Role of Akt Activation in PARP Inhibitor Resistance in Cancer
Abstract
1. Introduction
2. The PARP-Akt Interplay
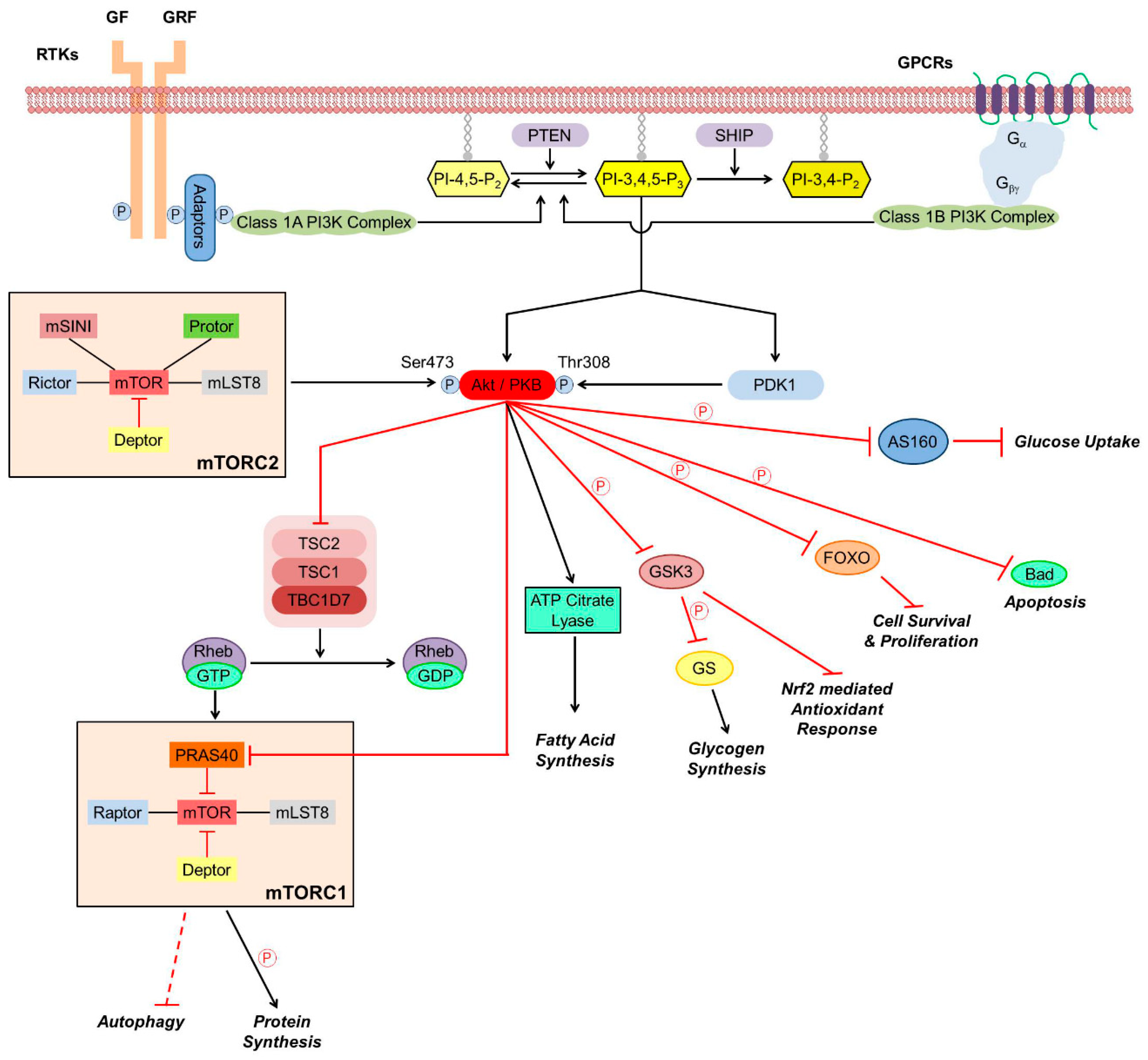
2.1. Cellular Role and Regulation of Akt
2.2. Nuclear Effects of PARP Activation
2.3. Role of the PARP-Akt Interplay in Shock and Inflammation
2.4. Role of the PARP-Akt Interplay in Reperfusion Injury
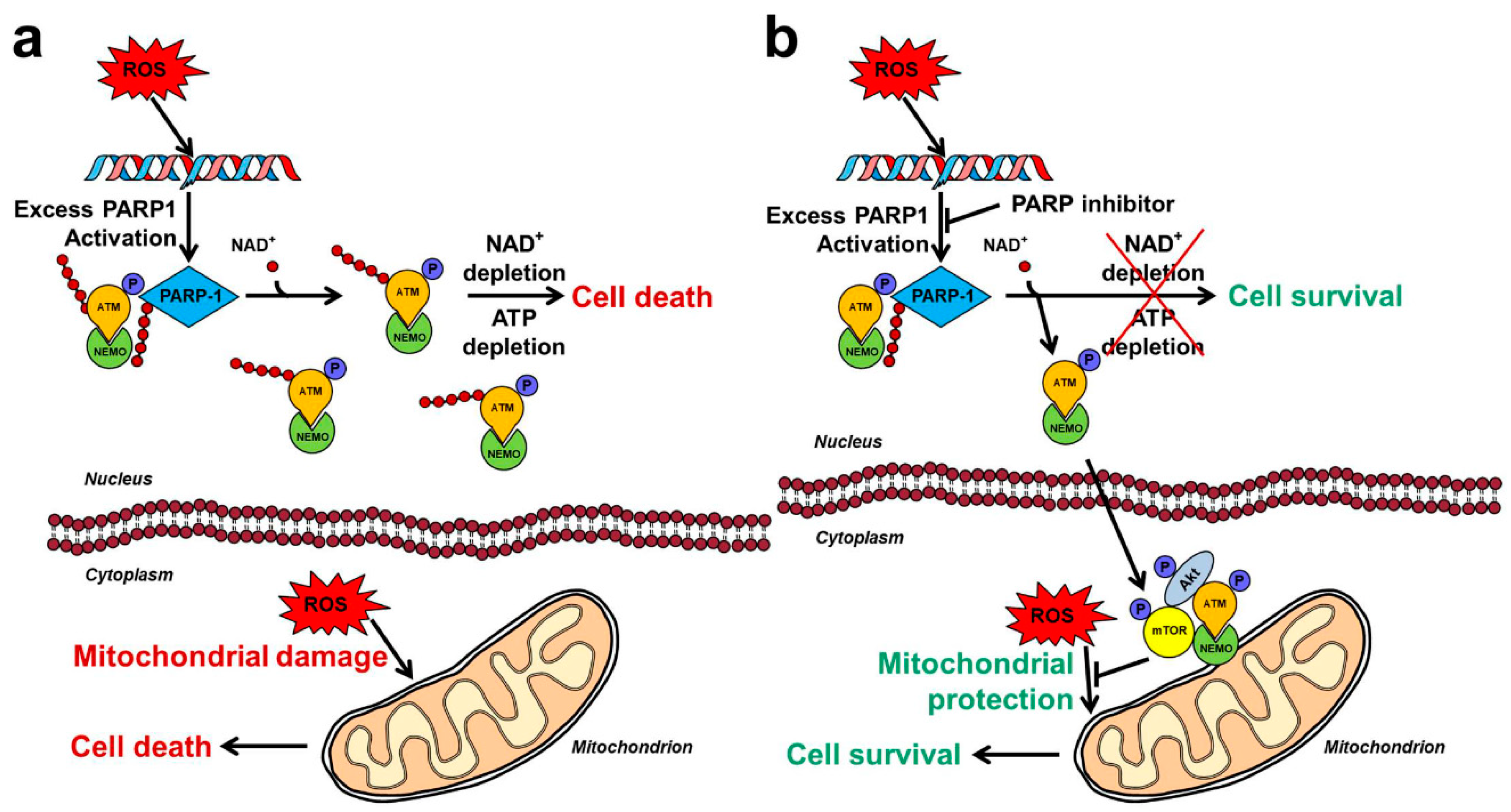
2.5. Mechanism of PARP Inhibition Induced Akt Activation
3. PARP-Akt Interactions in Cancer Biology
4. Open Questions and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yi, M.; Dong, B.; Qin, S.; Chu, Q.; Wu, K.; Luo, S. Advances and perspectives of PARP inhibitors. Exp. Hematol. Oncol. 2019, 11, 8–29. [Google Scholar] [CrossRef]
- Wu, J.; Starr, S. Low-fidelity compensatory backup alternative DNA repair pathways may unify current carcinogenesis theories. Future Oncol. 2014, 10, 1239–1253. [Google Scholar] [CrossRef]
- Aparicio, T.; Baer, R.; Gautier, J. DNA double-strand break repair pathway choice and cancer. DNA Repair 2014, 19, 169–175. [Google Scholar] [CrossRef]
- Kaniecki, K.; De Tullio, L.; Greene, E.C. A change of view: Homologous recombination at single-molecule resolution. Nat. Rev. Genet. 2018, 19, 191–207. [Google Scholar] [CrossRef]
- Bai, P.; Nagy, L.; Fodor, T.; Liaudet, L.; Pacher, P. Poly (ADP-ribose) polymerases as modulators of mitochondrial activity. Trends Endocrinol. Metab. 2015, 26, 75–83. [Google Scholar] [CrossRef]
- Henning, R.J.; Bourgeois, M.; Harbison, R.D. Poly (ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders. Cardiovasc. Toxicol. 2018, 18, 493–506. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Caruso, D.; Strudel, M.; Tomao, S.; Tomao, F. Update on poly-ADP-ribose polymerase inhibition for ovarian cancer treatment. J. Transl. Med. 2016, 14, 267. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tapodi, A.; Debreceni, B.; Hanto, K.; Bognar, Z.; Wittmann, I.; Gallyas, F., Jr.; Varbiro, G.; Sumegi, B. Pivotal Role of Akt Activation in Mitochondrial Protection and Cell Survival by Poly(ADP-ribose)polymerase-1 Inhibition in Oxidative Stress. J. Biol. Chem. 2005, 280, 35767–35775. [Google Scholar] [CrossRef] [PubMed]
- Szanto, A.; Hellebrand, E.E.; Bognar, Z.; Tucsek, Z.; Szabo, A.; Gallyas, F., Jr.; Sumegi, B.; Varbiro, G. PARP-1 inhibition-induced activation of PI-3-kinase-Akt pathway promotes resistance to taxol. Biochem. Pharmacol. 2009, 77, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Tapodi, A.; Bognar, Z.; Szabo, C.; Gallyas, F.; Sumegi, B. PARP inhibition induces Akt-mediated cytoprotective effects through the formation of a mitochondria-targeted phospho-ATM-NEMO-Akt-mTOR signalosome. Biochem. Pharmacol. 2019, 162, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Tschopp, O.; Baudry, A.; Dümmler, B.; Hynx, D.; Hemmings, B.A. Physiological functions of protein kinase B/Akt. Biochem. Soc. Trans. 2004, 32, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.; Parsons, R. PTEN: Life as a tumor suppressor. Exp. Cell Res. 2001, 264, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Balendran, A.; Casamayor, A.; Deak, M.; Paterson, A.; Gaffney, P.; Currie, R.; Downes, C.P.; Alessi, D.R. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol. 1999, 9, 393–404. [Google Scholar] [CrossRef]
- Liu, P.; Begley, M.; Michowski, W.; Inuzuka, H.; Ginzberg, M.; Gao, D.; Tsou, P.; Gan, W.; Papa, A.; Kim, B.M.; et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature 2014, 508, 541. [Google Scholar] [CrossRef]
- Delcommenne, M.; Tan, C.; Gray, V.; Rue, L.; Woodgett, J.; Dedhar, S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 11211–11216. [Google Scholar] [CrossRef]
- Yano, S.; Tokumitsu, H.; Soderling, T.R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 1998, 396, 584. [Google Scholar] [CrossRef]
- Pap, M.; Cooper, G.M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 1998, 273, 19929–19932. [Google Scholar] [CrossRef]
