Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study
Abstract
1. Introduction
2. Results
2.1. Cohorts
2.2. Biomarker Assessment in the Nivolumab Group
2.3. Baseline Biomarkers Level and Survival in the Nivolumab Group
2.4. Baseline Biomarkers among EGFR-Mutated NSCLC Patients
2.5. Biomarkers Kinetics and Survival in Nivolumab Group
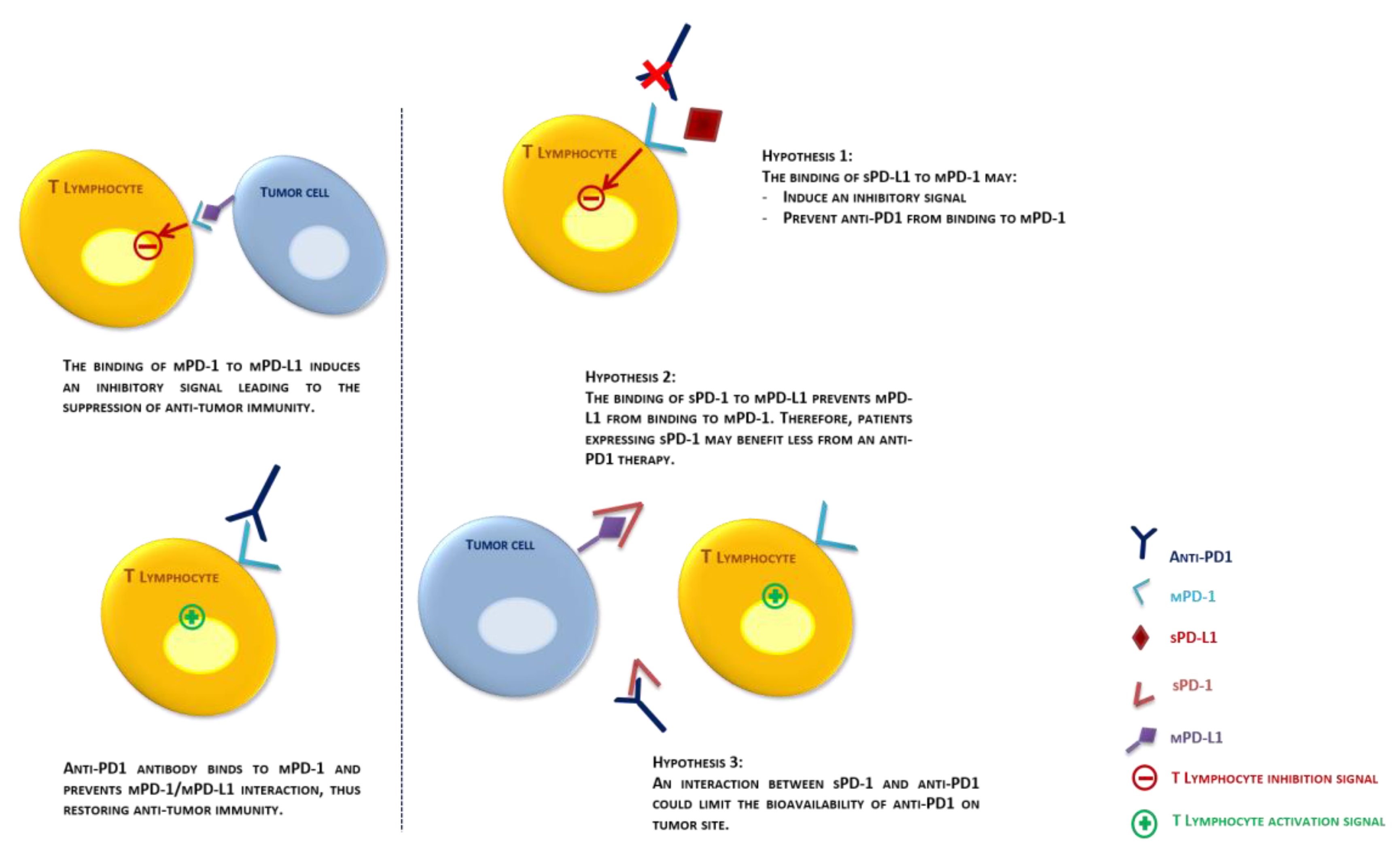
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Nivolumab Treatment
4.3. Blood Collection
4.4. Plasma Levels of Biomarkers
4.5. Clinical Endpoints
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 95%CI | 95% Confidence Interval |
| ALK | Anaplastic Lymphoma Kinase |
| APC | Antigen Presenting Cells |
| BOR | Best Objective Response |
| CRP | C-reactive Protein |
| CV | Coefficient of variation |
| DNA | Deoxyribonucleic Acid |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| EGFR | Epidermal Growth Factor Receptor |
| ELISA | Enzyme-linked immunosorbent assay |
| HR | Hazard Ratio |
| IQR | Interquartile Range |
| mRNA | Messenger RNA |
| NLR | Neutrophil to lymphocyte Ratio |
| NSCLC | Non-Small Cell Lung Cancer |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PD-1; mPD-1; sPD-1 | Programmed cell death 1; membrane-bound PD-1; Soluble PD-1 |
| PD-L1 TC | PD-L1 expression rate on tumor cells |
| PD-L1, PD-L2; mPD-L1, mPD-L2 sPD-L1, | Programmed death ligand 1, 2; membrane-bound PD-L1, PD-L2; Soluble PD-L1 |
| PFS | Progression-free Survival |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RPM | Revolutions per minute |
| TCR | T-cell Receptor |
| TILs | Tumor Infiltrating Lymphocytes |
| TMB | Tumor Mutational Burden |
References
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. Ann Oncol. 2018, 29, 192–237. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Gettinger, S.; Rizvi, N.A.; Chow, L.Q.; Borghaei, H.; Brahmer, J.; Ready, N.; Gerber, D.E.; Shepherd, F.A.; Antonia, S.; Goldman, J.W.; et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 2980–2987. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Nielsen, C.; Ohm-Laursen, L.; Barington, T.; Husby, S.; Lillevang, S.T. Alternative splice variants of the human PD-1 gene. Cell Immunol. 2005, 235, 109–116. [Google Scholar] [CrossRef]
