Imaging Features and Patterns of Metastasis in Non-Small Cell Lung Cancer with RET Rearrangements
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
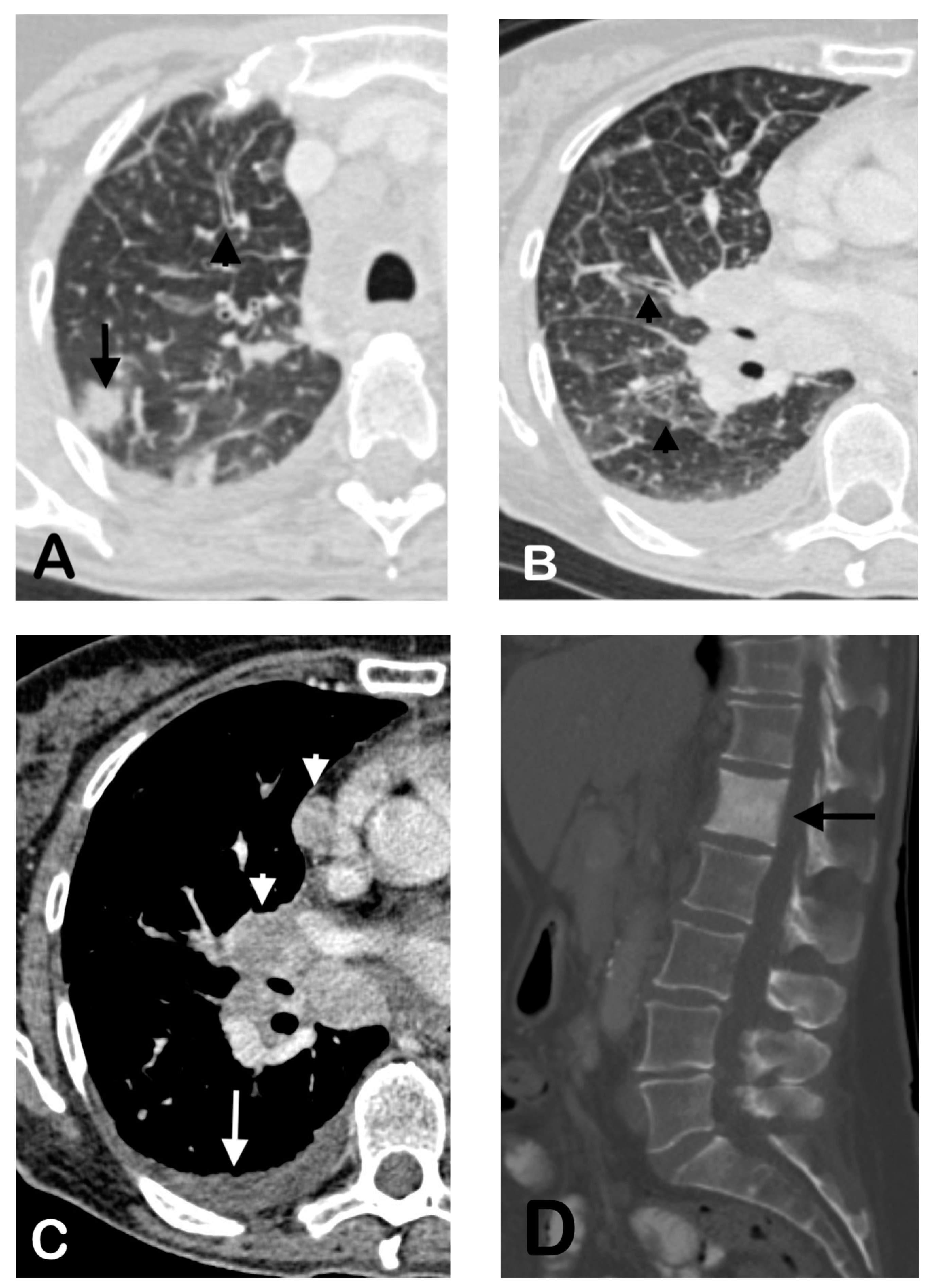
2.2. Imaging Features of the Primary Tumor
2.3. Patterns of Metastases
3. Discussion
4. Materials and Methods
4.1. Patient Identification
4.2. Imaging Review and Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Shaw, A.T. Recent advances in targeting ROS1 in lung cancer. J. Thorac. Oncol. 2017, 12, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Riely, G.J.; Shaw, A.T. Targeting ALK: Precision medicine takes on drug resistance. Cancer Discov. 2017, 7, 137–155. [Google Scholar] [CrossRef]
- Ju, Y.S.; Lee, W.-C.; Shin, J.-Y.; Lee, S.; Bleazard, T.; Won, J.-K.; Kim, Y.T.; Kim, J.-I.; Kang, J.-H.; Seo, J.-S. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012, 22, 436–445. [Google Scholar] [CrossRef]
- Kohno, T.; Ichikawa, H.; Totoki, Y.; Yasuda, K.; Hiramoto, M.; Nammo, T.; Sakamoto, H.; Tsuta, K.; Furuta, K.; Shimada, Y.; et al. KIF5B-RET fusions in lung adenocarcinoma. Nat. Med. 2012, 18, 375–377. [Google Scholar] [CrossRef]
- Lipson, D.; Capelletti, M.; Yelensky, R.; Otto, G.; Parker, A.; Jarosz, M.; Curran, J.A.; Balasubramanian, S.; Bloom, T.; Brennan, K.W.; et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat. Med. 2012, 18, 382–384. [Google Scholar] [CrossRef]
- Wang, R.; Hu, H.; Pan, Y.; Li, Y.; Ye, T.; Li, C.; Luo, X.; Wang, L.; Li, H.; Zhang, Y.; et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 4352–4359. [Google Scholar] [CrossRef]
- Bergethon, K.; Shaw, A.T.; Ignatius Ou, S.-H.; Katayama, R.; Lovly, C.M.; McDonald, N.T.; Massion, P.P.; Siwak-Tapp, C.; Gonzalez, A.; Fang, R.; et al. ROS1 Rearrangements define a unique molecular class of lung cancers. J. Clin. Oncol. 2012, 30, 863–870. [Google Scholar] [CrossRef]
- Gautschi, O.; Milia, J.; Filleron, T.; Wolf, J.; Carbone, D.P.; Owen, D.; Camidge, R.; Narayanan, V.; Doebele, R.C.; Besse, B.; et al. Targeting RET in patients with RET-rearranged lung cancers: Results from the global, multicenter RET registry. J. Clin. Oncol. 2017, 35, 1403–1410. [Google Scholar] [CrossRef]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Ordóñez, N.G.; Bastida, C.C.; Stephens, P.J.; Miller, V.A.; Gaido, L.; Jackson, T.; Karp, D.D. Effect of the RET inhibitor vandetanib in a patient with RET fusion-positive metastatic non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, e141–e144. [Google Scholar] [CrossRef] [PubMed]
