Abstract
Hepatocellular carcinoma (HCC) is among the ten most commonly diagnosed cancers and the fourth leading cause of cancer-related death. Patients with hepatitis B virus (HBV) infection are prone to developing chronic liver diseases (i.e., fibrosis and cirrhosis), and the HBV X antigen plays an important role in the development of HCC. The difficulty in detecting HCC at the early stages is one of the main reasons that the death rate approximates the incidence rate. The regulators controlling the downstream liver protein expression from HBV infection are unclear. Mass spectrometric techniques and customized programs were used to identify differentially expressed proteins which may be involved in the development of liver fibrosis and HCC progression in hepatitis B virus X protein transgenic mice (HBx mice). FSTL1, CTSB, and TGF-β enhanced the signaling pathway proteins during the pathogenesis of HBx. Missing proteins can be essential in cell growth, differentiation, apoptosis, migration, metastasis or angiogenesis. We found that LHX2, BMP-5 and GDF11 had complex interactions with other missing proteins and BMP-5 had both tumor suppressing and tumorigenic roles. BMP-5 may be involved in fibrosis and tumorigenic processes in the liver. These results provide us an understanding of the mechanism of HBx-induced disorders, and may serve as molecular targets for liver treatment.
1. Introduction
Globally, hepatocellular carcinoma (HCC) is among the top ten most commonly diagnosed cancers and the fourth leading cause of cancer-related death. In males, the mortality rate of HCC has been increasing and HCC is ranked as the second most common cause of cancer death [1]. Patients with hepatitis B virus (HBV) or hepatitis C virus (HCV) infection are at high risk of HCC development. An HBV infection can be acute or chronic. It has been reported that patients chronically infected with HBV may bear a 5- to 100-fold increased risk of HCC development [2]. In order to control HBV, an orally bioavailable antiviral prodrug of tenofovir named Tenofovir alafenamide (TAF, a nucleotide reverse transcriptase inhibitor) has been characterized with improved renal and bone safety in clinical trials [3]. Within the content of HBV, one of the cutting edge developments in HCC prevention is to use the Toronto HCC Risk Index (THRI) to assess the disease-specific incidence of HCC from cirrhosis patients for HCC risk prediction. The THRI combines clinical and laboratory parameters and has been validated by another cohort for its good predictive ability for HCC [4]. Additionally, HBV-related tumors have been characterized with high rates of chromosomal alterations and p53 inactivation, overactivation of fetal liver progenitor cells, and AKT pathway and β-catenin mutations [5].
HBV is a DNA virus and its genome can be converted into covalently closed circular DNA (cccDNA) and it can serve as a transcriptional template [6]. cccDNA integrates its DNA into the host genome resulting in both genomic instability and mutagenesis of diverse cancer-related genes. cccDNA constantly expresses the HBV regulatory HBx protein (Hepatitis B virus X antigen, a protein of 154 amino acids) and unleashes the cellular transcription program and proliferation control via oncogenic Ras signaling [7]. As a result, hepatocytes are sensitized to carcinogenic factors. HBx also induces anti-apoptotic activity and promotes the cell cycle progression of hepatocytes through the Akt/mTOR pathway [8]. HBx has been proposed as a multifunctional protein because HBx is required for virus replication and its effects on apoptosis and metabolism are likely to be essential for management of chronic HBV infection [9].
HBV infection is heavily involved in human hepatocarcinogenesis, and the Hepatitis B virus X antigen plays the lead role [10]. It has been reported that transgenic mice using the albumin promoter to express HBx were shown to develop HCC at 14 to 18 months without additional treatment [11,12]. Although there were no cancer cells detected in the liver of these HBx transgenic mice at the 11–13 months stage, liver cancer cells were detected later on. This is an ideal timing to study proteins involved in the transition from non-tumor to tumor and to investigate the critical changes of HBx-induced HCC carcinogenesis.
Biomarkers are biochemical responses which can be indicators of exposure to and/or effects of a stressor within an individual. Missing proteins altered in concentration or state according to a specific biological process or disease fit into this category since measurement of concentration changes, relative or absolute, is essential to the discovery of valid biomarkers [13]. Biomarkers found in vitro or in vivo require further validation, and biobanking for viral hepatitis research has been well established [14]. This will facilitate the translational process. There are several possible reasons for missing proteins to go undetected (without evidence at the protein level); these can be technical (such as ionization/detection failure or lack of uniquely identifiable tryptic peptides), biological (low abundance in sample, silent genes present only in the genome, expressed only developmentally regulated or only in a certain time or space) or informatics (erroneous annotation, parsimonious protein assembly of MS/MS identifications or expression in rare cell/tissue types) [15].
Missing proteins may be involved in various cellular activities in both normal and tumor cells. They may function in cell growth, division, differentiation, apoptosis, migration, metastasis, angiogenesis and adhesion [16]. Thus, missing proteins in tumor-developing tissue could be potential biomarkers associated with carcinogenesis. Additionally, disease-specific protein biomarkers could help us understand disease mechanisms in which proteins play a key role. Previously, we generated a database listing the identities of missing proteins in tumor cells and potential diagnostic cancer biomarkers [17]. In addition, an HBx-induced HCC mouse model has been demonstrated to identify potential chemopreventive agents of HBV-related hepatocarcinogenesis [18]. In this study, we used proteomic approaches to characterize proteins significant for the development of liver fibrosis and transition to HCC. The expression profile offers not only information on the nature of missing proteins related to liver physiology, but also potential diagnostic protein markers involved in the progression of HBx-induced HCC. These markers might serve as potential therapeutic target molecules for HCC treatment.
2. Results
2.1. Pathological Findings in HBx Mice
Figure 1 shows the immunohistochemistry staining of mouse liver samples from WT and HBx groups. Samples were stained with antibodies of HBx. Expression of HBx protein in the transgenic livers was confirmed by immunohistochemistry (IHC) using an HBx polyclonal antibody. Compared with liver tissue of WT littermate mice, a higher proportion of HBx protein-expressing levels was observed in HBx mice. Enzymes produced from the liver, such as ALT and AST, can profile liver function. Figure S1 shows the AST and ALT averages in wild-type and HBx mice. The AST and ALT averages were higher in HBx mice than in wild-type mice. AST is found in several organs, such as the liver, muscle and heart, whereas ALT is mainly found in the liver and kidneys. Also, ALT is mainly distributed in the cytoplasm of hepatocytes, while AST is mainly distributed in the cytosol and mitochondria of hepatocytes. Thus, the index of ALT in liver injury is more sensitive and specific than that of AST, and it is often used as one of the indicators during HBV assessment.
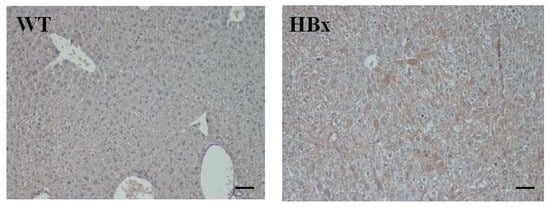
Figure 1.