- Kruger, S.; Legenstein, M.L.; Rösgen, V.; Haas, M.; Modest, D.P.; Westphalen, C.B.; Ormanns, S.; Kirchner, T.; Heinemann, V.; Holdenrieder, S.; et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. OncoImmunology 2017, 6, e1310358. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Kang, P.J.; Chuang, Y.H.; Wang, Y.H.; Jan, M.C.; Wu, C.F.; Lin, C.L.; Liu, C.J.; Liaw, Y.F.; Lin, S.M.; et al. Circulating Programmed Death-1 as a Marker for Sustained High Hepatitis B Viral Load and Risk of Hepatocellular Carcinoma. Ahn SH, editor. PLoS ONE 2014, 9, e95870. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, S.F.; Demuth, C.; Weber, B.; Sorensen, B.S.; Meldgaard, P. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR -mutated non-small cell lung cancer treated with erlotinib. Lung Cancer 2016, 100, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671. [Google Scholar] [CrossRef]
- Okuma, Y.; Hosomi, Y.; Nakahara, Y.; Watanabe, K.; Sagawa, Y.; Homma, S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer Amst Neth. 2017, 104, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Shi, B.; Xu, P.; Hu, Z.; Bai, L.; Zhang, X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 2011, 56, 231–238. [Google Scholar] [CrossRef]
- He, X.; Xu, L.; Liu, Y. Identification of a novel splice variant of human PD-L1 mRNA encoding an isoform-lacking Igv-like domain. Acta Pharmacol. Sin. 2005, 26, 462–468. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Li, Y.; Nie, J.; Dai, L.; Hu, W.; Chen, X.; Han, J.; Ma, X.; Tian, G.; et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics: Circulating PD-L1 in lung cancer patients. Thorac. Cancer 2015, 6, 534–538. [Google Scholar] [CrossRef]
- Costantini, A.; Julie, C.; Dumenil, C.; Hélias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.F.; et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. OncoImmunology 2018, 7, e1452581. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhao, H.; Ren, X.-B.; Li, Y.-L.; Zhao, H.; Ren, X.-B. Relationship of VEGF/VEGFR with immune and cancer cells: Staggering or forward? Cancer Biol. Med. 2016, 13, 206–214. [Google Scholar] [CrossRef]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Liu, W.; Wang, L.; Yang, M.; Du, J. High circulating VEGF level predicts poor overall survival in lung cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jochems, C.; Talaie, T.; Anderson, A.; Jales, A.; Tsang, K.Y.; Madan, R.A.; Gulley, J.L.; Schlom, J. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood 2012, 120, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Schlom, J.; Jochems, C.; Gulley, J.L.; Huang, J. The role of soluble CD40L in immunosuppression. OncoImmunology 2013, 2, e22546. [Google Scholar] [CrossRef]
- Chung, H.W.; Lim, J.-B. Clinical significance of elevated serum soluble CD40 ligand levels as a diagnostic and prognostic tumor marker for pancreatic ductal adenocarcinoma. J. Transl. Med. 2014, 12, 102. [Google Scholar] [CrossRef]
