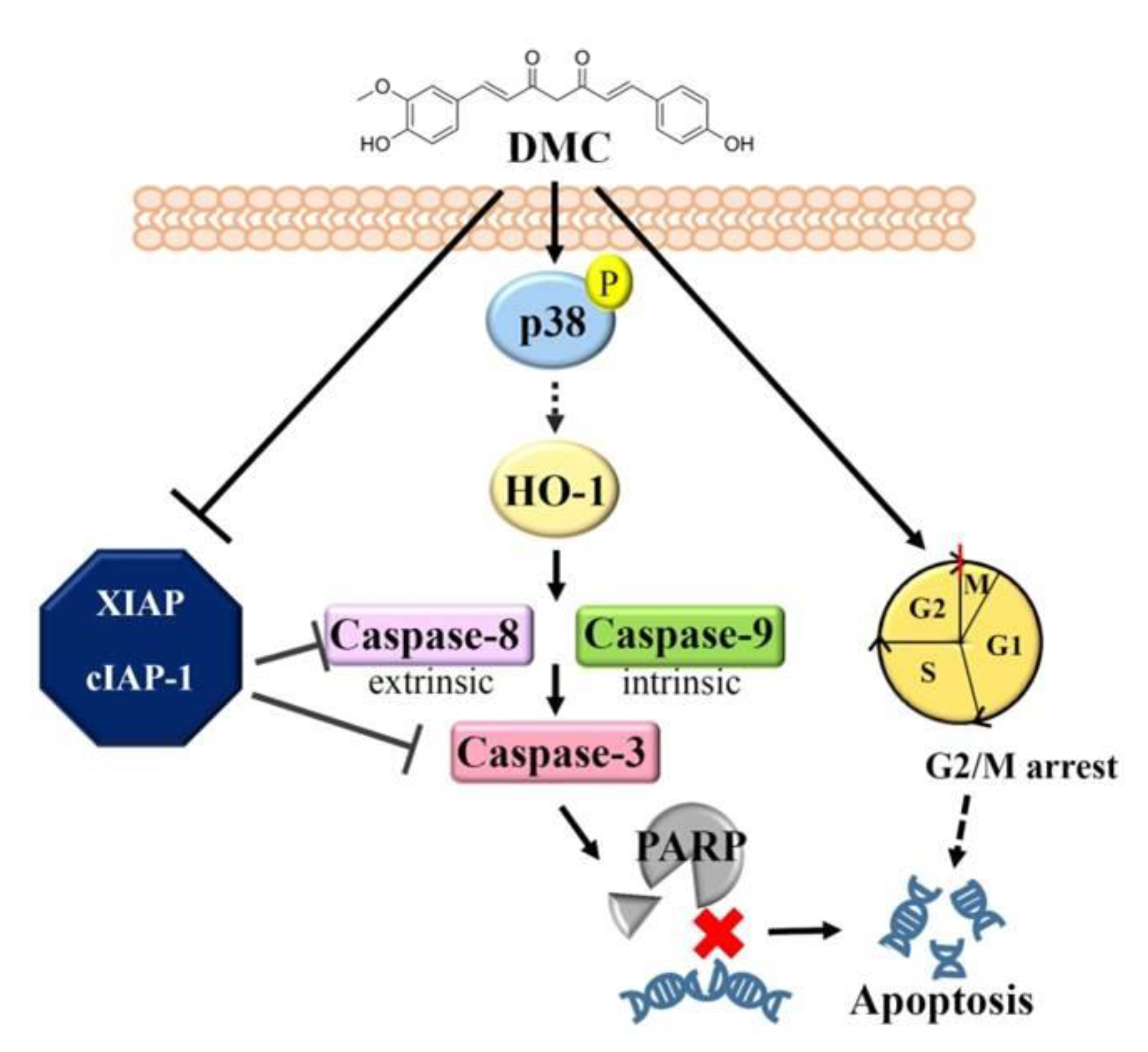
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Cell Viability Assay
4.3. Plate Colony-Formation Assay
4.4. Cell-Cycle Distribution Assay
4.5. Apoptosis Assays
4.6. Nuclear Morphological Analysis by Hoechst 33342
4.7. Human Apoptosis Proteome Profiler Array
4.8. Protein Lysate Preparation and Western Blot Analysis
4.9. Small Interfering (SI)RNA Transfection
4.10. Bioinformatics Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Le Campion, A.; Ribeiro, C.M.B.; Luiz, R.R.; da Silva Junior, F.F.; Barros, H.C.S.; Dos Santos, K.C.B.; Ferreira, S.J.; Goncalves, L.S.; Ferreira, S.M.S. Low Survival Rates of Oral and Oropharyngeal Squamous Cell Carcinoma. Int. J. Dent. 2017, 2017, e5815493. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, T.; Kirita, T. Hallmarks of Cancer-Related Newly Prognostic Factors of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 2413. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Nakayama, H.; Tanaka, T.; Yoshida, R.; Yoshitake, Y.; Fukuma, D.; Kawahara, K.; Nakagawa, Y.; Ota, K.; Hiraki, A.; et al. Overexpression of cIAP2 contributes to 5-FU resistance and a poor prognosis in oral squamous cell carcinoma. Br. J. Cancer 2011, 105, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Mogi, S.; Tsuda, H.; Tanaka, Y.; Kozaki, K.; Imoto, I.; Inazawa, J.; Hasegawa, S.; Omura, K. Expression of cIAP-1 correlates with nodal metastasis in squamous cell carcinoma of the tongue. Int. J. Oral Maxillofac. Surg. 2008, 37, 1047–1053. [Google Scholar] [CrossRef]
- Tanimoto, T.; Tsuda, H.; Imazeki, N.; Ohno, Y.; Imoto, I.; Inazawa, J.; Matsubara, O. Nuclear expression of cIAP-1, an apoptosis inhibiting protein, predicts lymph node metastasis and poor patient prognosis in head and neck squamous cell carcinomas. Cancer Lett. 2005, 224, 141–151. [Google Scholar] [CrossRef]
- Yang, X.H.; Feng, Z.E.; Yan, M.; Hanada, S.; Zuo, H.; Yang, C.Z.; Han, Z.G.; Guo, W.; Chen, W.T.; Zhang, P. XIAP is a predictor of cisplatin-based chemotherapy response and prognosis for patients with advanced head and neck cancer. PLoS ONE 2012, 7, e31601. [Google Scholar] [CrossRef]
- Scheurer, M.J.J.; Seher, A.; Steinacker, V.; Linz, C.; Hartmann, S.; Kubler, A.C.; Muller-Richter, U.D.A.; Brands, R.C. Targeting inhibitors of apoptosis in oral squamous cell carcinoma in vitro. J. Craniomaxillofac Surg. 2019. [Google Scholar] [CrossRef]