- Li, G.G.; Somwar, R.; Joseph, J.; Smith, R.S.; Hayashi, T.; Martin, L.; Franovic, A.; Schairer, A.; Martin, E.; Riely, G.J.; et al. Antitumor activity of RXDX-105 in multiple cancer types with RET rearrangements or mutations. Clin. Cancer Res. 2017, 23, 2981–2990. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Murayama, T.; Yoshimura, K.; Kawakami, T.; Takahara, S.; Imai, Y.; Kuribayashi, Y.; Nagase, K.; Goto, K.; Nishio, M.; et al. Phase I/II study of alectinib in lung cancer with RET fusion gene: Study protocol. J. Med. Investig. 2017, 64, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Velcheti, V.; Tuch, B.B.; Ebata, K.; Busaidy, N.L.; Cabanillas, M.E.; Wirth, L.J.; Stock, S.; Smith, S.; Lauriault, V.; et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol. 2018, 29, 1869–1876. [Google Scholar] [CrossRef]
- Drilon, A.; Rekhtman, N.; Arcila, M.; Wang, L.; Ni, A.; Albano, M.; Van Voorthuysen, M.; Somwar, R.; Smith, R.S.; Montecalvo, J.; et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016, 17, 1653–1660. [Google Scholar] [CrossRef]
- Horiike, A.; Takeuchi, K.; Uenami, T.; Kawano, Y.; Tanimoto, A.; Kaburaki, K.; Tambo, Y.; Kudo, K.; Yanagitani, N.; Ohyanagi, F.; et al. Sorafenib treatment for patients with RET fusion-positive non-small cell lung cancer. Lung Cancer 2016, 93, 43–46. [Google Scholar] [CrossRef]
- Lin, J.J.; Kennedy, E.; Sequist, L.V.; Brastianos, P.K.; Goodwin, K.E.; Stevens, S.; Wanat, A.C.; Stober, L.L.; Digumarthy, S.R.; Engelman, J.A.; et al. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J. Thorac. Oncol. 2016, 11, 2027–2032. [Google Scholar] [CrossRef]
- Drilon, A.; Fu, S.; Patel, M.R.; Fakih, M.; Wang, D.; Olszanski, A.J.; Morgensztern, D.; Liu, S.V.; Cho, B.C.; Bazhenova, L.; et al. A phase I/Ib trial of the VEGFR-sparing multikinase RET inhibitor RXDX-105. Cancer Discov. 2019, 9, 384–395. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, J.-K.; Ahn, M.-J.; Kim, D.-W.; Sun, J.-M.; Keam, B.; Kim, T.M.; Heo, D.S.; Ahn, J.S.; Choi, Y.-L.; et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: A phase II clinical trial. Ann. Oncol. 2017, 28, 292–297. [Google Scholar] [CrossRef]
- Yoh, K.; Seto, T.; Satouchi, M.; Nishio, M.; Yamamoto, N.; Murakami, H.; Nogami, N.; Matsumoto, S.; Kohno, T.; Tsuta, K.; et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): An open-label, multicentre phase 2 trial. Lancet Respir. Med. 2017, 5, 42–50. [Google Scholar] [CrossRef]
- Subbiah, V.; Gainor, J.F.; Rahal, R.; Brubaker, J.D.; Kim, J.L.; Maynard, M.; Hu, W.; Cao, Q.; Sheets, M.P.; Wilson, D.; et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018, 8, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Lee, D.H.; Curigliano, G.; Doebele, R.C.; Kim, D.-W.; Baik, C.S.; Tan, D.S.-W.; Lopes, G.; Gadgeel, S.M.; Cassier, P.A.; et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2019, 37, 9008. [Google Scholar] [CrossRef]