- Guo, Y.J.; Liu, G.; Wang, X.; Jin, D.; Wu, M.; Ma, J.; Sy, M.S. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994, 54, 422–426. [Google Scholar]
- Franzmann, E.J.; Reategui, E.P.; Pedroso, F.; Pernas, F.G.; Karakullukcu, B.M.; Carraway, K.L.; Hamilton, K.; Singal, R.; Goodwin, W.J. Soluble CD44 Is a Potential Marker for the Early Detection of Head and Neck Cancer. Cancer Epidemiol. Biomark. 2007, 16, 1348–1355. [Google Scholar] [CrossRef]
- Van Hal, N.L.; Van Dongen, G.A.; Ten Brink, C.B.; Heider, K.H.; Rech-Weichselbraun, I.; Snow, G.B.; Brakenhoff, R.H. Evaluation of soluble CD44v6 as a potential serum marker for head and neck squamous cell carcinoma. Clin. Cancer Res. 1999, 5, 3534–3541. [Google Scholar]
- Seyedmajidi, S.; Seyedmajidi, M.; Foroughi, R.; Zahedpasha, A.; Saravi, Z.Z.; Pourbagher, R.; Bijani, A.; Motallebnejad, M.; Shabestani, A.M.; Mostafazadeh, A. Comparison of Salivary and Serum Soluble CD44 Levels between Patients with Oral SCC and Healthy Controls. Asian Pac. J. Cancer Prev. 2018, 19, 3059–3063. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, D.H.; Lim, J.M.; Lee, J.W.; Jeong, S.J.; Kim, K.P.; Surh, Y.J. Breast cancer cell-derived soluble CD44 promotes tumor progression by triggering macrophage IL-1β production. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Bassanelli, M.; Sioletic, S.; Martini, M.; Giacinti, S.; Viterbo, A.; Staddon, A.; Liberati, F.; Ceribelli, A. Heterogeneity of PD-L1 Expression and Relationship with Biology of NSCLC. Anticancer Res. 2018, 38, 3789–3796. [Google Scholar] [CrossRef] [PubMed]
- Fancello, L.; Gandini, S.; Pelicci, P.G.; Mazzarella, L. Tumor mutational burden quantification from targeted gene panels: Major advancements and challenges. J. Immunother Cancer 2019, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Tartari, F.; Santoni, M.; Burattini, L.; Mazzanti, P.; Onofri, A.; Berardi, R. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: Recent insights and future challenges. Cancer Treat. Rev. 2016, 48, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Shen, J.; He, J.; Jiang, L.; Wang, W.; Guo, Z.; Peng, G.; Chen, G.; He, J.; et al. Prognostic Significance of Programmed Cell Death 1 (PD-1) or PD-1 Ligand 1 (PD-L1) Expression in Epithelial-Originated Cancer: A Meta-Analysis. Medicine 2015, 94, e515. [Google Scholar] [CrossRef]