- Jaldin-Fincati, J.R.; Pavarotti, M.; Frendo-Cumbo, S.; Bilan, P.J.; Klip, A. Update on GLUT4 Vesicle Traffic: A Cornerstone of Insulin Action. Trends Endocrinol. Metab. 2017, 28, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Sugden, P.H.; Fuller, S.J.; Weiss, S.; Clerk, A. Glycogen synthase kinase 3 (GSK3) in the heart: A point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br. J. Pharmacol. 2008, 153, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Cardone, M.H.; Roy, N.; Stennicke, H.R.; Salvesen, G.S.; Franke, T.F.; Stanbridge, E.; Frisch, S.; Reed, J.C. Regulation of cell death protease caspase-9 by phosphorylation. Science 1998, 282, 1318–1321. [Google Scholar] [CrossRef]
- Patel, S.; Woodgett, J. Glycogen synthase kinase-3 and cancer: Good cop, bad cop? Canc. Cell 2008, 14, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Zhu, J.; Lei, S.; Dong, Y.; Li, L.; Jiang, B.; Tan, L.; Wu, J.; Yu, S.; et al. GSK-3b downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci. Rep. 2016, 6, 20196. [Google Scholar] [CrossRef]
- Gotschel, F.; Kern, C.; Lang, S.; Sparna, T.; Markmann, C.; Schwager, J.; McNelly, S.; von Weizsacker, F.; Laufer, S.; Hecht, A.; et al. Inhibition of GSK3 differentially modulates NF-kB, CREB, AP-1 and b-catenin signaling in hepatocytes, but fails to promote TNF-a-induced apoptosis. Exp. Cell Res. 2008, 314, 1351–1366. [Google Scholar] [CrossRef]
- Martin, M.; Rehani, K.; Jope, R.S.; Michalek, S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005, 6, 777–784. [Google Scholar] [CrossRef]
- Meier, R.; Alessi, D.R.; Cron, P.; Andjelković, M.; Hemmings, B.A. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J. Biol. Chem. 1997, 272, 30491–30497. [Google Scholar] [CrossRef]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Du, K.; Montminy, M. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 1998, 273, 32377–32379. [Google Scholar] [CrossRef] [PubMed]
- Ozes, O.N.; Mayo, L.D.; Gustin, J.A.; Pfeffer, S.R.; Pfeffer, L.M.; Donner, D.B. NF-κB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature 1999, 401, 82. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Edelstein, L.C.; Chen, C.; Bash, J.; Gélinas, C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-κB that blocks TNFα-induced apoptosis. Genes Dev. 1999, 13, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann-Zeh, A.; Rodriguez-Viciana, P.; Ulrich, E.; Gilbert, C.; Coffer, P.; Downward, J.; Evan, G. Suppression of c-Myc-induced apoptosis by Ras signaling through PI(3)K and PKB. Nature 1997, 385, 544. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef]
- Amé, J.C.; Spenlehauer, C.; de Murcia, G. The PARP superfamily. Bioessays 2004, 26, 882–893. [Google Scholar] [CrossRef]
- Bouchard, V.J.; Rouleau, M.; Poirier, G.G. PARP-1, a determinant of cell survival in response to DNA damage. Exp. Hematol. 2003, 31, 446–454. [Google Scholar] [CrossRef]
- Miwa, M.; Saikawa, N.; Yamaizumi, Z.; Nishimura, S.; Sugimura, T. Structure of poly (adenosine diphosphate ribose): Identification of 2′-[1-ribosyl-2-(or 3-) (1′-ribosyl)] adenosine-5′,5,5′-tris (phosphate) as a branch linkage. Proc. Natl. Acad. Sci. USA 1979, 76, 595–599. [Google Scholar] [CrossRef]
- D’Amours, D.; Desnoyers, S.; D’Silva, I.; Poirier, G.G. Poly (ADP-ribosyl) ation reactions in the regulation of nuclear functions. Biochem. J. 1999, 342, 249–268. [Google Scholar] [CrossRef]
- Steffen, J.D.; Pascal, J.M. New players to the field of ADP-ribosylation make the final cut. EMBO J. 2013, 32, 1205–1207. [Google Scholar] [CrossRef]
- Huber, A.; Bai, P.; de Murcia, J.M.; de Murcia, G. PARP-1, PARP-2 and ATM in the DNA damage response: Functional synergy in mouse development. DNA Repair 2004, 3, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Brunyanszki, A.; Szczesny, B.; Virág, L.; Szabo, C. Mitochondrial poly (ADP-ribose) polymerase: The Wizard of Oz at work. Free Radic. Biol. Med. 2016, 100, 257–270. [Google Scholar] [CrossRef]
- Kraus, W.L.; Lis, J.T. PARP goes transcription. Cell 2003, 113, 677–683. [Google Scholar] [CrossRef]
- Bürkle, A. Poly (APD-ribosyl) ation, a DNA damage-driven protein modification and regulator of genomic instability. Cancer Lett. 2001, 163, 1–5. [Google Scholar] [CrossRef]