- Subbiah, V.; Taylor, M.; Lin, J.; Hu, M.; Ou, S.-H.I.; Brose, M.S.; Garralda, E.; Clifford, C.; Palmer, M.; Evans, E.; et al. Abstract CT043: Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in a phase I study of advanced, RET-altered solid tumors. Cancer Res. 2018, 78, CT043. [Google Scholar]
- Drilon, A.E.; Subbiah, V.; Oxnard, G.R.; Bauer, T.M.; Velcheti, V.; Lakhani, N.; Besse, B.; Park, K.; Patel, J.D.; Cabanillas, M.E.; et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J. Clin. Oncol. 2018, 36, 102. [Google Scholar] [CrossRef]
- Mendoza, D.P.; Dagogo-Jack, I.; Chen, T.; Padole, A.; Shepard, J.-A.O.; Shaw, A.T.; Digumarthy, S.R. Imaging characteristics of BRAF-mutant non-small cell lung cancer by functional class. Lung Cancer 2019, 129, 80–84. [Google Scholar] [CrossRef]
- Mendoza, D.P.; Stowell, J.; Muzikansky, A.; Shepard, J.-A.O.; Shaw, A.T.; Digumarthy, S.R. Computed tomography imaging characteristics of non-small-cell lung cancer with anaplastic lymphoma kinase rearrangements: A systematic review and meta-analysis. Clin. Lung Cancer 2019, 20, 339–349. [Google Scholar] [CrossRef]
- Digumarthy, S.R.; Mendoza, D.P.; Padole, A.; Chen, T.; Peterson, P.G.; Piotrowska, Z.; Sequist, L.V. Diffuse lung metastases in EGFR-mutant non-small cell lung cancer. Cancers 2019, 11, 1360. [Google Scholar] [CrossRef]
- Mendoza, D.P.; Lin, J.J.; Rooney, M.M.; Chen, T.; Sequist, L.V.; Shaw, A.T.; Digumarthy, S.R. Imaging features and metastatic patterns of advanced ALK-rearranged non-small cell lung cancer. Am. J. Roentgenol. 2019. [Google Scholar] [CrossRef]
- Digumarthy, S.R.; Mendoza, D.P.; Zhang, E.W.; Lennerz, J.K.; Heist, R.S. Clinicopathologic and imaging features of non-small-cell lung cancer with MET exon 14 skipping mutations. Cancers 2019, 11, 2033. [Google Scholar] [CrossRef]
- Yoon, H.J.; Sohn, I.; Cho, J.H.; Lee, H.Y.; Kim, J.-H.; Choi, Y.-L.; Kim, H.; Lee, G.; Lee, K.S.; Kim, J. Decoding tumor phenotypes for ALK, ROS1, and RET fusions in lung adenocarcinoma using a radiomics approach. Medicine (Baltimore) 2015, 94, e1753. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Yatabe, Y.; Kobayashi, Y.; Tomizawa, K.; Ito, S.; Hatooka, S.; Matsuo, K.; Mitsudomi, T. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer 2012, 77, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kobayashi, Y.; Urayama, K.Y.; Yamaura, H.; Yatabe, Y.; Hida, T. Imaging characteristics of driver mutations in EGFR, KRAS, and ALK among treatment-naïve patients with advanced lung adenocarcinoma. PLoS ONE 2016, 11, e0161081. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Tseng, D.; Yoda, S.; Dagogo-Jack, I.; Friboulet, L.; Lin, J.J.; Hubbeling, H.G.; Dardaei, L.; Farago, A.F.; Schultz, K.R.; et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non–small-cell lung cancer. JCO Precis. Oncol. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Lin, J.J.; Filleron, T.; Ni, A.; Milia, J.; Bergagnini, I.; Hatzoglou, V.; Velcheti, V.; Offin, M.; Li, B.; et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J. Thorac. Oncol. 2018, 13, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Digumarthy, S.R.; Padole, A.M.; Gullo, R.L.; Sequist, L.V.; Kalra, M.K. Can CT radiomic analysis in NSCLC predict histology and EGFR mutation status? Medicine (Baltimore) 2019, 98, e13963. [Google Scholar] [CrossRef]
- Yamamoto, S.; Korn, R.L.; Oklu, R.; Migdal, C.; Gotway, M.B.; Weiss, G.J.; Iafrate, A.J.; Kim, D.-W.; Kuo, M.D. ALK molecular phenotype in non-small cell lung cancer: CT radiogenomic characterization. Radiology 2014, 272, 568–576. [Google Scholar] [CrossRef]
- Plodkowski, A.J.; Drilon, A.; Halpenny, D.F.; O’Driscoll, D.; Blair, D.; Litvak, A.M.; Zheng, J.; Moskowitz, C.S.; Ginsberg, M.S. From genotype to phenotype: Are there imaging characteristics associated with lung adenocarcinomas harboring RET and ROS1 rearrangements? Lung Cancer 2015, 90, 321–325. [Google Scholar] [CrossRef][Green Version]
- Saiki, M.; Kitazono, S.; Yoshizawa, T.; Dotsu, Y.; Ariyasu, R.; Koyama, J.; Sonoda, T.; Uchibori, K.; Nishikawa, S.; Yanagitani, N.; et al. Characterization of computed tomography imaging of rearranged during transfection-rearranged lung cancer. Clin. Lung Cancer 2018, 19, 435–440. [Google Scholar] [CrossRef]
- Digumarthy, S.R.; Mendoza, D.P.; Lin, J.J.; Chen, T.; Rooney, M.M.; Chin, E.; Sequist, L.V.; Lennerz, J.K.; Gainor, J.F.; Shaw, A.T. Computed tomography imaging features and distribution of metastases in ROS1-rearranged non-small-cell lung cancer. Clin. Lung Cancer 2020, 21, 153–159. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Aisner, D.L.; Wood, D.E.; Akerley, W.; Bauman, J.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; Dilling, T.J.; Dobelbower, M.; et al. NCCN guidelines insights: Non-small cell lung cancer, version 5.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Halpenny, D.F.; Riely, G.J.; Hayes, S.; Yu, H.; Zheng, J.; Moskowitz, C.S.; Ginsberg, M.S. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements? Lung Cancer 2014, 86, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Shaw, A.T. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 2013, 18, 865–875. [Google Scholar] [CrossRef]
- Kodama, T.; Tsukaguchi, T.; Satoh, Y.; Yoshida, M.; Watanabe, Y.; Kondoh, O.; Sakamoto, H. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol. Cancer Ther. 2014, 13, 2910–2918. [Google Scholar] [CrossRef]
- Manhire, A.; Charig, M.; Clelland, C.; Gleeson, F.; Miller, R.; Moss, H.; Pointon, K.; Richardson, C.; Sawicka, E. BTS Guidelines for radiologically guided lung biopsy. Thorax 2003, 58, 920–936. [Google Scholar] [CrossRef]