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L.Q.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R.F.; O’Donnell, P.H. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017, 18, 212–220. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Rizvi, N.A.; Goldman, J.W.; Gettinger, S.N.; Borghaei, H.; Brahmer, J.R.; Ready, N.E.; Gerber, D.E.; Chow, L.Q.; Juergens, R.A.; et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-Based Tumor Mutational Burden as a Predictor of Clinical Benefit in Non-Small-Cell Lung Cancer Patients Treated with Atezolizumab. Nat. Med. 2018, 24, 1441–1448. Available online: http://www.nature.com/articles/s41591-018-0134-3 (accessed on 13 August 2018). [CrossRef]
- Noé, G.; Bellesoeur, A.; Golmard, L.; Thomas-Schoemann, A.; Boudou-Rouquette, P.; Tiako Meyo, M.; Puszkiel, A.; Arrondeau, J.; Alexandre, J.; Goldwasser, F.; et al. Differential Kinase Activation in Peripheral Blood Mononuclear Cells from Non-Small-Cell Lung Cancer Patients Treated with Nivolumab. Cancers 2019, 11, 762. [Google Scholar] [CrossRef]
- Cavanna, L.; Citterio, C.; Orlandi, E. Immune Checkpoint Inhibitors in EGFR-Mutation Positive TKI-Treated Patients with Advanced Non-Small-Cell Lung Cancer Network Meta-Analysis. Oncotarget 2019, 10, 209. Available online: http://www.oncotarget.com/fulltext/26541 (accessed on 13 August 2019). [CrossRef] [PubMed][Green Version]
- Carbognin, L.; Pilotto, S.; Milella, M.; Vaccaro, V.; Brunelli, M.; Caliò, A.; Cuppone, F.; Sperduti, I.; Giannarelli, D.; Chilosi, M.; et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. Santini D, editor. PLoS ONE 2015, 10, e0130142. [Google Scholar] [CrossRef] [PubMed]
- Abu Hejleh, T.; Furqan, M.; Ballas, Z.; Clamon, G. The clinical significance of soluble PD-1 and PD-L1 in lung cancer. Crit. Rev. Oncol. Hematol. 2019, 143, 148–152. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, G.; He, Y.; Zhu, H.; Zhang, H.; Feng, Z. Blockade of B7-H1 with sPD-1 Improves Immunity against Murine Hepatocarcinoma. Anticancer Res. 2005, 25, 3309–3313. [Google Scholar] [PubMed]
- Elhag, O.A.; Hu, X.J.; Wen-Ying, Z.; Li, X.; Yuan, Y.Z.; Deng, L.F.; Liu, D.L.; Liu, Y.L.; Hui, G. Reconstructed Adeno-Associated Virus with the Extracellular Domain of Murine PD-1 Induces Antitumor Immunity. Asian Pac. J. Cancer Prev. 2012, 13, 4031–4036. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Nie, H.; Liu, A.; Feng, G.; He, D.; Xu, R.; Zhang, Q.; Dong, C.; Zhang, J.Z. Aberrant Regulation of Synovial T Cell Activation by Soluble Costimulatory Molecules in Rheumatoid Arthritis. J. Immunol. 2006, 177, 8844–8850. [Google Scholar] [CrossRef] [PubMed]