- Stilmann, M.; Hinz, M.; Arslan, S.Ç.; Zimmer, A.; Schreiber, V.; Scheidereit, C. A Nuclear Poly (ADP-Ribose)-Dependent Signalosome Confers DNA Damage-Induced IkB Kinase Activation. Mol. Cell. 2009, 36, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.V.; Graham, M.E.; Jakob, B.; Tobias, F.; Kijas, A.W.; Tanuji, M.; Chen, P.; Robinson, P.J.; Taucher-Scholz, G.; Suzuki, K.; et al. Autophosphorylation and ATM activation: Additional sites add to the complexity. J. Biol. Chem. 2011, 286, 9107–9119. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.A. Poly (ADP-ribose) in the cellular response to DNA damage. Radiat. Res. 1985, 101, 4–15. [Google Scholar] [CrossRef]
- Virág, L.; Salzman, A.L.; Szabó, C. Poly (ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J. Immunol. 1998, 161, 3753–3759. [Google Scholar] [PubMed]
- Ha, H.C.; Snyder, S.H. Poly (ADPribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl. Acad. Sci. USA 1999, 69, 13978–13982. [Google Scholar] [CrossRef]
- Shieh, W.M.; Amé, J.C.; Wilson, M.V.; Wang, Z.Q.; Koh, D.W.; Jacobson, M.K.; Jacobson, E.L. Poly (ADPribose) polymerase null mouse cells synthesize ADP-ribose polymers. J. Biol. Chem. 1998, 273, 30069–30072. [Google Scholar] [CrossRef]
- Küpper, J.H.; Müller, M.; Wolf, I. NAD consumption in carcinogen-treated hamster cells overexpressing a dominant negative mutant of poly (ADPribose) polymerase. Biochem. Biophys. Res. Commun. 1999, 265, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, P.; Szabo, C. Poly (ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Discov. 2005, 4, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Poly (ADP-ribose) polymerase activation and circulatory shock. Novartis Found. Symp. 2007, 280, 92–103. [Google Scholar] [PubMed]
- Giansanti, V.; Donà, F.; Tillhon, M.; Scovassi, A.I. PARP inhibitors: New tools to protect from inflammation. Biochem. Pharmacol. 2010, 80, 1869–1877. [Google Scholar] [CrossRef]
- Luo, X.; Kraus, W.L. On PAR with PARP: Cellular stress signaling through poly (ADP-ribose) and PARP-1. Genes Dev. 2012, 26, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Virág, L. Role of poly (ADP-ribose) polymerases in the regulation of inflammatory processes. FEBS Lett. 2012, 586, 3771–3777. [Google Scholar] [CrossRef]
- García, S.; Conde, C. The role of poly (ADP-ribose) polymerase-1 in rheumatoid arthritis. Mediat. Inflamm. 2015, 2015, 837250. [Google Scholar] [CrossRef]
- Sethi, G.S.; Dharwal, V.; Naura, A.S. Poly (ADP-Ribose) Polymerase-1 in Lung Inflammatory Disorders: A Review. Front. Immunol. 2017, 8, 1172. [Google Scholar] [CrossRef]
- Berger, N.A.; Besson, V.C.; Boulares, A.H.; Bürkle, A.; Chiarugi, A.; Clark, R.S.; Curtin, N.J.; Cuzzocrea, S.; Dawson, T.M.; Dawson, V.L.; et al. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br. J. Pharmacol. 2018, 175, 192–222. [Google Scholar] [CrossRef]
- Veres, B.; Gallyas, F., Jr.; Varbiro, G.; Berente, Z.; Osz, E.; Szekeres, G.; Szabo, C.; Sumegi, B. Decrease of the inflammatory response and induction of the Akt/protein kinase B pathway by poly-(ADP-ribose) polymerase 1 inhibitor in endotoxin-induced septic shock. Biochem. Pharmacol. 2003, 65, 1373–1382. [Google Scholar] [CrossRef]
- Veres, B.; Radnai, B.; Gallyas, F., Jr.; Varbiro, G.; Berente, Z.; Osz, E.; Sumegi, B. Regulation of kinase cascades and transcription factors by a poly (ADP-ribose) polymerase-1 inhibitor, 4-hydroxyquinazoline, in lipopolysaccharide-induced inflammation in mice. J. Pharmacol. Exp. Ther. 2004, 310, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Veto, S.; Acs, P.; Bauer, J.; Lassmann, H.; Berente, Z.; Setalo, G., Jr.; Borgulya, G.; Sumegi, B.; Komoly, S.; Gallyas, F., Jr.; et al. Inhibiting poly(ADP-ribose) polymerase: A potential therapy against oligodendrocyte death. Brain 2010, 133, 822–834. [Google Scholar] [CrossRef] [PubMed]
- García, S.; Mera, A.; Gómez-Reino, J.J.; Conde, C. Poly (ADP-ribose) polymerase suppression protects rheumatoid synovial fibroblasts from Fas-induced apoptosis. Rheumatology Oxford 2009, 48, 483–489. [Google Scholar] [CrossRef] [PubMed]
- García, S.; Liz, M.; Gómez-Reino, J.J.; Conde, C. Akt activity protects rheumatoid synovial fibroblasts from Fas-induced apoptosis by inhibition of Bid cleavage. Arthritis Res. Ther. 2010, 12, R33. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, N.; Xiao, Z.; Cheng, J.; Mei, Y.; Zheng, P.; Wang, L.; Zhang, X.; Zhou, X.; Zhou, W.; et al. Effects of poly (ADP-ribose) polymerase-1 (PARP-1) inhibition on sulfur mustard-induced cutaneous injuries in vitro and in vivo. PeerJ 2016, 4, e1890. [Google Scholar] [CrossRef][Green Version]