- Xiao, R.; An, Y.; Ye, W.; Derakhshan, A.; Cheng, H.; Yang, X.; Allen, C.; Chen, Z.; Schmitt, N.C.; Van Waes, C. Dual Antagonist of cIAP/XIAP ASTX660 Sensitizes HPV(−) and HPV(+) Head and Neck Cancers To TNFalpha, TRAIL, and Radiation Therapy. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Podkalicka, P.; Mucha, O.; Jozkowicz, A.; Dulak, J.; Loboda, A. Heme oxygenase inhibition in cancers: Possible tools and targets. Contemp. Oncol. (Pozn) 2018, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Berberat, P.O.; Dambrauskas, Z.; Gulbinas, A.; Giese, T.; Giese, N.; Kunzli, B.; Autschbach, F.; Meuer, S.; Buchler, M.W.; Friess, H. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin. Cancer Res. 2005, 11, 3790–3798. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Cichon, T.; Smolarczyk, R.; Rudnicka, D.; Stopa, M.; Chevalier, C.; Leger, J.J.; Lackowska, B.; Grochot, A.; Bojkowska, K.; et al. Overexpression of heme oxygenase-1 in murine melanoma: Increased proliferation and viability of tumor cells, decreased survival of mice. Am. J. Pathol. 2006, 169, 2181–2198. [Google Scholar] [CrossRef] [PubMed]
- Ciesla, M.; Marona, P.; Kozakowska, M.; Jez, M.; Seczynska, M.; Loboda, A.; Bukowska-Strakova, K.; Szade, A.; Walawender, M.; Kusior, M.; et al. Heme Oxygenase-1 Controls an HDAC4-miR-206 Pathway of Oxidative Stress in Rhabdomyosarcoma. Cancer Res. 2016, 76, 5707–5718. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zou, C.; Cheng, W.; Li, Q.; Han, Z.; Wang, X.; Jin, J.; Zou, J.; Liu, Z.; Zhou, Z.; et al. Heme oxygenase-1 retards hepatocellular carcinoma progression through the microRNA pathway. Oncol. Rep. 2016, 36, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Pereira, V.; Chauveau, C.; Zagani, R.; Remy, S.; Tesson, L.; Mazal, D.; Ubillos, L.; Brion, R.; Asghar, K.; et al. Heme oxygenase-1 inhibits rat and human breast cancer cell proliferation: Mutual cross inhibition with indoleamine 2,3-dioxygenase. FASEB J. 2005, 19, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Tertil, M.; Golda, S.; Ciesla, M.; Weglarczyk, K.; Collet, G.; Guichard, A.; Kozakowska, M.; Boczkowski, J.; Was, H.; et al. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid. Redox Signal. 2013, 19, 644–660. [Google Scholar] [CrossRef]
- Yanagawa, T.; Omura, K.; Harada, H.; Nakaso, K.; Iwasa, S.; Koyama, Y.; Onizawa, K.; Yusa, H.; Yoshida, H. Heme oxygenase-1 expression predicts cervical lymph node metastasis of tongue squamous cell carcinomas. Oral Oncol. 2004, 40, 21–27. [Google Scholar] [CrossRef]
- Chen, H.W.; Huang, H.C. Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br. J. Pharmacol. 1998, 124, 1029–1040. [Google Scholar] [CrossRef]
- Diaz Osterman, C.J.; Gonda, A.; Stiff, T.; Sigaran, U.; Valenzuela, M.M.; Ferguson Bennit, H.R.; Moyron, R.B.; Khan, S.; Wall, N.R. Curcumin Induces Pancreatic Adenocarcinoma Cell Death Via Reduction of the Inhibitors of Apoptosis. Pancreas 2016, 45, 101–109. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Tastan, H.; Ozercan, I.H.; Guler, O.; Kahraman, N.; Kucuk, O.; Ozpolat, B. Chemopreventive and Antitumor Efficacy of Curcumin in a Spontaneously Developing Hen Ovarian Cancer Model. Cancer Prev. Res. (Phila) 2018, 11, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar] [PubMed]