- Greisen, S.R.; Rasmussen, T.K.; Stengaard-Pedersen, K.; Hetland, M.L.; Hørslev-Petersen, K.; Hvid, M.; Deleuran, B. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand. J. Rheumatol. 2014, 43, 101–108. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, H.; Chai, Y.; Song, H.; Tong, Z.; Wang, Q.; Qi, J.; Wong, G.; Zhu, X.; Liu, W.J.; et al. An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat. Commun. 2017, 8, 14369. [Google Scholar] [CrossRef]
- Higel, F. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. Pharm. Biopharm. 2016, 100, 94–100. [Google Scholar] [CrossRef]
- Frigola, X.; Inman, B.A.; Krco, C.J.; Liu, X.; Harrington, S.M.; Bulur, P.A.; Dietz, A.B.; Dong, H.; Kwon, E.D. Soluble B7-H1: Differences in production between dendritic cells and T cells. Immunol. Lett. 2012, 142, 78–82. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Canli, Ö.; Tal, A.; Pleli, T.; Trojan, J.; Schmidt, M.; Kronenberger, B.; Zeuzem, S.; Piiper, A.; Greten, F.R.; et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur. J. Cancer 2016, 59, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Iwasa, S.; Sasaki, Y.; Shoji, H.; Honma, Y.; Takashima, A.; Okita, N.T.; Kato, K.; Hamaguchi, T.; Yamada, Y. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Nam, A.R.; Bang, J.H.; Park, J.E.; Kim, T.Y.; Lee, K.H.; Han, S.W.; Im, S.A.; Kim, T.Y.; Bang, Y.J.; et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget 2016, 7, 76604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mahoney, K.M.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.H.; et al. Soluble PD-L1 as a Biomarker in Malignant Melanoma Treated with Checkpoint Blockade. Cancer Immunol. Res. 2017, 5, 480–492. [Google Scholar] [CrossRef]
- Takeuchi, M.; Doi, T.; Obayashi, K.; Hirai, A.; Yoneda, K.; Tanaka, F.; Iwai, Y. Soluble PD-L1 with PD-1-binding capacity exists in the plasma of patients with non-small cell lung cancer. Immunol. Lett. 2018, 196, 155–160. [Google Scholar] [CrossRef]
- Khan, M.; Lin, J.; Liao, G.; Tian, Y.; Liang, Y.; Li, R.; Liu, M.; Yuan, Y. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e11936. [Google Scholar] [CrossRef]
- Bonomi, M.; Ahmed, T.; Addo, S.; Kooshki, M.; Palmieri, D.; Levine, B.J.; Ruiz, J.; Grant, S.; Petty, W.J.; Triozzi, P.L. Circulating immune biomarkers as predictors of the response to pembrolizumab and weekly low dose carboplatin and paclitaxel in NSCLC and poor PS: An interim analysis. Oncol. Lett. 2019, 17, 1349–1356. Available online: http://www.spandidos-publications.com/10.3892/ol.2018.9724 (accessed on 30 January 2020). [CrossRef]