Immunohistochemistry (IHC) of mouse liver samples from wide type (WT) and hepatitis B virus X protein transgenic (HBx) mice. Samples were stained with antibodies of HBx. The scale bars indicate 30 μm.
All the liver tissues were processed for Hematoxylin and Eosin (H&E) staining for histological analysis while only representative results are shown in Figure 2. There are some microscopic lesions in the livers of mice, including (1) changes in hepatocyte focus (clear cell lesions), as shown in Figure 2A, (as indicated by*); (2) inflammatory cell infiltration in the liver, as shown in Figure 2B (as indicated by bold arrows); (3) hepatocyte hypertrophy (as indicated by arrows) and nuclear giant cell hepatocytes (as indicated by a circle), as shown in Figure 2C; and (4) hepatic ellipsoidal hyperplasia. Other observations include the accumulation of glycogen in the liver, cell swelling, granular or cell-like appearance of the cytoplasm, and vacuoles lacking uniform size. Figure 3 shows the Masson’s trichrome staining for the liver histopathological examination of the livers from WT and HBx mice. The HBx liver sample showed diffuse fibrosis of hepatic subcapsule and fibrosis expansion without septa formation.

Figure 2.
Hematoxylin and eosin staining histopathological examination of the livers from WT and HBx mice. (A) Focus of hepatocellular alteration (Clear cell foci); (B) inflammatory cell infiltration; (C) hepatocellular hypertrophy. The scale bars indicate 30 μm.
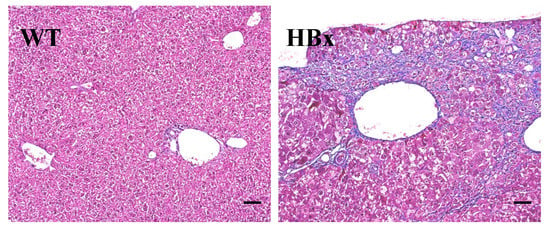
Figure 3.
Masson’s trichrome staining for the liver histopathological examination of the livers from WT and HBx mice. (HBx) Diffuse fibrosis of hepatic subcapsule and fibrosis expansion without septa formation.
2.2. Proteomic Analysis
Thousands of unique peptides were identified in pooled liver samples from the MASCOT database. These proteins were identified at minimal confidence levels as only one unique peptide sequence was matched. Studies reported a total of 541 protein identifications with high confidence levels. In this study, HBx mice were selected to profile HBV-related proteins. Hepatic proteins from both HBx and wild-type mice were extracted, digested and applied to a nano-UPLC-ESI-MS/MS system for analyzing fragmentation patterns of tryptic peptides. Each sample was subjected to three replicate runs. A total of 16 proteins were related to HBV-infection, tumorgenesis and inflammation. The search results of the liver samples from HBx group showed that Cathepsin B (CTSB) and Follistatin-related protein 1 (FSTL1) were the only proteins involved in tumorigenesis (Table 1 and Table 2), while Cytochrome c (CYC), Dihydrolipoyl dehydrogenase (DLDH), Exocyst complex component 4 (EXOC4), Glutamate–cysteine ligase catalytic subunit (GSH1) and Ubiquitin-40S ribosomal protein S27a (RS27A) were involved in HBV-infection (Table 3 and Table 4). heat shock 70 kDa protein 1-like (HS71L), heat shock protein HSP 90-beta (HS90B) and heat shock cognate 71 kDa protein (HSP7C) were involved in inflammation (Table 5 and Table 6). Among these proteins, only transforming growth factor-beta receptor-associated protein 1 (TGF-β) was associated with tumorigenesis, inflammation, and HBV-infection (Figure 4).

Table 1.
Summary of proteins corresponding to tumorigenesis in HBx group.

Table 2.
Summary of protein function corresponding to tumorigenesis in HBx group.

Table 3.
Summary of proteins corresponding to HBV infection.

Table 4.
Summary of protein function corresponding to HBV infection.

Table 5.
Summary of proteins corresponding to inflammation.

Table 6.
Summary of protein function corresponding to inflammation.

Figure 4.
The MASCOT results indicate that 11 proteins were associated with HBV, 7 proteins were associated with inflammation, and 7 proteins were associated with tumorigenesis. Among these proteins, Cathepsin B (CTSB) and Follistatin-related protein 1 (FSTL1) were involved in tumorgenesis, but not HBV nor inflammation. Transforming growth factor-beta receptor-associated protein 1 (TGF-β) was the only protein associated with tumor, inflammation, and HBV.
The expressions of three tumor-associated proteins, TGF-β, CTSB and FSTL1, were examined using immunohistochemical staining and Western blotting (Figure 5 and Figure 6). Samples from HBx mice were more hypertrophic and had larger nuclei than those from WT mice; the antibodies of these three proteins were also evident in the liver cells of these HBx mice.
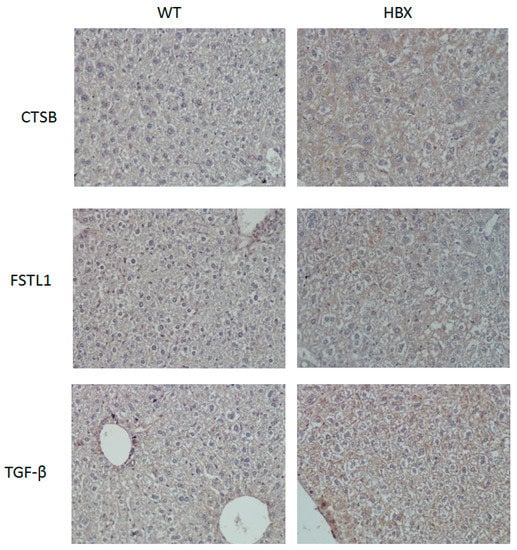
Figure 5.
Immunohistochemistry (IHC) of mouse liver samples from WT and HBx groups. Samples were stained with antibodies of Cathepsin B (CTSB), Follistatin-related protein 1 (FSTL1) or transforming growth factor-beta receptor-associated protein 1 (TGF-β.)
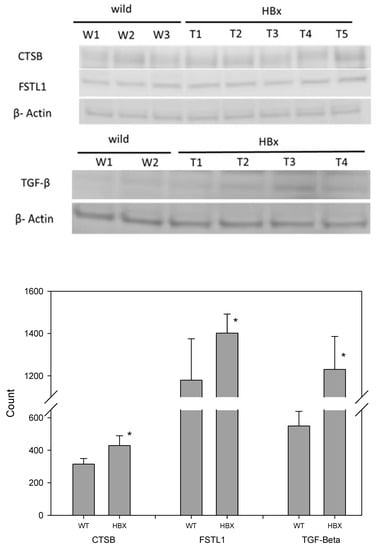
Figure 6.