- Gilad, E.; Zingarelli, B.; Salzman, A.L.; Szabo, C. Protection by inhibition of poly (ADP-ribose) synthetase against oxidant injury in cardiac myoblasts In vitro. J. Mol. Cell Cardiol. 1997, 29, 2585–2597. [Google Scholar] [CrossRef]
- Zingarelli, B.; Cuzzocrea, S.; Zsengellér, Z.; Salzman, A.L.; Szabo, C. Protection against myocardial ischemia and reperfusion injury by 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase. Cardiovasc. Res. 1997, 36, 205–215. [Google Scholar] [CrossRef]
- Zingarelli, B.; Salzman, A.L.; Szabo, C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ. Res. 1998, 83, 85–94. [Google Scholar] [CrossRef]
- Kovacs, K.; Toth, A.; Deres, P.; Kalai, T.; Hideg, K.; Sumegi, B. Myocardial protection by selective poly(ADP-ribose) polymerase inhibitors. Exp. Clin. Cardiol. 2004, 9, 17–20. [Google Scholar]
- Kovacs, K.; Toth, A.; Deres, P.; Kalai, T.; Hideg, K.; Gallyas, F., Jr.; Sumegi, B. Critical role of PI3-kinase/Akt activation in the PARP inhibitor induced heart function recovery during ischemia-reperfusion. Biochem. Pharmacol. 2006, 71, 441–452. [Google Scholar] [CrossRef]
- Pálfi, A.; Tóth, A.; Kulcsár, G.; Hantó, K.; Deres, P.; Bartha, E.; Halmosi, R.; Szabados, E.; Czopf, L.; Kálai, T.; et al. The role of Akt and mitogen-activated protein kinase systems in the protective effect of poly (ADP-ribose) polymerase inhibition in Langendorff perfused and in isoproterenol-damaged rat hearts. J. Pharmacol. Exp. Ther. 2005, 315, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.F.; Chen, D.Y.; Du, B.; Ji, X.P. Poly (ADP-ribose) polymerase inhibitor reduces heart ischaemia/reperfusion injury via inflammation and Akt signalling in rats. Chin. Med. J. 2013, 126, 1913–1917. [Google Scholar] [PubMed]
- Park, E.S.; Kang, J.C.; Kang, D.H.; Jang, Y.C.; Yi, K.Y.; Chung, H.J.; Park, J.S.; Kim, B.; Feng, Z.P.; Shin, H.S. 5-AIQ inhibits H2O2-induced apoptosis through reactive oxygen species scavenging and Akt/GSK-3β signaling pathway in H9c2 cardiomyocytes. Toxicol. Appl. Pharmacol. 2013, 268, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Kang, D.H.; Kang, J.C.; Jang, Y.C.; Lee, M.J.; Chung, H.J.; Yi, K.Y.; Kim, D.E.; Kim, B.; Shin, H.S. Cardioprotective effect of KR-33889, a novel PARP inhibitor, against oxidative stress-induced apoptosis in H9c2 cells and isolated rat hearts. Arch. Pharm. Res. 2017, 40, 640–654. [Google Scholar] [CrossRef]
- Garcia Soriano, F.; Virág, L.; Jagtap, P.; Szabó, E.; Mabley, J.G.; Liaudet, L.; Marton, A.; Hoyt, D.G.; Murthy, K.G.; Salzman, A.L.; et al. Diabetic endothelial dysfunction: The role of poly(ADP-ribose) polymerase activation. Nat. Med. 2001, 7, 108–113. [Google Scholar] [CrossRef]
- Szabó, G.; Bährle, S.; Stumpf, N.; Sonnenberg, K.; Szabó, E.E.; Pacher, P.; Csont, T.; Schulz, R.; Dengler, T.J.; Liaudet, L.; et al. Poly (ADP-Ribose) polymerase inhibition reduces reperfusion injury after heart transplantation. Circ. Res. 2002, 90, 100–106. [Google Scholar] [CrossRef]
- Pacher, P.; Liaudet, L.; Bai, P.; Virag, L.; Mabley, J.G.; Haskó, G.; Szabo, C. Activation of poly (ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J. Pharmacol. Exp. Ther. 2002, 300, 862–867. [Google Scholar] [CrossRef]
- Pacher, P.; Liaudet, L.; Mabley, J.G.; Komjáti, K.; Szabo, C. Pharmacologic inhibition of poly (adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J. Am. Coll. Cardiol. 2002, 40, 1006–1016. [Google Scholar] [CrossRef]
- Pacher, P.; Liaudet, L.; Soriano, F.G.; Mabley, J.G.; Szabó, E.; Szabo, C. The role of poly (ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes 2002, 51, 514–521. [Google Scholar] [CrossRef]
- Szabo, C.; Pacher, P.; Zsengellér, Z.; Vaslin, A.; Komjáti, K.; Benkö, R.; Chen, M.; Mabley, J.G.; Kollai, M. Angiotensin II-mediated endothelial dysfunction: Role of poly (ADP-ribose) polymerase activation. Mol. Med. 2004, 10, 28–35. [Google Scholar] [CrossRef]