- Zhang, M.; Li, G.; Wang, Y.; Wang, Y.; Zhao, S.; Haihong, P.; Zhao, H. PD-L1 expression in lung cancer and its correlation with driver mutations: A meta-analysis. Sci. Rep. 2017, 7, 10255. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fang, W.; Zhang, Y.; Hong, S.; Kang, S.; Yan, Y.; Chen, N.; Zhan, J.; He, X.; Qin, T.; et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 2015, 6, 14209. Available online: http://www.oncotarget.com/fulltext/3694 (accessed on 5 February 2020). [CrossRef] [PubMed]
- Puszkiel, A.; Noé, G.; Boudou-Rouquette, P.; Le-Cossec, C.; Arrondeau, J.; Giraud, J.S.; Thomas-Schoemann, A.; Alexandre, J.; Vidal, M.; Goldwasser, F.; et al. Development and validation of an ELISA method for the quantification of nivolumab in plasma from non-small-cell lung cancer patients. J. Pharm. Biomed. Anal. 2017, 139, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
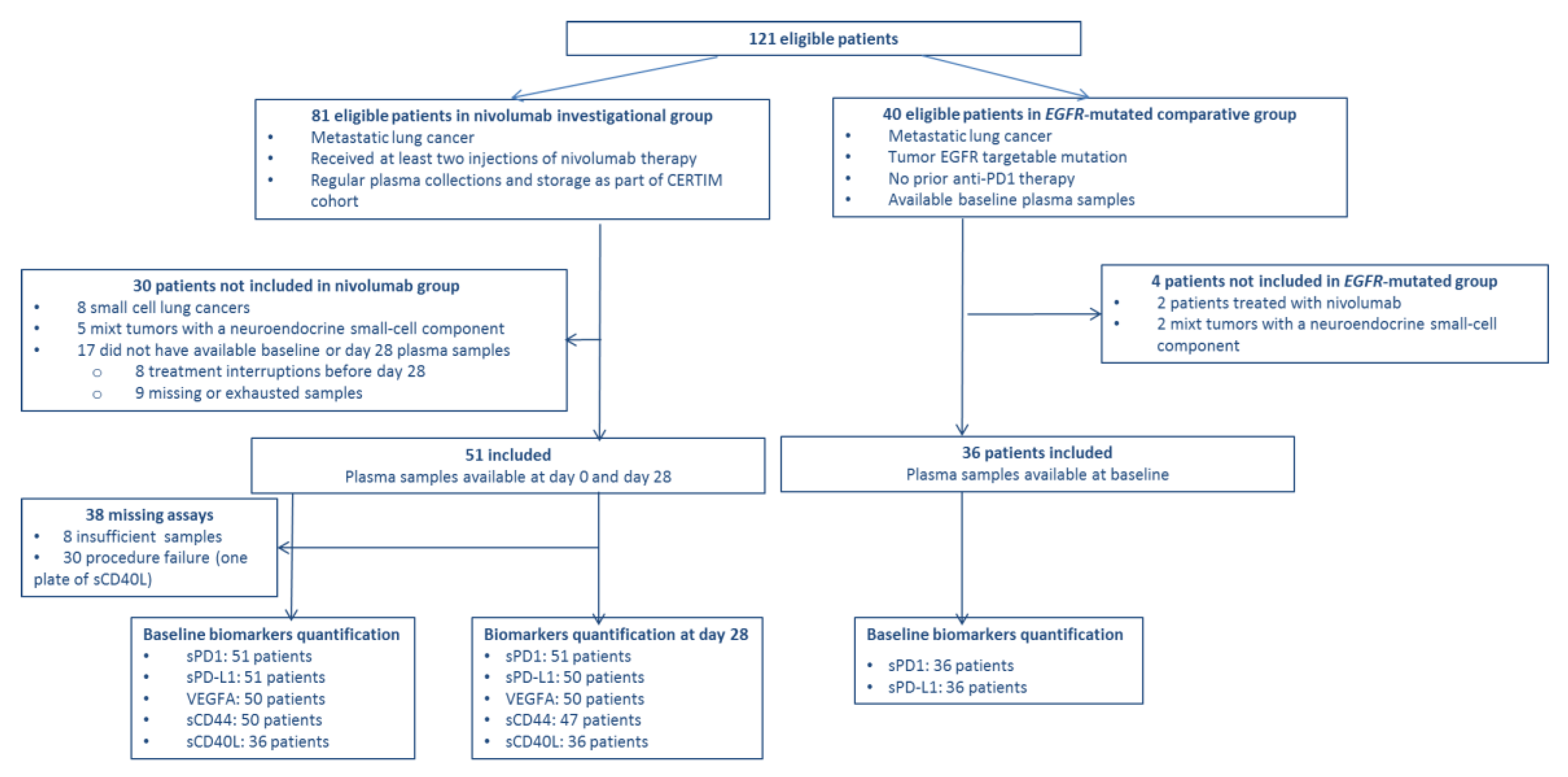


| Characteristics | Nivolumab (n = 51) | EGFR-Mutated (n = 36) |
|---|---|---|
| Sex, n (%) | ||
| Males | 29 (56.9) | 7 (19.4) |
| Females | 22 (43.1) | 29 (80.6) |
| Median age, years (IQR) | 66 (60–69) | 45 (36–54) |
| Smoking habit, n (%) | ||
| Never | 2 (4) | 25 (69.4) |
| Former | 37 (72.5) | 10 (27.8) |
| Active | 12 (23.5) | 1 (2.8) |
| ECOG, n (%) | ||
| 0 | 1 (2) | 9 (25) |
| 1 | 29 (56.8) | 19 (52.8) |
| 2 | 19 (37.2) | 8 (22.2) |
| 3 | 2 (4) | 0 (0) |
| Type of cancer, n (%) | ||
| Adenocarcinoma | 40 (78.4) | 34 (94.4) |
| Squamous cell carcinoma | 11 (21.6) | 0 (0) |
| Large cell | 0 (0) | 2 (5.6) |
| Metastases, n (%) | ||
| Synchronous | 35 (68.6) | 30 (83.3) |
| Metachronous | 16 (31.4) | 6 (16.7) |
| Number of metastatic sites, n (%) | ||
| 1 | 21 (41.2) | 18 (50) |
| 2 | 15 (29.4) | 4 (11.1) |
| 3 | 11 (21.6) | 9 (25) |
| > 3 | 4 (7.8) | 5 (13.9) |
| Mutations, n (%) | ||
| EGFR | 2 (4) | 36 (100) |
| ALK | 2 (4) | 0 (0) |
| KRAS | 12 (23.5) | 0 (0) |
| Others | 4 (7.8) | 0 (0) |
| Tumor PD-L1 expression, n (%) | ||
| 0 | 14 (27.5) | - |
| 1–4% | 3 (5.9) | - |
| 5–9% | 6 (11.7) | - |
| 10–24% | 5 (9.8) | - |
| 25–49% | 3 (5.9) | - |
| ≥ 50% | (17.6) | - |
| Unknown | 11 (21.6) | - |
| Prior antiangiogenic therapy, n (%) | ||
| Yes | 15 (29.4) | 0 (0) |
| No | 36 (70.6) | 36 (100) |
| Number of prior lines before nivolumab, n (%) | ||
| 1 | 35 (68.6) | - |
| 2 | 7 (13.7) | - |
| 3 | 6 (11.8) | - |
| > 3 | 3 (5.9) | - |
| VARIABLES | PFS HR (95%CI) Univariate | p | PFS HR (95%CI) Multivariate | p | OS HR (95%IC) Univariate | p | OS HR (95%IC) Multivariate | p |
|---|---|---|---|---|---|---|---|---|
| Patients’ characteristics (n = 51) | ||||||||
| Age | 1.00 (0.96–1.04) | 0.899 | 0.99(0.94–1.04) | 0.695 | ||||
| ECOG 2 or 3 vs. 0 or 1 | 1.09 (0.59–2.00) | 0.783 | 1.23 (0.59–2.57) | 0.583 | 1.13 (0.56–2.27) | 0.733 | 1.03 (0.45–2.36) | 0.944 |