Western blotting of mouse liver samples from WT and HBx groups. Samples were stained with antibodies of CTSB, FSTL1, TGF-β and β-actin (N = 8, * p < 0.05).
The differential protein expressions of CSTB, FSTL1 and TGFb were detected by three methods. First, they were distinguished by mass spectrometry as a qualitative analysis. Liver lysates of HBx mice and those of WT mice were pooled to reduce individual differences. Then, an immunohistochemical (IHC) technique was applied. Each liver was sliced into several sections and the differential protein expression between HBx and WT groups were easily visualized. Western blotting was also performed. Liver lysates of HBx mice and those of WT mice were also pooled. Since Western blot is a semi-quantitative analysis and the abnormal hepatic area was much smaller than the normal area, the differentially expressed protein signals may have been diluted. Moreover, the appearance of the liver was not significantly different between the two groups, and not all livers of HBX mice were diseased uniformly. IHC photos represent tissue microenvironments. The images taken were those of abnormal liver tissues, but that does not mean that the entire liver was abnormal. IHC is very different from Western blot, as Western blot is based on tiny pieces of liver grinded for proteins. Because some of the abnormal liver tissues were only pre-cancerous, they were hard to distinguish from normal liver tissue grossly. The livers of HBX mice were mostly normal. Proteins that appeared to be uniform under the microscope were only partly mottled in the entire liver. Normal tissue may have been sampled, so the specific protein differences may have been diluted in Western blot. The images of Western blots are not easy to identify the density with the naked eyes, but the image software analysis can still identify the difference in density of each band. In this study, Western blots of eight pairs of liver tissues of HBX and WT mice were compared, and were repeated three times for each liver sample. The concentrations of CSTB, FSTL1 and TGF-β were significantly different (p < 0.05). Mass spectrometry and immunohistochemistry showed that the protein expressions of CSTB, FSTL1 and TGFb were notably different between WT mice and HBx mice.
Previously, our group reported that the expression of hepatic glycine n-methyltransferase (GNMT) was down-regulated in NAFLD and HCC [19,20]. We also found that an oncoprotein–Phosphatidylinositol-3,4,5-Trisphosphate Dependent Rac Exchange Factor 2 (PREX2) was a GNMT-interacting protein. The degradation of PREX2 was much slower in the absence of GNMT, and this could lead to HCC development. Assessment of the hepatic PREX2 expression in HBx mice showed that the expression of PREX2 in HBx transgenic mice was higher than that in WT mice. (Figure S2).
2.3. Missing Protein Analysis
In this study, the peptides identified from MS/MS spectra were applied to our search program made by JAVA programming with the gene expression sequences downloaded from the HPA database. This database provides rapid analysis of suspected missing and non-missing proteins from chromosomes. A missing protein was positively identified when two or more product ion mass spectra of peptides completely matched the sequence of a missing protein in the database.
After utilizing the program, there were 29 possible missing proteins in the HBx group (Table 7). A further investigation found that 19 of them were missing proteins at the PE2 level. Among those 29 proteins, 13 proteins were related to tumor study, in which eight proteins were tumorigenic. CCDC67-002, GDF11-001, and LMX1A-001 were identified at the protein level and BMP5, FEZF2-004, HS6ST3, KIAA2022-001 and SP5 were missing proteins. Three were tumor suppressors (ADAMTS15, OSR1 and USP27X) and one was down-regulated in HCC (SRCIN1-001). In addition, LHX2-004 was related to HCC fibrosis (Table 8).

Table 7.
Summary of proteins identified by the human protein atlas (HPA) database.

Table 8.
Summary of protein function corresponding to tumorigenesis.
3. Discussion
Liver enzymes, such as ALT and AST, can sketch the liver function. Our data showed the AST and ALT averages were higher than in wild-type mice. For example, mild ALT elevation (5 times higher than normal) was reported in unspecific liver diseases, and moderate ALT elevation (normally 5 to 20 times) was common in acute viral hepatitis or drug-induced hepatitis, and severe ALT elevation (normally 20 to 50 times) was mainly seen in acute viral hepatitis. However, it has been stated that AST is also a good predictor in evaluating the performance of AST in low-infectious hepatitis B [21]. Since damaged livers secrete more AST and ALT, the abnormally high levels of AST and ALT indicate liver damage. The AST level was elevated in 34% of HBx mice. In addition, the ALT level was elevated in 90% of HBx mice. These were suggestive of either liver fibrosis or chronic hepatitis.
Our H&E staining of the liver tissues from HBx mice showed some microscopic lesions. These were consistent with the results mentioned in the literature [22]. These HBx mice have liver damage with cell morphology changes, but no liver tumors. Thus, these HBx mice were still in the stage where HBx virus was still replicating and none of the liver tumors had been formed.
Hepatocellular carcinoma has been described by Debes and coworkers as a unique tumor lacking biomarkers due to multifaceted complexities. This includes highly variable genetic components and variable liver disease-dependent protein expression as well as the unique individual immune system since an inflammatory environment leads to cirrhosis before forming HCC [23]. In this study, hepatic proteins from HBx mice have been characterized for biomarkers. Cathepsin B is a lysosomal cysteine protease and is one of 11 cysteine cathepsins [24]. Cathepsin B can function both as an endopeptidase and an exopeptidase (with carboxydipeptidase activity) [25]. Elevated expression of Cathepsin B protein was found in studies of breast, thyroid, and colorectal cancers [26,27,28]. Such high expression of cathepsin B in many different cancers led to the assumption that this enzyme played a fundamental role in progression of these tumors. Indeed, experimental down-regulation of Cathepsin B demonstrated reduced motility and invasion of cancer cells [29]. However, the role of Cathepsin B in HCC is still unknown. Ruan and colleagues reported that the over-expression of cathepsin B in HCC was associated with poor prognosis while Qin and coworkers stated that HCC patients with low expression levels of CTSB had poor survival [30,31].
Follistatin-like 1 (FSTL1) has calcium ion and heparin binding functions and five domains including a FS-like and a Kazal-like domain, two EF-hand domains, and a von Willebrand factor type C domain [32]. It belongs to the secreted protein acid and rich in cysteine (SPARC) family and it exhibits variable expression during pathological conditions including cancer. Multiple signaling pathways and biological processes are involved in FSTL1-mediated vascularization and regulation of immune responses. However, results from cancer progression studies of the proliferative, apoptotic, migratory, and inflammatory effects of FSTL1 were inconsistent [33]. Increased expression of FSTL1 was observed in most cases of HCC when compared to healthy controls. Overexpression of FSTL1 resulted in a HCC cell line (Huh7) expansion, seen as increased proliferation and inhibited apoptosis. AKT/GSK-3β signaling was activated, and the expression of pro-apoptotic BIM and BAX proteins decreased [34].