- Pacher, P.; Vaslin, A.; Benko, R.; Mabley, J.G.; Liaudet, L.; Haskó, G.; Marton, A.; Bátkai, S.; Kollai, M.; Szabó, C. A new, potent poly (ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J. Pharmacol. Exp. Ther. 2004, 311, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Szenczi, O.; Kemecsei, P.; Holthuijsen, M.F.; van Riel, N.A.; van der Vusse, G.J.; Pacher, P.; Szabo, C.; Kollai, M.; Ligeti, L.; Ivanics, T. Poly (ADP-ribose) polymerase regulates myocardial calcium handling in doxorubicin-induced heart failure. Biochem. Pharmacol. 2005, 69, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.Y.; Chen, M.; Zsengellér, Z.; Li, H.; Kiss, L.; Kollai, M.; Szabó, C. Poly (ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J. Pharmacol. Exp. Ther. 2005, 312, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Liaudet, L.; Mabley, J.G.; Cziráki, A.; Haskó, G.; Szabo, C. Beneficial effects of a novel ultrapotent poly (ADP-ribose) polymerase inhibitor in murine models of heart failure. Int. J. Mol. Med. 2006, 17, 369–375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korkmaz-Icöz, S.; Radovits, T.; Loganathan, S.; Li, S.; Ruppert, M.; Benke, K.; Brlecic, P.; Szabó, C.; Karck, M.; Szabó, G. Prolonging hypothermic ischaemic cardiac and vascular storage by inhibiting the activation of the nuclear enzyme poly(adenosine diphosphate-ribose) polymerase. Eur. J. Cardiothorac. Surg. 2017, 51, 829–835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, L.; Kwan, J.C.; Macdonald, P.S.; Yang, L.; Preiss, T.; Hicks, M. Improved poststorage cardiac function by poly (ADP-ribose) polymerase inhibition: Role of phosphatidylinositol 3-kinase Akt pathway. Transplantation 2007, 84, 380–386. [Google Scholar] [CrossRef]
- Bartha, E.; Solti, I.; Szabo, A.; Olah, G.; Magyar, K.; Szabados, E.; Kalai, T.; Hideg, K.; Toth, K.; Gero, D.; et al. Regulation of kinase cascade activation and heat shock protein expression by poly (ADP-ribose) polymerase inhibition in doxorubicin-induced heart failure. J. Cardiovasc. Pharmacol. 2011, 58, 380–391. [Google Scholar] [CrossRef]
- Sarszegi, Z.; Bognar, E.; Gaszner, B.; Kónyi, A.; Gallyas, F., Jr.; Sumegi, B.; Berente, Z. BGP-15, a PARP-inhibitor, prevents imatinib-induced cardiotoxicity by activating Akt and suppressing JNK and p38 MAP kinases. Mol. Cell Biochem. 2012, 365, 129–137. [Google Scholar] [CrossRef]
- Qin, W.D.; Liu, G.L.; Wang, J.; Wang, H.; Zhang, J.N.; Zhang, F.; Ma, Y.; Ji, X.Y.; Li, C.; Zhang, M.X. Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy. Oncotarget 2016, 7, 35618–35631. [Google Scholar] [CrossRef]
- Palfi, A.; Toth, A.; Hanto, K.; Deres, P.; Szabados, E.; Szereday, Z.; Kulcsar, G.; Kalai, T.; Hideg, K.; Gallyas, F., Jr.; et al. PARP inhibition prevents postinfarction myocardial remodeling and heart failure via the protein kinase C/glycogen synthase kinase-3beta pathway. J. Mol. Cell Cardiol. 2006, 41, 149–159. [Google Scholar] [CrossRef]
- Magyar, K.; Deres, L.; Eros, K.; Bruszt, K.; Seress, L.; Hamar, J.; Hideg, K.; Balogh, A.; Gallyas, F., Jr.; Sumegi, B.; et al. A quinazoline-derivative compound with PARP inhibitory effect suppresses hypertension-induced vascular alterations in spontaneously hypertensive rats. Biochim. Biophys. Acta 2014, 1842, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Deres, L.; Bartha, E.; Palfi, A.; Eros, K.; Riba, A.; Lantos, J.; Kalai, T.; Hideg, K.; Sumegi, B.; Gallyas, F.; et al. PARP-inhibitor treatment prevents hypertension induced cardiac remodeling by favorable modulation of heat shock proteins, Akt-1/GSK-3β and several PKC isoforms. PLoS ONE 2014, 9, e102148. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc. Res. 2006, 70, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, T.S.; Gidday, J.M. Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2573–H2581. [Google Scholar] [CrossRef]
- Kovacs, K.; Hanto, K.; Bognar, Z.; Tapodi, A.; Bognar, E.; Kiss, G.N.; Szabo, A.; Rappai, G.; Kiss, T.; Sumegi, B.; et al. Prevalent role of Akt and ERK activation in cardioprotective effect of Ca(2+) channel- and beta-adrenergic receptor blockers. Mol. Cell Biochem. 2009, 321, 155–164. [Google Scholar] [CrossRef]
- Covington, S.M.; Bauler, L.D.; Toledo-Pereyra, L.H. Akt: A therapeutic target in hepatic ischemia-reperfusion injury. J. Investig. Surg. 2017, 30, 47–55. [Google Scholar] [CrossRef]