- Ireson, C.R.; Jones, D.J.; Orr, S.; Coughtrie, M.W.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomark. Prev. 2002, 11, 105–111. [Google Scholar]
- Luthra, P.M.; Kumar, R.; Prakash, A. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem. Biophys. Res. Commun. 2009, 384, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Yodkeeree, S.; Chaiwangyen, W.; Garbisa, S.; Limtrakul, P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. J. Nutr. Biochem. 2009, 20, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hatamipour, M.; Ramezani, M.; Tabassi, S.A.S.; Johnston, T.P.; Ramezani, M.; Sahebkar, A. Demethoxycurcumin: A naturally occurring curcumin analogue with antitumor properties. J. Cell Physiol. 2018, 233, 9247–9260. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, A.; Chen, Z.; Van Waes, C. Therapeutic Small Molecules Target Inhibitor of Apoptosis Proteins in Cancers with Deregulation of Extrinsic and Intrinsic Cell Death Pathways. Clin. Cancer Res. 2017, 23, 1379–1387. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tan, M.G.; Wong, P.T.; Herr, D.R.; Lai, M.K. Andrographolide induces Nrf2 and heme oxygenase 1 in astrocytes by activating p38 MAPK and ERK. J. Neuroinflammation 2016, 13, 251. [Google Scholar] [CrossRef]
- Nakashima, K.; Sato, T.; Shigemori, S.; Shimosato, T.; Shinkai, M.; Kaneko, T. Regulatory role of heme oxygenase-1 in silica-induced lung injury. Respir. Res. 2018, 19, e144. [Google Scholar] [CrossRef]
- Hung, C.M.; Su, Y.H.; Lin, H.Y.; Lin, J.N.; Liu, L.C.; Ho, C.T.; Way, T.D. Demethoxycurcumin modulates prostate cancer cell proliferation via AMPK-induced down-regulation of HSP70 and EGFR. J. Agric. Food Chem. 2012, 60, 8427–8434. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hung, C.C.; Wang, C.C.N.; Lin, H.Y.; Huang, S.H.; Sheu, M.J. Demethoxycurcumin sensitizes the response of non-small cell lung cancer to cisplatin through downregulation of TP and ERCC1-related pathways. Phytomedicine 2019, 53, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hatamipour, M.; Ramezani, M.; Tabassi, S.A.S.; Johnston, T.P.; Sahebkar, A. Demethoxycurcumin: A naturally occurring curcumin analogue for treating non-cancerous diseases. J. Cell Physiol. 2019, 234, 19320–19330. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Chae, H.D.; Yun, J.; Jung, M.; Kim, Y.S.; Kim, S.H.; Han, M.H.; Shin, D.Y. Constitutive activation of cyclin B1-associated cdc2 kinase overrides p53-mediated G2-M arrest. Cancer Res. 2000, 60, 542–545. [Google Scholar]