The TGF-beta (β) family is not only multifunctional, but also essential for survival. It plays important roles in regulation of growth, development of tissues, and maintenance of homeostasis [35]. This family has three members –TGF-β1, β2 and β3 and TGF-β 1 is the most abundant and universally expressed member. TGF-β 1, a secreted cytokine inactive during storage in the extracellular matrix, regulates growth and differentiation in a variety of cell types and is involved in normal development and immune function. However, it also triggers the epithelial-mesenchymal transition, angiogenesis, invasion, and connective tissue growth factor production [36,37,38,39]. These features promote HCC tumor progression. Fibrosis, epithelial-mesenchymal-transition and TGF-beta are closely related to cancer progression, and cancer progression is mediated by cancer-associated fibroblasts (CAF) rendering drug resistance. The tumor microenvironment may play a key role in drug resistance and the central element of the tumor microenvironment is a cancer-associated fibroblast (CAF). The mechanisms of CAF-mediated therapeutic resistance include HGF/MET by restoring survival signaling pathway (PI3K or MAPK), PDGF/PDGF-R by improving IFP, IL-6 by activating of STAT3, SDF-1/CXCR4 by BCL-2 / BCL-XL or activating NOTCH pathway to regulate CSCs and AnXA1/TGR-beta/CA IX/ MMP-2/MMP-9 to regulate EMT [40]. Indeed, it has been shown that CAFs and TGF-β signaling activation contribute to chemotherapy resistance in cancer [41]. Another study showed that HCC patients receiving sorafenib may benefit from immune modulation strategies [42]. Argentiero and colleagues reported that a specific WNT pathway inhibitor may instruct the immune system to increase cytotoxicity in tumor and re-establish the anti-PDAC immunity [43]. Such immune-based therapeutic strategies targeting WNT may be adapted to hepatic fibrosis. However, HCC can be classified by immune status, where roughly 30% of HCCs belong to the "immune class” (inflamed hot tumors) and the rest are “non-inflamed” or “cold tumors” [44]. These hot HCC tumors are more likely to be effective if treated with immune checkpoint inhibitors. Half of the cold tumors belong to the "immune exclusion class", which is characterized by Wnt/CTNNB1 mutations and might have innate resistance to anti–PD-1/PD-L1 inhibitors. The rest, about one third of HCCs, are categorized as "immune intermediate class" (noninflamed tumors with wild-type CTNNB1 and intermediate intensities of immune infiltration). Since the molecular pathways and gene signatures are not defined, the patients may or may not respond to immunotherapy. Thus, selections of patients and trials are definitely very important for HCC treatments because intra-individual differences may influence the outcome. For example, the importance of adequate patient selection in sorafenib treatment for HCC has been shown in a recent clinical trial where sorafenib usage was restricted to Child–Pugh A patients [45].
TGF-β is also heavily involved in tumor microenvironment. There is increasing attention to the role of the tumor microenvironment in cancer development and progression. Because cancer cells build up a complex relationship within the surrounding environment and the host immune response is important to the success of immunotherapy, the tumor vasculature is the key to successful treatment response. Tumor microenvironment may be affected by the types of tumors. In liquid type cancers, endothelial cells play an important role. Endothelial cells regulate the microenvironment through immune cell trafficking and immune response modulation. Such response can be a powerful treatment approach [46]. In fact, endothelial cells are important components of the bone marrow microvasculature and are in close contact with CD8+ T cells. Moreover, endothelial cells trap and present antigen to CD8+ T cells as well as produce high amounts of IL-10 and TGF-β. However, multiple myeloma patient bone marrows produce tumor-specific CD8+ T cells that are unable to control the proliferation of the malignant plasma cells. Furthermore, there are two CD8+ T cell populations in multiple myeloma patient’s bone marrow. One is sustained by endothelial cells and produce TGF-β to promote the development of tumors and to counteract the activity of the other population. It illustrates an unpredicted immune regulatory mechanism that inhibits the development of antitumor immunity and may diminish the effect of immunotherapy [47]. In agreement with the idea of microenvironment, targeting the TGF-β pathway with a TGFβRI small molecule inhibitor (galunisertib) has been proven to support anti-tumor immunity in a solid tumor study [48]. The treatment with galunisertib showed strong dose-dependence with anti-tumor activities where T cell proliferation mediated by TGF-β was suppressed. Because this effect was CD8+ T cell dependent and experimental results showed a successful establishment of immunological memory, utilizing a secondary immune response to effectively target tumor has become possible. Moreover, the combination of galunisertib with PD-L1 blockade showed a synergic anti-tumor effect [48]. Dissimilar to TGFβ-dependent environment, bone metastasis development starts with colonization of the bone marrow microenvironment. Cells may survive or be dormant depending on the locality. If cells survived a long-lived dormant state, some will re-activate and grow to modify the microenvironment. Eventually, tumor cells become microenvironment independent. Thus, targeting the dependency between colonizing tumor cells and the cells of bone opens new therapeutic opportunities [49].
HBx, CTSB, TGF-β1 and FSTL1 are heavily involved in the TGF-β signal transmission pathway (Figure 7). SMAD4 is recognized as an important tumor suppressor, but nuclear SMAD4 levels were significantly increased in HCC tumors [50]. HBx induces TGF-β1 to activate Smad and non-Smad pathways where CTSB up-regulates MMP expression and FSTL1 activates RSmad3. FSTL1 induces liver fibrosis through hepatic stellate cell activation; CTSB plays key roles in extracellular matrix (ECM) degradation and tissue remodeling, which drive hepatic stellate cell proliferation and promote fibrogenic potential [51]. Moreover, Manchanda and co-workers suggested the potential of using CTSB as a diagnostic biomarker for chronic liver diseases [52]. Indeed, these three proteins play important roles in liver fibrosis and our HBx mice did not develop liver tumors.
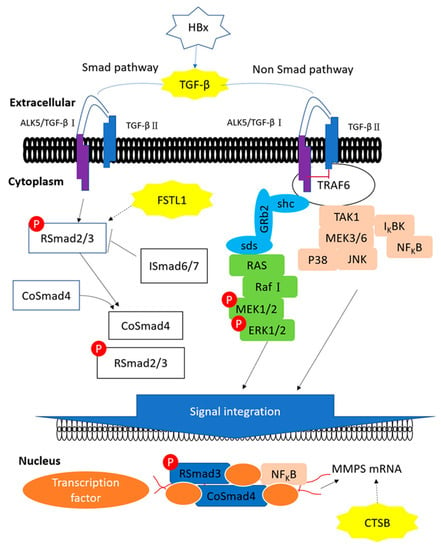
Figure 7.
The proposed influences of HBx in TGF-β pathway. HBx, CTSB, TGF-β1 and FSTL1 are heavily involved in the TGF-β signal transmission pathway. HBx induces TGF-β1 to activate Smad and non-Smad pathways where CTSB up-regulates MMP expression and FSTL1 activates RSmad3.