| Smoking: former vs. never | 0.65 (0.15–2.73) | 0.552 | 0.66 (0.09–4.93) | 0.683 | ||||
| Smoking: active vs. never | 0.59 (0.13–2.77) | 0.504 | 0.53 (0.06–4.51) | 0.564 | ||||
| Biology (n = 51) | ||||||||
| CRP | 1.01 (1.00–1.02) | 0.029 | 1.00 [0.99–1.02] | 0.578 | 1.01 (0.99–1.02) | 0.309 | 0.99 (0.98–1.01) | 0.709 |
| NLR | 1.06 (0.98–1.15) | 0.146 | 1.12 (1.02–1.24) | 0.020 | ||||
| Lymphocytes | 1.00 (0.99–1.00) | 0.132 | 0.99 (0.99–1.00) | 0.012 | ||||
| Pathology (n = 40) | ||||||||
| PD–L1 TC | 0.99 (0.98–1.00) | 0.067 | 0.99 [0.98–1.00] | 0.051 | 0.99 (0.97–0.99) | 0.060 | 0.99 (0.97–1.00) | 0.058 |
| PD–L1 TC: 1% cut–off | 0.62 (0.31–1.22) | 0.166 | 0.62 (0.29–1.36) | 0.236 | ||||
| PD-L1 TC: 10% cut-off | 0.47 (0.23–0.97) | 0.040 | 0.35 (0.14–0.85) | 0.021 | ||||
| PD-L1 TC: 50% cut-off | 0.51 (0.21–1.24) | 0.136 | 0.47 (0.16–1.38) | 0.170 | ||||
| Plasma biomarkers | ||||||||
| sPD-1 | 3.03 (1.01–9.12) | 0.049 | 2.33 (0.76–7.18) | 0.141 | ||||
| sPD-1 positive (n = 15) vs. negative (n = 36) | 2.59 (1.29–5.21) | 0.007 | 2.28 (1.11–4.68) | 0.025 | ||||
| sPD-L1 | 1.89 (0.72–4.95) | 0.194 | 2.15 (0.77–6.01) | 0.145 | ||||
| sPD-L1 positive (n = 27) vs. negative (n = 24) | 2.68 (1.36–5.28) | 0.004 | 2.68 (1.23–5.84) | 0.013 | ||||
| sPD-1 and/or sPD-L1 | ||||||||
| 1 positive biomarker (n = 20) vs. 0 (n = 20) | 4.13 (1.89–9.02) | 0.0004 | 4.00 (1.54–10.40) | 0.004 | ||||
| 2 positive biomarkers (n = 11) vs. 0 (n = 20) | 4.11 (1.64–10.3) | 0.003 | 3.99 (1.44–11.00) | 0.008 | ||||
| sCombo positive (n = 31) vs. negative (n = 20) | 4.12 (1.95–8.71) | 0.0002 | 2.66 [1.17–6.08] | 0.020 | 3.99 (1.63–9.80) | 0.003 | 2.17 (0.86–5.45) | 0.101 |
| VEGFA | 1.00 (1.00–1.00) | 0.17 | 1.00 (0.99–1.00) | 0.525 | ||||
| sCD44 | 1.02 (0.97–1.07) | 0.399 | 1.01 (0.96–1.07) | 0.650 | ||||
| sCD40L | 2.04 (0.56–7.97) | 0.278 | 1.00 (0.99–1.00) | 0.515 | ||||
| sCD40L positive (n = 13) vs. negative (n = 23) | 1.43 (0.67–3.03) | 0.355 | 1.59 (0.35–7.14) | 0.546 |
| VARIABLES | PFS HR (95%CI) Univariate | p | PFS HR (95%CI) Multivariate | p | OS HR (95%CI) Univariate | p | OS HR (95%CI) Multivariate | p |
|---|---|---|---|---|---|---|---|---|
| ECOG 2 or 3 vs. 0 or 1 (n = 51) | 1.09 (0.59–2.00) | 0.783 | 1.08 (0.53–2.23) | 0.827 | 1.13 (0.56–2.27) | 0.733 | 0.78 (0.33–1.83) | 0.566 |
| CRP | 1.01 (1.00–1.02) | 0.029 | 1.01 (0.99–1.02) | 0.230 | 1.01 (0.99–1.02) | 0.309 | 1.00 (0.99–1.02) | 0.878 |
| PD-L1 TC (n = 40) | 0.99 (0.98–1.00) | 0.067 | 0.99 (0.98–1.00) | 0.058 | 0.99 (0.97–0.99) | 0.060 | 0.99 (0.97–1.00) | 0.075 |
| Delta sPD-1 | ||||||||