- Ni, X.; Zhang, A.; Zhao, Z.; Shen, Y.; Wang, S. Demethoxycurcumin inhibits cell proliferation, migration and invasion in prostate cancer cells. Oncol. Rep. 2012, 28, 85–90. [Google Scholar] [CrossRef]
- Lal, N.; Nemaysh, V.; Luthra, P.M. Proteasome mediated degradation of CDC25C and Cyclin B1 in Demethoxycurcumin treated human glioma U87 MG cells to trigger G2/M cell cycle arrest. Toxicol. Appl. Pharmacol. 2018, 356, 76–89. [Google Scholar] [CrossRef]
- Hsiao, Y.T.; Kuo, C.L.; Chueh, F.S.; Liu, K.C.; Bau, D.T.; Chung, J.G. Curcuminoids Induce Reactive Oxygen Species and Autophagy to Enhance Apoptosis in Human Oral Cancer Cells. Am. J. Chin. Med. 2018, 46, 1145–1168. [Google Scholar] [CrossRef]
- Chen, Y.K.; Huse, S.S.; Lin, L.M. Expression of inhibitor of apoptosis family proteins in human oral squamous cell carcinogenesis. Head Neck 2011, 33, 985–998. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakayama, H.; Yoshitake, Y.; Irie, A.; Nagata, M.; Kawahara, K.; Takamune, Y.; Yoshida, R.; Nakagawa, Y.; Ogi, H.; et al. Selective inhibition of nuclear factor-kappaB by nuclear factor-kappaB essential modulator-binding domain peptide suppresses the metastasis of highly metastatic oral squamous cell carcinoma. Cancer Sci. 2012, 103, 455–463. [Google Scholar] [CrossRef]
- Almeida, L.O.; Abrahao, A.C.; Rosselli-Murai, L.K.; Giudice, F.S.; Zagni, C.; Leopoldino, A.M.; Squarize, C.H.; Castilho, R.M. NFkappaB mediates cisplatin resistance through histone modifications in head and neck squamous cell carcinoma (HNSCC). FEBS Open Bio. 2014, 4, 96–104. [Google Scholar] [CrossRef]
- Duffey, D.C.; Crowl-Bancroft, C.V.; Chen, Z.; Ondrey, F.G.; Nejad-Sattari, M.; Dong, G.; Van Waes, C. Inhibition of transcription factor nuclear factor-kappaB by a mutant inhibitor-kappaBalpha attenuates resistance of human head and neck squamous cell carcinoma to TNF-alpha caspase-mediated cell death. Br. J. Cancer 2000, 83, 1367–1374. [Google Scholar] [CrossRef][Green Version]
- Pae, H.O.; Jeong, G.S.; Jeong, S.O.; Kim, H.S.; Kim, S.A.; Kim, Y.C.; Yoo, S.J.; Kim, H.D.; Chung, H.T. Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp. Mol. Med. 2007, 39, 267–277. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Chu, L.C.; Hua, K.F.; Chao, L.K. Heme oxygenase-1 mediates the anti-inflammatory effect of Curcumin within LPS-stimulated human monocytes. J. Cell Physiol. 2008, 215, 603–612. [Google Scholar] [CrossRef]
- Lee, W.Y.; Chen, Y.C.; Shih, C.M.; Lin, C.M.; Cheng, C.H.; Chen, K.C.; Lin, C.W. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol. Appl. Pharmacol. 2014, 274, 55–62. [Google Scholar] [CrossRef]