The protein-protein interactions among those 29 proteins are shown in Figure 8. LHX2, BMP5 and GDF11 had complex interactions with other proteins. In the liver, LIM homeobox 2 (LHX2), a transcription factor, is involved in mesoderm development and may function to inhibit the activation of hepatic stellate cells (HSC); such activation is essential to develop hepatic fibrosis and cirrhosis [53]. Growth differentiation factor 11 (GDF11) is also involved in mesoderm and embryonic development. In hepatic tumorigenesis, GDF11 was reported to reduce the viability of liver cancer cells and its mRNA expression was down-regulated in tumor tissues compared with that in the matching normal tissues [54]. After a careful functional assessment of the database, two were found to be related to liver fibrosis and ten were associated with tumorigenesis. Among these 11 proteins, only seven were evident at the transcription level and defined as missing proteins. Bone morphogenetic protein 5 (BMP-5), which was one of the missing proteins (PE2), plays roles in both fibrosis and tumorigenesis.
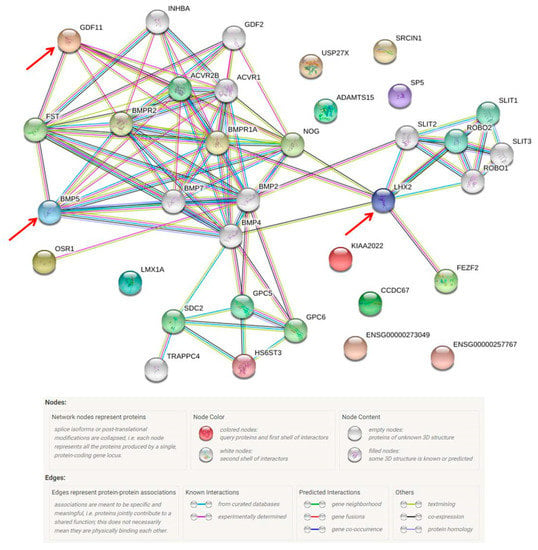
Figure 8.
The protein–protein interaction pathways are illustrated. Twenty-nine proteins were identified from the missing protein database to be related with HBX. LHX2, BMP5 and GDF11 are inter-connected with other proteins.
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily known for their roles in dorsal-ventral patterning, organogenesis and cell differentiation [55]. According to their sequence homology and function, the BMP/growth and differentiation factor (GDF) subfamily can be subdivided into seven groups, of which BMP-5, -6, -7 and -8 belong to the same group [56]. It has been shown that BMPs regulate various biological activities, including proliferation, differentiation, migration and cell death. Thus, BMP dysregulation can be pathological.
Indeed, multifunctional signaling molecules such as BMPs have attracted attention in cancer research, and BMP-5 does have a role in cancer. For example, higher BMP-5 expression is linked to female patients and lung adenocarcinoma (compared to male patients and squamous cell carcinoma), although the overall expression of BMP-5 is inhibited in non-small-cell lung cancer (NSCLC) tissues compared with adjacent normal tissues [57]. As a result, this differential expression pattern might be a potential prognostic biomarker or therapeutic target for patients with NSCLC. In breast cancer, TGF-β1-induced epithelial-to-mesenchymal transition is mediated by Blimp-1-dependent repression of BMP-5. In addition, BMP-5 mRNA levels in breast cancer cell lines and in primary breast tumors were lowered when compared with normal tissues, and correlated with cancer recurrence [58]. In peripheral nerve and neoplastic lesions of nerve sheath tumors, BMP-5 contributed to the maintenance of health, control of proliferation, and neoplastic transformation of the peripheral nervous system. In addition, the BMP-5 mRNA level in malignant schwannoma was relatively lower than that in benign lesions [59]. In adrenocortical carcinoma, BMP-5 was likely to be involved in the modulation of the malignant and functional phenotype of adrenocortical cancer cells. Down-regulation of BMP-5 mRNA expression was found in tissue samples from adrenocortical carcinoma and adrenocortical tumor cell lines when compared with normal adrenal glands [60]. In the digestive system, BMP-5 plays similar roles in both colorectal cancer (CRC) and pancreatic cancer. The loss of BMP-5 expression takes place as an early event in CRC where BMP-5 may dysregulate E-cadherin and then trigger tumor initiation and development [61]. Also, miR32 may target BMP-5 to help CRC development [62]. Low expression levels of BMP-5 were detected in various pancreatic cancer cells compared to normal samples [63]. BMP-5 displayed a biphasic role as being both detrimental and beneficial: BMP-5 treatment inhibited the growth of pancreatic cancer cells but promoted migration and invasion [63].
In the liver, BMPs take part in key functions, including liver specification (BMP-4), control of glucose homeostasis (BMP-4 and -6), liver bud formation (BMP-6), embryonic stem cell differentiation into hepatocyte-like cells (BMP-6), control of iron homeostasis (BMP-6) and enhancement of hepatocyte proliferation during regeneration (BMP-7) [64]. Although the function of BMP-5 in the liver is not clear, it could be similar to that of its groupmates (BMPs 6-8). Our study detected the up-regulation of BMP-5 in liver samples of HBx mice, and these livers had fibrosis but no tumor formation. This oncogenic role is very different from the one previously reported. Jin et al. reported that BMP-5 was down-regulated and acted as a tumor suppressor, and overexpression of BMP-5 was associated with malignancy of the oral epithelium, and especially with metastatic carcinoma cells in lymph nodes [65]. Protein expression of BMP-4 and BMP-7 was upregulated in HBV-related human cirrhosis/HCC samples, and the overexpression of BMP-4 and BMP-7 increased cell viability and enhanced cell migration in HCC cell-lines. [22] Moreover, Chen and coworkers reported that gene amplification and increased gene expression of BMP-5 were significant in HCC samples [66]. This is in agreement with our finding that BMP-5 may contribute to the fibrogenic and tumorigenic processes in the liver.
4. Materials and Methods
4.1. Hepatocyte-Specific HBx Transgenic and Wild-Type Mice
The generation of HBx transgenic mice has been described previously [11]. The wild-type C57BL/6 mice were provided by National Laboratory Animal Center (NLAC), NARLabs, Taiwan. All mice were maintained in specific pathogen-free areas of the animal holding facilities and were treated according to protocols approved by the Animal Care and Use Committee of Kaohsiung Medical University. All mice were kept in a 12-hour light–dark cycle room with water and standard mouse pellet chow. The mice sacrificed in this study were 11–13 months old (n = 10 for WT; n = 15 for HBx). All the mice had been fasting for at least 8 hours before sacrifice.
4.2. Serum Alanine Aminotransferase and Aspartate Aminotransferase Tests
Serum samples without hemolysis were collected for determination of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities. Liver transaminases were measured by Hitachi 7080 automatic biochemistry analyzer and were biomarkers of liver injury with some degree of intact liver function.