- Koh, S.H.; Park, Y.; Song, C.W.; Kim, J.G.; Kim, K.; Kim, J.; Kim, M.H.; Lee, S.R.; Kim, D.W.; Yu, H.J.; et al. The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur. J. Neurosci. 2004, 20, 1461–1472. [Google Scholar] [CrossRef]
- Szabo, C. Nicotinamide: A jack of all trades (but master of none?). Intensive Care Med. 2003, 29, 863–866. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Lin, S.H.; Li, F.; Maiese, K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr. Neurovasc. Res. 2005, 2, 271–285. [Google Scholar] [CrossRef]
- Li, W.J.; Oh, S.J. PARP inhibition prevents oxidative injury of bladder induced by acute urinary retention and subsequent emptying. Apoptosis 2011, 16, 574–580. [Google Scholar] [CrossRef]
- Kalmar-Nagy, K.; Degrell, P.; Szabo, A.; Sumegi, K.; Wittmann, I.; Gallyas, F., Jr.; Sumegi, B. PARP inhibition attenuates acute kidney allograft rejection by suppressing cell death pathways and activating PI-3K-Akt cascade. PLoS ONE 2013, 8, e81928. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, K.; Vaczy, A.; Fekete, K.; Kovari, P.; Atlasz, T.; Reglodi, D.; Gabriel, R.; Gallyas, F.; Sumegi, B. PARP inhibitor protects against chronic hypoxia/reoxygenation-induced retinal injury by regulation of MAPKs, HIF1α, Nrf2, and NFκB. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Quesada, R.; Munoz-Gamez, J.A.; Martin-Oliva, D.; Peralta, A.; Valenzuela, M.T.; Matinez-Romero, R.; Quiles-Perez, R.; Menissier-de Murcia, J.; de Murcia, G.; Ruiz de Almodovar, M.; et al. Interaction between ATM and PARP-1 in response to DNA damage and sensitization of ATM deficient cells through PARP inhibition. BMC Mol. Biol. 2007, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Halaby, M.J.; Hibma, J.C.; He, J.; Yang, D.Q. ATM protein kinase mediates full activation of Akt and regulates glucose transporter 4 translocation by insulin in muscle cells. Cell Signal. 2008, 20, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Stagni, V.; Manni, I.; Oropallo, V.; Mottolese, M.; Di Benedetto, A.; Piaggio, G.; Falcioni, R.; Giaccari, D.; Di Carlo, S.; Sperati, F.; et al. ATM kinase sustains HER2 tumorigenicity in breast cancer. Nat. Commun. 2015, 6, 6886. [Google Scholar] [CrossRef]
- Yang, D.Q.; Halaby, M.J.; Li, Y.; Hibma, J.C.; Burn, P. Cytoplasmic ATM protein kinase: An emerging therapeutic target for diabetes, cancer and neuronal degeneration. Drug Discov. Today 2011, 16, 332–338. [Google Scholar] [CrossRef]
- Lee, S.S.; Bohrson, C.; Pike, A.M.; Wheelan, S.J.; Greider, C.W. ATM kinase is required for telomere elongation in mouse and human cells. Cell Rep. 2015, 13, 1623–1632. [Google Scholar] [CrossRef]
- Wu, Z.H.; Shi, Y.; Tibbetts, R.S.; Miyamoto, S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 2006, 311, 1141–1146. [Google Scholar] [CrossRef]
- Williamson, C.T.; Muzik, H.; Turhan, A.G.; Zamo, A.; O’Connor, M.J.; Bebb, D.G.; Lees-Miller, S.P. ATM deficiency sensitizes mantle cell lymphoma cells to poly (ADPribose) polymerase-1 inhibitors. Mol. Cancer Ther. 2010, 9, 347–357. [Google Scholar] [CrossRef]
- Rajawat, J.; Shukla, N.; Mishra, D.P. Therapeutic targeting of poly (ADP-ribose) polymerase-1 (PARP1) in cancer: Current developments, therapeutic strategies, and future opportunities. Med. Res. Rev. 2017, 37, 1461–1491. [Google Scholar] [CrossRef]
- Wang, D.; Wang, M.; Jiang, N.; Zhang, Y.; Bian, X.; Wang, X.; Roberts, T.M.; Zhao, J.J.; Liu, P.; Cheng, H. Effective use of PI3K inhibitor BKM120 and PARP inhibitor Olaparib to treat PIK3CA mutant ovarian cancer. Oncotarget 2016, 7, 13153–13166. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.J.; Curtin, N.J.; Newell, D.R.; Golding, B.T.; Durkacz, B.W.; Calvert, A.H. The role of inhibitors of poly (ADP-ribose) polymerase as resistance-modifying agents in cancer therapy. Biochimie 1995, 77, 408–422. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly (ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Murai, J.; Huang, S.Y.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef]
- Kim, G.; Ison, G.; McKee, A.E.; Zhang, H.; Tang, S.; Gwise, T.; Sridhara, R.; Lee, E.; Tzou, A.; Philip, R.; et al. FDA approval summary. Olaparib monotherapy in patients with deleterious germline BRCA mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin. Cancer Res. 2015, 21, 4257–4261. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef]