- Wu, M.S.; Chien, C.C.; Chang, J.; Chen, Y.C. Pro-apoptotic effect of haem oxygenase-1 in human colorectal carcinoma cells via endoplasmic reticular stress. J. Cell Mol. Med. 2019, 23, 5692–5704. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lee, Y.R.; Huang, C.C.; Li, Y.Z.; Chang, Y.S.; Yang, C.Y.; Wu, J.D.; Liu, Y.W. Curcumin-induced heme oxygenase-1 expression plays a negative role for its anti-cancer effect in bladder cancers. Food Chem. Toxicol. 2012, 50, 3530–3536. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Arch. Biochem. Biophys. 2019, 678, e108186. [Google Scholar] [CrossRef]
- Almeida, A.S.; Queiroga, C.S.; Sousa, M.F.; Alves, P.M.; Vieira, H.L. Carbon monoxide modulates apoptosis by reinforcing oxidative metabolism in astrocytes: Role of Bcl-2. J. Biol. Chem. 2012, 287, 10761–10770. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Chakraborty, I.; Mascharak, P.K. Synthesis and assessment of CO-release capacity of manganese carbonyl complexes derived from rigid alpha-diimine ligands of varied complexity. Eur. J. Inorg. Chem. 2015, 2015, 5021–5026. [Google Scholar] [CrossRef] [PubMed]
- Lundvig, D.M.; Pennings, S.W.; Brouwer, K.M.; Mtaya-Mlangwa, M.; Mugonzibwa, E.; Kuijpers-Jagtman, A.M.; Wagener, F.A.; Von den Hoff, J.W. Cytoprotective responses in HaCaT keratinocytes exposed to high doses of curcumin. Exp. Cell Res. 2015, 336, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, X.; Wang, Z.; Zeng, X.; Hu, Z.; Ye, Z.; Shen, G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des. Devel. Ther. 2017, 11, 431–439. [Google Scholar] [CrossRef] [PubMed]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 2007, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.A.; Noguti, J.; Oshima, C.T.; Ribeiro, D.A. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral cancer: A promising approach. Anticancer Res. 2014, 34, 1547–1552. [Google Scholar] [PubMed]
- Hoch, M.A.; Cousins, K.; Nartey, R.; Riley, K.; Hartranft, M. Two cases of combination therapy with cetuximab, paclitaxel, and cisplatin for advanced head and neck cancer. J. Oncol. Pharm. Pract. 2018, 24, 553–554. [Google Scholar] [CrossRef]
- Guigay, J.; Even, C.; Mayache-Badis, L.; Debbah, M.; Saada-Bouzid, E.; Tao, Y.; Deschamps, F.; Janot, F.; Lezghed, N.; Michel, C. Long-term response in patient with recurrent oropharyngeal carcinoma treated with cetuximab, docetaxel and cisplatin (TPEx) as first-line treatment followed by cetuximab maintenance. Oral Oncol. 2017, 68, 114–118. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.M.; Chang, G.C.; Yu, S.L.; Hsieh, W.Y.; Chen, J.J.; Chen, H.W.; Yang, P.C. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: The versatile adjuvant for gefitinib therapy. PLoS ONE 2011, 6, e23756. [Google Scholar] [CrossRef]