4.3. Histopathological Examination and Immunohistochemical Staining
Liver specimens were scored for fibrosis by examining Masson’s trichrome stained slides, and all other parameters, such as degeneration/inflammation, were observed by examining hematoxylin and eosin (H&E) stained slides. The tissues were fixed in 10% formalin, processed, and embedded in paraffin. The tissue slides were sectioned at 3–5 μm in thickness, and stained with H&E or Masson’s trichrome stain using standard procedure. The pathological reports were done by the National Laboratory Animal Center in Taiwan. For immunohistochemical staining analysis, tissue sections were de-paraffinized and subsequent blockage of the endogenous peroxidase activity was achieved by incubation with 2.5% methanolic hydrogen peroxide for 30 min. The tissue sections were stained with antibodies against Hepatitis B Virus X antigen (ab39716), FSTL1 (20182-1-AP), Cathepsin B (PA5-47975) or TGF-β (GTX 108510). The immunohistochemical assays were performed according to the manufacturer’s instructions and counterstained with hematoxylin (Dako).
4.4. Sample Digestion and Preparation
The mouse livers were homogenized, and then the proteins were extracted with RIPA buffer. Protein samples (100 μL) were transferred into 1.5 mL Eppendorf tubes and incubated at 37 °C for 3 h after mixing with 25 μL of 1 M dithiothreitol (DTT, USB Corporation, 15397). The samples were reduced and alkylated in the dark at room temperature for 30 min after the addition of 25 μL of 1 M iodoacetamide (IAA, Amersham Biosciences, RPN6302V) in 25 mM ammonium bicarbonate. Approximately 10 μL of 0.1 μg/μL modified trypsin digestion buffer (Trypsin Gold, Mass Spectrometry Grade, V5280, Promega, WI, USA) in 25 mM ammonium bicarbonate was added to the protein samples, which were then incubated at 37 °C for at least 12 h in a water bath. Two microliters of formic acid were added to each sample before mass spectrometric analysis for protein identification.
4.5. Immunoblotting
Each tissue lysate sample was electrophoresed through a precast gel (NuPAGE, 4–12% Bis-Tris Gel, 1.5 mm, Invitrogen, Carlsbad, CA). Proteins were transferred from the gel to a polyvinyldifluoride (PVDF) membrane (Millipore, Bedford, CA) and blocked with 5% milk in PBS (adjusted to pH 7.4) containing 0.05% Tween-20. The membranes were then separately incubated overnight with primary antibodies (1 ug/ mL) of anti- FSTL1, anti- Cathepsin B, anti- TGF-β, anti-PREX2 and anti-β-actin. After washing, the membrane was incubated with alkaline peroxidase-conjugated AffiniPure goat anti-rabbit IgG (111-035-003, Immuno Research) for 1 h (1:10,000). Proteins were detected with an enhanced chemiluminescent (ECL) system, and quantitative analysis of Western blotting was carried out using the ImageQuant-TL-7.0 software, version 2010 (Amersham Biosciences).
4.6. Proteomic Analysis
The protein tryptic digests were fractionated using a flow rate of 400 nL/min with a nano-UPLC system (nanoACQUITY UPLC, Waters, Milford, MA, USA) coupled to an ion trap mass spectrometer (LTQ Orbitrap Discovery Hybrid FTMS, Thermo, San Jose, CA, USA) equipped with an electrospray ionization source. For reverse phase nano-UPLC-ESI-MS/MS analyses, a sample (2 μL) of the desired peptide digest was loaded into the trapping column (Symmetry C18, 5 μm, 180 μm × 20 mm) by an autosampler. Reverse phase separation was performed using a linear acetonitrile gradient from 99% buffer A (100% D.I. water/0.1% formic acid) to 85% buffer B (100% acetonitrile/0.1% formic acid) in 100 min using the micropump at a flow rate of approximately 400 nL/min. Separation was performed on a C18 microcapillary column (BEH C18, 1.7 μm, 75 μm × 100 mm) using the nano separation system. As peptides were eluted from the micro-capillary column, they were electrosprayed into the ESI-MS/MS with the application of a distal 2.1 kV spraying voltage with heated capillary temperature of 200°C. Each scan cycle contained one full-scan mass spectrum (m/z range: 400–2000) and was followed by three data dependent tandem mass spectra. The collision energy of MS/MS analysis was set at 35%.
4.7. Protein Database Search and STRING Database for Protein–Protein Interaction Network Analysis
Mascot software (Version 2.2.1, Matrix Science, London, UK) was used to search the Swiss-Prot protein sequence database. For proteolytic cleavages, only tryptic cleavage was allowed, and the number of maximal internal (missed) cleavage sites was set to 2. There was no modification allowed. Mass tolerances of the precursor peptide ion and fragment ion were set to 10 ppm and 0.5 Da, respectively. In the case of a missing protein identification, we downloaded all gene expression sequences from the human protein atlas web (HPA, www.proteinatlas.org). Search for the presence of any missing protein utilized JAVA programming based on the HPA. The peptide sequences performed by Mascot software were uploaded to our program for missing protein identification and the search algorithm was set to 100% match. When a protein was identified by two or more unique peptides, the protein was considered to be present in the sample. The STRING database accumulates and integrates functional interactions among expressed proteins and consists of direct (physical) and indirect (functional) interactions. The latest version of STRING covers 5090 organisms with 24.6 million proteins. This database is committed to have the maximum protein interactions including primary and predicted interactions, such as annotated pathways, text-mining outcomes, inter-organism transfers or other accessory information [67]. In addition, STRING database was uploaded with differentially expressed genes to the protein-protein interaction (PPI) network. The association evidence of the STRING database can be classified into 7 categories: co-expression, text-mining, experimental data, previously curated pathway and protein-complex knowledge and three predicted categories (neighborhood, fusion, and gene co-occurrence). The STRING version 11 was used for the analysis.
5. Conclusions
Proteins significant for the development of liver fibrosis and transition to HCC were suggested and identified by proteomic approaches. The information provided here may shed light on the elucidation of molecular mechanisms involved in HBx-induced HCC. Our approach allows us to identify the critical proteins in hepatocarcinogenesis from an HBx-induced mouse model. These differentially expressed proteins and key missing proteins may serve as useful molecular markers for the early-stage diagnosis or treatment of HCC.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/2/409/s1, Figure S1: Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) level measurements showed that the averages of AST and ALT in the HBx transgenic mice were significantly higher than that in the wild-type mice. Figure S2. Immunoblotting of mouse liver samples. The PREX2 protein expression levels from HBx transgenic mice are higher than that from WT mice.