- Sachdev, E.; Tabatabai, R.; Roy, V.; Rimel, B.J.; Mita, M.M. PARP Inhibition in Cancer: An Update on Clinical Development. Target. Oncol. 2019, 14, 657–679. [Google Scholar] [CrossRef]
- Shao, N.; Shi, Y.; Yu, L.; Ye, R.; Shan, Z.; Zhang, Z.; Zhang, Y.; Lin, Y. Prospect for Application of PARP Inhibitor in Patients with HER2 Negative Breast Cancer. Int. J. Biol. Sci. 2019, 15, 962–972. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Smith, K.A.; Waypa, G.B.; Schumacker, P.T. Redox signaling during hypoxia in mammalian cells. Redox Biol. 2017, 13, 228–234. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute GDC Data Portal. Available online: https://portal.gdc.cancer.gov/exploration?facetTab=genes&filters=%7B%22content%22%3A%5B%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22genes.biotype%22%2C%22value%22%3A%5B%22protein_coding%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22genes.gene_id%22%2C%22value%22%3A%5B%22ENSG00000082701%22%2C%22ENSG00000121879%22%2C%22ENSG00000142208%22%2C%22ENSG00000171862%22%2C%22ENSG00000198793%22%5D%7D%7D%2C%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22genes.is_cancer_gene_census%22%2C%22value%22%3A%5B%22true%22%5D%7D%7D%5D%2C%22op%22%3A%22and%22%7D&searchTableTab=genes (accessed on 14 February 2020).
- Lawrence, M.S.; Stojanov, P.; Mermel, C.H.; Robinson, J.T.; Garraway, L.A.; Golub, T.R.; Meyerson, M.; Gabriel, S.B.; Lander, E.S.; Getz, G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014, 505, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Revathidevi, S.; Munirajan, A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, D.; Longy, M. Mutations of the human PTEN gene. Hum. Mutat. 2000, 16, 109–122. [Google Scholar] [CrossRef]
- Nakatani, N.; Thompson, D.A.; Barthel, A.; Sakaue, H.; Liu, W.; Weigel, R.J.; Roth, R.A. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen independent prostate cancer lines. J. Biol. Chem. 1999, 274, 21528–21532. [Google Scholar] [CrossRef]
- Xu, X.; Sakon, M.; Nagano, H.; Hiraoka, N.; Yamamoto, H.; Hayashi, N.; Dono, K.; Nakamori, S.; Umeshita, k.; Ito, Y.; et al. Akt2 expression correlates with prognosis of human hepatocellular carcinoma. Oncol. Rep. 2004, 11, 25–32. [Google Scholar] [CrossRef]
- Roy, H.K.; Olusola, B.F.; Clemens, D.L.; Karolski, W.J.; Ratashak, A.; Lynch, H.T.; Smyrk, T.C. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis 2002, 23, 201–205. [Google Scholar] [CrossRef]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11. [Google Scholar] [CrossRef]
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res. 2005, 94, 29–86. [Google Scholar]
- Gewinner, C.; Wang, Z.C.; Richardson, A.; Teruya-Feldstein, J.; Etemadmoghadam, D.; Bowtell, D.; Barretina, J.; Lin, W.M.; Rameh, L.; Salmena, L.; et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009, 16, 115–125. [Google Scholar] [CrossRef]
- Nakakido, M.; Deng, Z.; Suzuki, T.; Dohmae, N.; Nakamura, Y.; Hamamoto, R. Dysregulation of AKT pathway by SMYD2-mediated lysine methylation on PTEN. Neoplasia 2015, 17, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Petta, S.; Martinelli, G.; Barbieri, E.; Santucci, M.A. RAD 001 (everolimus) prevents mTOR and Akt late re-activation in response to imatinib in chronic myeloid leukemia. J. Cell Biochem. 2010, 109, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Okoh, V.O.; Felty, Q.; Parkash, J.; Poppiti, R.; Roy, D. Reactive oxygen species via redox signaling to PI3K/AKT pathway contribute to the malignant growth of 4-hydroxyestradiol-transformed mammary epithelial cells. PLoS ONE 2013, 8, e54206. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, S.R.; Yang, K.S.; Ahn, Y.; Kim, Y.J.; Stadtman, E.R.; Rhee, S.G. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA 2004, 101, 16419–16424. [Google Scholar] [CrossRef]
- Noordermeer, S.M.; van Attikum, H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol. 2019, 29, 820–834. [Google Scholar] [CrossRef]
- Cseh, A.M.; Fabian, Z.; Quintana-Cabrera, R.; Szabo, A.; Eros, K.; Soriano, M.E.; Gallyas, F.; Scorrano, L.; Sumegi, B. PARP Inhibitor PJ34 Protects Mitochondria and Induces DNA-Damage Mediated Apoptosis in Combination With Cisplatin or Temozolomide in B16F10 Melanoma Cells. Front. Physiol. 2019, 10, 538. [Google Scholar] [CrossRef]