- Chen, C.F.; Lu, C.C.; Chiang, J.H.; Chiu, H.Y.; Yang, J.S.; Lee, C.Y.; Way, T.D.; Huang, H.J. Synergistic inhibitory effects of cetuximab and curcumin on human cisplatin-resistant oral cancer CAR cells through intrinsic apoptotic process. Oncol. Lett. 2018, 16, 6323–6330. [Google Scholar] [CrossRef]
- Wada, K.; Lee, J.Y.; Hung, H.Y.; Shi, Q.; Lin, L.; Zhao, Y.; Goto, M.; Yang, P.C.; Kuo, S.C.; Chen, H.W.; et al. Novel curcumin analogs to overcome EGFR-TKI lung adenocarcinoma drug resistance and reduce EGFR-TKI-induced GI adverse effects. Bioorg. Med. Chem. 2015, 23, 1507–1514. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, W.; Da, J.; Xu, M.; Wang, Y.; Zhou, J.; Wang, Z. Bisdemethoxycurcumin in combination with alpha-PD-L1 antibody boosts immune response against bladder cancer. Onco Targets Ther. 2017, 10, 2675–2683. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Chin, M.C.; Lin, C.C.; His, Y.T.; Lo, Y.S.; Chuang, Y.C.; Chen, M.K. Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway. Cell Physiol. Biochem. 2018, 50, 911–923. [Google Scholar] [CrossRef]
- Chien, M.H.; Lee, W.J.; Yang, Y.C.; Tan, P.; Pan, K.F.; Liu, Y.C.; Tsai, H.C.; Hsu, C.H.; Wen, Y.C.; Hsiao, M.; et al. N-alpha-acetyltransferase 10 protein promotes metastasis by stabilizing matrix metalloproteinase-2 protein in human osteosarcomas. Cancer Lett. 2018, 433, 86–98. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, M.-H.; Yang, W.-E.; Yang, Y.-C.; Ku, C.-C.; Lee, W.-J.; Tsai, M.-Y.; Lin, C.-W.; Yang, S.-F. Dual Targeting of the p38 MAPK-HO-1 Axis and cIAP1/XIAP by Demethoxycurcumin Triggers Caspase-Mediated Apoptotic Cell Death in Oral Squamous Cell Carcinoma Cells. Cancers 2020, 12, 703. https://doi.org/10.3390/cancers12030703
Chien M-H, Yang W-E, Yang Y-C, Ku C-C, Lee W-J, Tsai M-Y, Lin C-W, Yang S-F. Dual Targeting of the p38 MAPK-HO-1 Axis and cIAP1/XIAP by Demethoxycurcumin Triggers Caspase-Mediated Apoptotic Cell Death in Oral Squamous Cell Carcinoma Cells. Cancers. 2020; 12(3):703. https://doi.org/10.3390/cancers12030703
Chicago/Turabian StyleChien, Ming-Hsien, Wei-En Yang, Yi-Chieh Yang, Chia-Chi Ku, Wei-Jiunn Lee, Meng-Ying Tsai, Chiao-Wen Lin, and Shun-Fa Yang. 2020. "Dual Targeting of the p38 MAPK-HO-1 Axis and cIAP1/XIAP by Demethoxycurcumin Triggers Caspase-Mediated Apoptotic Cell Death in Oral Squamous Cell Carcinoma Cells" Cancers 12, no. 3: 703. https://doi.org/10.3390/cancers12030703
APA StyleChien, M.-H., Yang, W.-E., Yang, Y.-C., Ku, C.-C., Lee, W.-J., Tsai, M.-Y., Lin, C.-W., & Yang, S.-F. (2020). Dual Targeting of the p38 MAPK-HO-1 Axis and cIAP1/XIAP by Demethoxycurcumin Triggers Caspase-Mediated Apoptotic Cell Death in Oral Squamous Cell Carcinoma Cells. Cancers, 12(3), 703. https://doi.org/10.3390/cancers12030703