Author Contributions
Conceived and designed the experiments: Y.-C.T., M.-H.Y., Y.-M.A.C.; Data analysis: M.-H.Y., M.C., H.-H.M., Y.-C.C., C.-C.C., W.-C.T.; Anatomical pathology analysis: K.-C.C.; Protein identification: H.-Y.W., C.-H.Y.; Protein extraction and sample preparation: H.-Y.W., C.-H.L.; Paper writing: Y.-C.T., M.-H.Y., M.C., Y.-M.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by research grants: MOST 108-2221-E-037-003 from the Ministry of Science and Technology; NSYSUKMU109-P014 from NSYSU-KMU Research Project; KMU-TC108A04 from Kaohsiung Medical University Research Center Grant, AS-KPQ-105-TPP Taiwan Protein Project and the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Acknowledgments
The authors thank the Center for Research Resources and Development in Kaohsiung Medical University for the assistance in protein identification, and National Laboratory Animal Center (NLAC), NARLabs, Taiwan, for technical support in pathology analysis. We would like to thank Ting-Fen Tsai (Department of Life Sciences and Institute of Genome Sciences, National Yang-Ming University) for providing the HBx mice.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Brunetto, M.; Seto, W.K.; Lim, Y.S.; Fung, S.; Marcellin, P.; Ahn, S.H.; Izumi, N.; Chuang, W.L.; Bae, H.; et al. 96weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J. Hepatol. 2018, 68, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.A.; Kowgier, M.; Hansen, B.E.; Brouwer, W.P.; Maan, R.; Wong, D.; Shah, H.; Khalili, K.; Yim, C.; Heathcote, E.J.; et al. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J. Hepatol. 2018, 68, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, F.; Belloni, L.; Pediconi, N.; Levrero, M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin. Liver Dis. 2013, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Trivedi, A.; Johnson, D.L. Regulation of RNA polymerase I-dependent promoters by the hepatitis B virus X protein via activated Ras and TATA-binding protein. Mol. Cell. Biol. 1998, 18, 7086–7094. [Google Scholar] [CrossRef]
- Wang, X.; Huo, B.; Liu, J.; Huang, X.; Zhang, S.; Feng, T. Hepatitis B virus X reduces hepatocyte apoptosis and promotes cell cycle progression through the Akt/mTOR pathway In Vivo. Gene 2019, 691, 87–95. [Google Scholar] [CrossRef]
- Slagle, B.L.; Bouchard, M.J. Role of HBx in hepatitis B virus persistence and its therapeutic implications. Curr. Opin. Virol. 2018, 30, 32–38. [Google Scholar] [CrossRef]
- Murakami, S. Hepatitis B virus X protein: A multifunctional viral regulator. J. Gastroenterol. 2001, 36, 651–660. [Google Scholar] [CrossRef]
- Wu, B.K.; Li, C.C.; Chen, H.J.; Chang, J.L.; Jeng, K.S.; Chou, C.K.; Hsu, M.T.; Tsai, T.F. Blocking of G1/S transition and cell death in the regenerating liver of Hepatitis B virus X protein transgenic mice. Biochem. Biophys. Res. Commun. 2006, 340, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Fu, S.L.; Kao, C.H.; Yang, C.W.; Lin, C.H.; Hsu, M.T.; Tsai, T.F. Chemopreventive effect of silymarin on liver pathology in HBV X protein transgenic mice. Cancer Res. 2008, 68, 2033–2042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seibert, V.; Ebert, M.P.; Buschmann, T. Advances in clinical cancer proteomics: SELDI-ToF-mass spectrometry and biomarker discovery. Brief. Funct. Genom. Proteom. 2005, 4, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Van Hees, S.; Goethals, S.; Smits, E.; Huizing, M.; Francque, S.; De Winter, B.; Michielsen, P.; Vanwolleghem, T. Biobanking for Viral Hepatitis Research. Front. Med. (Lausanne) 2019, 6, 183. [Google Scholar] [CrossRef]
- Horvatovich, P.; Végvári, Á.; Saul, J.; Park, J.G.; Qiu, J.; Syring, M.; Pirrotte, P.; Petritis, K.; Tegeler, T.J.; Aziz, M.; et al. In Vitro Transcription/Translation System: A Versatile Tool in the Search for Missing Proteins. J. Proteome Res. 2015, 14, 3441–3451. [Google Scholar] [CrossRef]
- Adkins, J.N.; Varnum, S.M.; Auberry, K.J.; Moore, R.J.; Angell, N.H.; Smith, R.D.; Springer, D.L.; Pounds, J.G. Toward a human blood serum proteome: Analysis by multidimensional separation coupled with mass spectrometry. Mol. Cell. Proteom. 2002, 1, 947–955. [Google Scholar] [CrossRef]
- Yang, M.H.; Chen, W.J.; Fu, Y.S.; Huang, B.; Tsai, W.C.; Chen, Y.M.; Lin, P.C.; Yuan, C.H.; Tyan, Y.C. Utilizing glycine N-methyltransferasegene knockout mice as a model for identification of missing proteins in hepatocellular carcinoma. Oncotarget 2018, 9, 442–452. [Google Scholar] [CrossRef]
- Teng, C.F.; Yu, C.H.; Chang, H.Y.; Hsieh, W.C.; Wu, T.H.; Lin, J.H.; Wu, H.C.; Jeng, L.B.; Su, I.J. Chemopreventive effect of phytosomal curcumin on hepatitis b virus-related hepatocellular carcinoma in a transgenic mouse model. Sci. Rep. 2019, 9, 10338. [Google Scholar] [CrossRef]
- Liao, Y.J.; Chen, T.L.; Lee, T.S.; Wang, H.A.; Wang, C.K.; Liao, L.Y.; Liu, R.S.; Huang, S.F.; Chen, Y.M. Glycine N-methyltransferase deficiency affects Niemann-Pick type C2 protein stability and regulates hepatic cholesterol homeostasis. Mol. Med. 2012, 18, 412–422. [Google Scholar] [CrossRef]
- Li, C.H.; Yen, C.H.; Chen, Y.F.; Lee, K.J.; Fang, C.C.; Zhang, X.; Lai, C.C.; Huang, S.F.; Lin, H.K.; Chen, Y.M. Characterization of the GNMT-HectH9-PREX2 tripartite relationship in the pathogenesis of hepatocellular carcinoma. Int. J. Cancer 2017, 140, 2284–2297. [Google Scholar] [CrossRef]
- Ijaz, B.; Ahmad, W.; Javed, F.T.; Gull, S.; Hassan, S. Revised cutoff values of ALT and HBV DNA level can better differentiate HBeAg (-) chronic inactive HBV patients from active carriers. Virol. J. 2011, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Hsia, Y.; Yang, W.Y.; Lin, Y.I.; Li, C.C.; Tsai, T.F.; Chang, K.W.; Shieh, G.S.; Tsai, S.F.; Wang, H.D.; et al. Identification of the common regulators for hepatocellular carcinoma induced by hepatitis B virus X antigen in a mouse model. Carcinogenesis 2012, 33, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; Carrera, E.; Mattos, A.Z.; Prieto, J.E.; Boonstra, A.; ESCALON investigators. Hepatocellular carcinoma, a unique tumor with a lack of biomarkers. Ann. Hepatol. 2019, 18, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D.; Sloane, B.F. Cathepsin B: Multiple enzyme forms from a single gene and their relation to cancer. Enzyme Protein 1996, 49, 94–105. [Google Scholar] [CrossRef]