- Hou, W.H.; Chen, S.H.; Yu, X. Poly-ADP ribosylation in DNA damage response and cancer therapy. Mutat. Res. 2019, 780, 82–91. [Google Scholar] [CrossRef]
- Yazinski, S.A.; Comaills, V.; Buisson, R.; Genois, M.M.; Nguyen, H.D.; Ho, C.K.; Todorova Kwan, T.; Morris, R.; Lauffer, S.; Nussenzweig, A.; et al. ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes. Dev. 2017, 31, 318–332. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Sedukhina, A.S.; Okamoto, N.; Nagasawa, S.; Suzuki, N.; Ohta, T.; Hattori, H.; Roche-Molina, M.; Narváez, A.J.; Jeyasekharan, A.D.; et al. NF-kappaB signaling mediates acquired resistance after PARP inhibition. Oncotarget 2015, 6, 3825–3839. [Google Scholar] [CrossRef]
- Koh, D.W.; Lawler, A.M.; Poitras, M.F.; Sasaki, M.; Wattler, S.; Nehls, M.C.; Stöger, T.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Failure to degrade poly(ADP-ribose) causes increased sensitivity to cytotoxicity and early embryonic lethality. Proc. Natl. Acad. Sci. USA 2004, 101, 17699–17704. [Google Scholar] [CrossRef]
- Fathers, C.; Drayton, R.M.; Solovieva, S.; Bryant, H.E. Inhibition of poly (ADP-ribose) glycohydrolase (PARG) specifically kills BRCA2-deficient tumor cells. ABBV Cell Cycle 2012, 11, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, R.; Morra, R.; Appel, C.D.; Tallis, M.; Chioza, B.; Jankevicius, G.; Simpson, M.A.; Matic, I.; Ozkan, E.; Golia, B.; et al. Deficiency of terminal ADP-ribose protein glycohydrolase TARG1/C6orf130 in neurodegenerative disease. EMBO J. 2013, 32, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Chabanon, R.M.; Muirhead, G.; Krastev, D.B.; Adam, J.; Morel, D.; Garrido, M.; Lamb, A.; Hénon, C.; Dorvault, N.; Rouanne, M.; et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J. Clin. Investig. 2019, 129, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Tutuncuoglu, B.; Krogan, N.J. Mapping genetic interactions in cancer: A road to rational combination therapies. Genome Med. 2019, 11, 62. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. Comprehensive Outline of Whole Exome Sequencing Data Analysis Tools Available in Clinical Oncology. Cancers 2019, 11, 1725. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Valente, A.; Wade, K. Personalized Medicine in Gynecologic Cancer: Fact or Fiction? Surg. Oncol. Clin. 2020, 29, 105–113. [Google Scholar] [CrossRef]
| Target | Name | Company | Cancer Type |
|---|---|---|---|
| mTOR | Everolimus | Novartis | Neuroendocrine tumor, breast cancer, gastrointestinal and lung neuroendocrine tumor |
| mTOR | Sirolimus | Pfizer | Lymphangioleiomyomatosis |
| mTOR | Temsirolimus | Wyeth | Renal cell carcinoma |
| PI3Kδ | Idelalisib | Gilead Sciences | Chronic lymphocytic leukemia, follicular B-cell non-Hodgkin lymphoma, small lymphocytic lymphoma |
| PI3Kδγ | Duvelisib | Intellikine | Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| PI3Kα | Alpelisib | Novartis | HR-positive and HER2/neu-negative breast cancer |
| PI3Kαδ | Copanlisib | Bayer | Relapsed follicular lymphoma |
| PARP1/2 | Oliparib | AstraZeneca | BRCA1/2 mutated ovarian, breast, and prostate cancer |
| PARP1/2 | Rucaparib | Clovis | BRCA1/2 mutated ovarian cancer |
| PARP1/2 | Niraparib | Tesaro | Recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer |
| PARP1/2 | Talazoparib | Pfizer | BRCA1/2 mutated metastatic breast cancer |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallyas, F., Jr.; Sumegi, B.; Szabo, C. Role of Akt Activation in PARP Inhibitor Resistance in Cancer. Cancers 2020, 12, 532. https://doi.org/10.3390/cancers12030532
Gallyas F Jr., Sumegi B, Szabo C. Role of Akt Activation in PARP Inhibitor Resistance in Cancer. Cancers. 2020; 12(3):532. https://doi.org/10.3390/cancers12030532
Chicago/Turabian StyleGallyas, Ferenc, Jr., Balazs Sumegi, and Csaba Szabo. 2020. "Role of Akt Activation in PARP Inhibitor Resistance in Cancer" Cancers 12, no. 3: 532. https://doi.org/10.3390/cancers12030532
APA StyleGallyas, F., Jr., Sumegi, B., & Szabo, C. (2020). Role of Akt Activation in PARP Inhibitor Resistance in Cancer. Cancers, 12(3), 532. https://doi.org/10.3390/cancers12030532