- British Thoracic Society Society of Cardiothoracic Surgeons of Great Britain Ireland Working Party. Guidelines on the selection of patients with lung cancer for surgery. Thorax 2001, 56, 89–108. [Google Scholar] [CrossRef]
- Román, A.; Perez-Rozos, A.; Otero, A.; Jodar, C.; García-Ríos, I.; Lupiañez-Perez, Y.; Antonio Medina, J.; Gomez-Millan, J. Efficacy and safety of a simplified SBRT regimen for central and peripheral lung tumours. Clin. Transl. Oncol. 2019, 22, 144–150. [Google Scholar] [CrossRef]
- Ali Mohammed Hammamy, R.; Farooqui, K.; Ghadban, W. Sclerotic bone metastasis in pulmonary adenocarcinoma. Case Rep. Med. 2018, 2018, 1903757. [Google Scholar] [CrossRef]
- Haghighatkhah, H.R.; Sanei Taheri, M.; Kharrazi, S.M.H.; Ghazanfari Amlashi, D.; Haddadi, M.; Pourabdollah, M. An unusual case of pulmonary adenocarcinoma with multiple and extraordinary metastases. Iran. J. Radiol. 2012, 9, 93–98. [Google Scholar] [CrossRef]
| Clinical Feature | RET | ALK | ROS1 | RET vs. ALK | RET vs. ROS1 |
|---|---|---|---|---|---|
| (N = 32) | (N = 116) | (N = 67) | p-Value | p-Value | |
| Age | |||||
| Median | 64 | 51 | 54 | <0.0001 | 0.042 |
| Range | (42–83) | (19–75) | (23–89) | ||
| Sex | |||||
| Female | 14 (44%) | 66 (57%) | 49 (73%) | 0.230 | 0.007 |
| Male | 18 (56%) | 50 (43%) | 18 (27%) | ||
| Race | |||||
| Caucasian | 26 (81%) | 90 (78%) | 49 (73%) | 0.868 | 0.378 |
| Asian | 5 (16%) | 17 (15%) | 10 (15%) | ||
| Other | 1 (3%) | 9 (8%) | 8 (12%) | ||
| Smoking history | |||||
| Never | 23 (72%) | 87 (75%) | 45 (67%) | 0.820 | 0.817 |
| Prior/current | 9 (28%) | 29 (25%) | 22 (33%) | ||
| Tumor histology | |||||
| Adenocarcinoma | 28 (88%) | 108 (93%) | 67 (100%) | 0.025 | 0.010 |
| Neuroendocrine | 4 (12%) | 2 (2%) | 0 | ||
| Other | 0 | 6 (5%) | 0 | ||
| Stage | |||||
| I-II | 8 (25%) | 7 (6%) | 5 (7%) | 0.0044 | 0.023 |
| III | 2 (6%) | 22 (19%) | 13 (19%) | ||
| IV | 22 (69%) | 87 (75%) | 49 (73%) |
| Imaging Feature | RET | ALK | ROS1 | RET vs. ALK | RET vs. ROS1 |
|---|---|---|---|---|---|
| (N = 32) | (N = 116) | (N = 67) | p-Value | p-Value | |
| Tumor size (mm) | |||||
| Median | 32 | 41 | 33 | 0.258 | 0.836 |
| Range | (9–89) | (5–115) | (10–126) | ||
| ≥3 cm | 19 (59%) | 80 (69%) | 37 (55%) | 0.396 | 0.829 |
| Density | |||||
| Solid | 30 (94%) | 115 (99%) | 64 (96%) | 0.118 | 0.657 |
| Subsolid | 2 (6%) | 1 (1%) | 3 (4%) | ||
| Other features | |||||
| Air bronchograms | 2 (6%) | 7 (6%) | 3 (4%) | 1.000 | 0.657 |
| Cavitation | 0 | 7 (6%) | 2 (3%) | 0.347 | 1.000 |
| Calcification | 0 | 0 | 0 | ||
| Tumor location | |||||
| RUL | 4 (12%) | 28 (24%) | 14 (21%) | 0.249 | 0.477 |