| Positive or null (n = 44) vs. negative (n = 7) | 0.29 (0.12–0.68) | 0.005 | 0.49 (0.30–0.80] | 0.004 | 0.28 (0.11–0.70) | 0.007 | 0.39 (0.21–0.71) | 0.002 |
| Positive (n = 9) vs. null or negative (n = 42) | 0.93 (0.429–2.02) | 0.855 | 0.71 (0.29–1.73) | 0.447 | ||||
| Delta sPD-L1 | ||||||||
| Positive or null (n = 44) vs. negative (n = 7) | 0.69 (0.34–1.38) | 0.291 | 0.41 (0.20–0.87) | 0.020 | ||||
| Positive (n = 7) vs. null or negative (n = 43) | 1.05 (0.44–2.51) | 0.908 | 0.59 (0.19–1.94) | 0.383 | ||||
| Delta VEGFA | ||||||||
| Positive or null (n = 38) vs. negative (n = 12 | 1.15 (0.55–2.42) | 0.709 | 1.16 (0.52–2.58) | 0.725 | ||||
| Positive (n = 15) vs. null or negative (n = 35) | 0.71 (0.36–1.43) | 0.338 | 0.91 (0.42–1.97) | 0.803 | ||||
| Delta sCD44 | ||||||||
| Positive or null (n = 38) vs. negative (n = 8) | 0.90 (0.39–2.05) | 0.796 | 0.87 (0.30–2.52) | 0.797 | ||||
| Positive (n = 18) vs. null or negative (n = 28) | 0.53 (0.26–1.06) | 0.074 | 0.55(0.26–1.19) | 0.129 | ||||
| Delta sCD40L | ||||||||
| Positive or null (n = 26) vs. negative (n = 7) | 0.94 (0.39–2.25) | 0.883 | 0.71 (0.26–1.97) | 0.509 | ||||
| Positive (n = 10) vs. null or negative (n = 23) | 1.07 (0.47–2.43) | 0.869 | 1.14 (0.48–2.68) | 0.766 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiako Meyo, M.; Jouinot, A.; Giroux-Leprieur, E.; Fabre, E.; Wislez, M.; Alifano, M.; Leroy, K.; Boudou-Rouquette, P.; Tlemsani, C.; Khoudour, N.; et al. Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study. Cancers 2020, 12, 473. https://doi.org/10.3390/cancers12020473
Tiako Meyo M, Jouinot A, Giroux-Leprieur E, Fabre E, Wislez M, Alifano M, Leroy K, Boudou-Rouquette P, Tlemsani C, Khoudour N, et al. Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study. Cancers. 2020; 12(2):473. https://doi.org/10.3390/cancers12020473
Chicago/Turabian StyleTiako Meyo, Manuela, Anne Jouinot, Etienne Giroux-Leprieur, Elizabeth Fabre, Marie Wislez, Marco Alifano, Karen Leroy, Pascaline Boudou-Rouquette, Camille Tlemsani, Nihel Khoudour, and et al. 2020. "Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study" Cancers 12, no. 2: 473. https://doi.org/10.3390/cancers12020473
APA StyleTiako Meyo, M., Jouinot, A., Giroux-Leprieur, E., Fabre, E., Wislez, M., Alifano, M., Leroy, K., Boudou-Rouquette, P., Tlemsani, C., Khoudour, N., Arrondeau, J., Thomas-Schoemann, A., Blons, H., Mansuet-Lupo, A., Damotte, D., Vidal, M., Goldwasser, F., Alexandre, J., & Blanchet, B. (2020). Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study. Cancers, 12(2), 473. https://doi.org/10.3390/cancers12020473