| RML | 6 (19%) | 10 (9%) | 10 (15%) | ||
| RLL | 8 (25%) | 24 (21%) | 19 (28%) | ||
| LUL | 8 (25%) | 22 (19%) | 8 (12%) | ||
| LLL | 6 (19%) | 32 (28%) | 16 (24%) | ||
| Lobar level | |||||
| Lower | 14 (44%) | 56 (48%) | 35 (52%) | 0.693 | 0.521 |
| Upper | 18 (56%) | 60 (52%) | 32 (48%) | ||
| Laterality | |||||
| Left | 14 (44%) | 54 (47%) | 24 (36%) | 0.843 | 0.510 |
| Right | 18 (56%) | 62 (53%) | 43 (64%) | ||
| Axial location | |||||
| Central | 10 (31%) | 62 (53%) | 43 (64%) | 0.029 | 0.003 |
| Peripheral | 22 (69%) | 54 (47%) | 24 (36%) |
| Metastatic Site | RET | ALK | ROS1 | RET vs. ALK | RET vs. ROS1 |
|---|---|---|---|---|---|
| (N = 22) | (N = 87) | (N = 49) | p-Value | p-Value | |
| Intrathoracic | 14 (64%) | 64 (74%) | 41 (84%) | 0.429 | 0.074 |
| Lung | 4 (18%) | 18 (21%) | 18 (37%) | 1.000 | 0.167 |
| Pleural | 10 (45%) | 35 (40%) | 20 (41%) | 0.809 | 0.797 |
| Lymphangitic carcinomatosis | 6 (27%) | 35 (40%) | 21 (43%) | 0.329 | 0.292 |
| Pericardium | 1 (5%) | 2 (2%) | 2 (4%) | 0.495 | 1.000 |
| Extrathoracic | 17 (77%) | 65 (75%) | 29 (59%) | 1.000 | 0.183 |
| Bone | 10 (45%) | 41 (47%) | 16 (33%) | 1.000 | 0.425 |
| Sclerotic metastasis | 8 (80%) | 28 (68%) | 9 (56%) | 0.703 | 0.399 |
| Liver | 3 (14%) | 21 (24%) | 10 (20%) | 0.393 | 0.741 |
| Brain | 7 (32%) | 22 (25%) | 5 (10%) | 0.592 | 0.039 |
| Distant lymph nodes | 5 (23%) | 17 (20%) | 8 (16%) | 0.769 | 0.524 |
| Adrenal | 4 (18%) | 6 (7%) | 7 (14%) | 0.114 | 0.729 |
| Soft tissue | 1 (5%) | 5 (6%) | 1 (2%) | 1.000 | 0.527 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Digumarthy, S.R.; Mendoza, D.P.; Lin, J.J.; Rooney, M.; Do, A.; Chin, E.; Yeap, B.Y.; Shaw, A.T.; Gainor, J.F. Imaging Features and Patterns of Metastasis in Non-Small Cell Lung Cancer with RET Rearrangements. Cancers 2020, 12, 693. https://doi.org/10.3390/cancers12030693
Digumarthy SR, Mendoza DP, Lin JJ, Rooney M, Do A, Chin E, Yeap BY, Shaw AT, Gainor JF. Imaging Features and Patterns of Metastasis in Non-Small Cell Lung Cancer with RET Rearrangements. Cancers. 2020; 12(3):693. https://doi.org/10.3390/cancers12030693
Chicago/Turabian StyleDigumarthy, Subba R., Dexter P. Mendoza, Jessica J. Lin, Marguerite Rooney, Andrew Do, Emily Chin, Beow Y. Yeap, Alice T. Shaw, and Justin F. Gainor. 2020. "Imaging Features and Patterns of Metastasis in Non-Small Cell Lung Cancer with RET Rearrangements" Cancers 12, no. 3: 693. https://doi.org/10.3390/cancers12030693
APA StyleDigumarthy, S. R., Mendoza, D. P., Lin, J. J., Rooney, M., Do, A., Chin, E., Yeap, B. Y., Shaw, A. T., & Gainor, J. F. (2020). Imaging Features and Patterns of Metastasis in Non-Small Cell Lung Cancer with RET Rearrangements. Cancers, 12(3), 693. https://doi.org/10.3390/cancers12030693