- McKerrow, J.H.; Bhargava, V.; Hansell, E.; Huling, S.; Kuwahara, T.; Matley, M.; Coussens, L.; Warren, R. A functional proteomics screen of proteases in colorectal carcinoma. Mol. Med. 2000, 6, 450–460. [Google Scholar] [CrossRef]
- Srisomsap, C.; Subhasitanont, P.; Otto, A.; Mueller, E.C.; Punyarit, P.; Wittmann-Liebold, B.; Svasti, J. Detection of cathepsin B up-regulation in neoplastic thyroid tissues by proteomic analysis. Proteomics 2002, 2, 706–712. [Google Scholar] [CrossRef]
- Wulfkuhle, J.D.; Sgroi, D.C.; Krutzsch, H.; McLean, K.; McGarvey, K.; Knowlton, M.; Chen, S.; Shu, H.; Sahin, A.; Kurek, R.; et al. Proteomics of human breast ductal carcinoma in situ. Cancer Res. 2002, 62, 6740–6749. [Google Scholar]
- Krueger, S.; Haeckel, C.; Buehling, F.; Roessner, A. Inhibitory effects of antisense cathepsin B cDNA transfection on invasion and motility in a human osteosarcoma cell line. Cancer Res. 1999, 59, 6010–6014. [Google Scholar]
- Ruan, J.; Zheng, H.; Rong, X.; Rong, X.; Zhang, J.; Fang, W.; Zhao, P.; Luo, R. Over-expression of cathepsin B in hepatocellular carcinomas predicts poor prognosis of HCC patients. Mol. Cancer. 2016, 15, 17. [Google Scholar] [CrossRef]
- Qin, L.; Chen, J.; Wang, J.; Ye, J.; Tan, H.; Xu, L. Expression of cathepsin B in human hepatocellular carcinoma and its prognostic significance. Int. J. Clin. Exp. Pathol. 2016, 9, 1343–1350. [Google Scholar]
- Hambrock, H.O.; Kaufmann, B.; Müller, S.; Hanisch, F.; Nose, K.; Paulsson, M.; Maurer, P.; Hartmann, U. Structural characterization of TSC-36/Flik: Analysis of two charge isoforms. J. Biol. Chem. 2004, 279, 11727–11735. [Google Scholar] [CrossRef]
- Mattiotti, A.; Prakash, S.; Barnett, P.; van den Hoff, M.J.B. Follistatin-like 1 in development and human diseases. Cell. Mol. Life Sci. 2018, 75, 2339–2354. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, Y.; Wang, C.; Liu, Z.; Xu, M.; Zheng, X. FSTL1 contributes to tumor progression via attenuating apoptosis in a AKT/GSK-3beta-dependent manner in hepatocellular carcinoma. Cancer Biomark. 2017, 20, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Kale, V.P. TGF-beta signaling and its role in the regulation of hematopoietic stem cells. Syst. Synth. Boil. 2015, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fransvea, E.; Angelotti, U.; Antonaci, S.; Giannelli, G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology 2008, 47, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.; Fransvea, E.; Lavezzari, G.; Antonaci, S.; Giannelli, G. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology 2009, 50, 1140–1151. [Google Scholar] [CrossRef]
- Fransvea, E.; Mazzocca, A.; Antonaci, S.; Giannelli, G. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology 2009, 49, 839–850. [Google Scholar] [CrossRef]
- Bergamini, C.; Sgarra, C.; Trerotoli, P.; Lupo, L.; Azzariti, A.; Antonaci, S.; Giannelli, G. Laminin-5 stimulates hepatocellular carcinoma growth through a different function of alpha6beta4 and alpha3beta1 integrins. Hepatology 2007, 46, 1801–1809. [Google Scholar] [CrossRef]
- Li, X.Y.; Hu, S.Q.; Xiao, L. The cancer-associated fibroblasts and drug resistance. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2112–2119. [Google Scholar]
- Porcelli, L.; Iacobazzi, R.M.; Di Fonte, R.; Serratì, S.; Intini, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Leonetti, F.; Azzariti, A.; et al. CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Casadei Gardini, A.; Scarpi, E.; Faloppi, L.; Scartozzi, M.; Silvestris, N.; Santini, D.; de Stefano, G.; Marisi, G.; Negri, F.V.; Foschi, F.G.; et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget 2016, 7, 67142–67149. [Google Scholar] [PubMed]
- Argentiero, A.; De Summa, S.; Di Fonte, R.; Iacobazzi, R.M.; Porcelli, L.; Da Vià, M.; Brunetti, O.; Azzariti, A.; Silvestris, N.; Solimando, A.G. Gene Expression Comparison between the Lymph Node-Positive and -Negative Reveals a Peculiar Immune Microenvironment Signature and a Theranostic Role for WNT Targeting in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Cancers 2019, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Pinyol, R.; Sia, D.; Llovet, J.M. Immune Exclusion-Wnt/CTNNB1 Class Predicts Resistance to Immunotherapies in HCC. Clin. Cancer Res. 2019, 25, 2021–2023. [Google Scholar] [CrossRef] [PubMed]
- Labeur, T.A.; Ten Cate, D.W.G.; Bart Takkenberg, R.; Azahaf, H.; van Oijen, M.G.H.; van Delden, O.M.; de Man, R.A.; van Vugt, J.L.A.; IJzermans, J.N.M.; Eskens, F.A.L.M.; et al. Are we SHARP enough? The importance of adequate patient selection in sorafenib treatment for hepatocellular carcinoma. Acta Oncol. 2018, 57, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.A.; Farnsworth, R.H.; Solomon, B.; Achen, M.G.; Stacker, S.A.; Fox, S.B. The Role of the Tumor Vasculature in the Host Immune Response: Implications for Therapeutic Strategies Targeting the Tumor Microenvironment. Front. Immunol. 2016, 7, 621. [Google Scholar] [CrossRef]
- OLeone, P.; Di Lernia, G.; Solimando, A.G.; Cicco, S.; Saltarella, I.; Lamanuzzi, A.; Ria, R.; Frassanito, M.A.; Ponzoni, M.; Ditonno, P.; et al. Bone marrow endothelial cells sustain a tumor-specific CD8+ T cell subset with suppressive function in myeloma patients. Oncoimmunology 2018, 8, e1486949. [Google Scholar]
- Holmgaard, R.B.; Schaer, D.A.; Li, Y.; Castaneda, S.P.; Murphy, M.Y.; Xu, X.; Inigo, I.; Dobkin, J.; Manro, J.R.; Iversen, P.W.; et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J. Immunother. Cancer. 2018, 6, 47. [Google Scholar] [CrossRef]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef]
- Hernanda, P.Y.; Chen, K.; Das, A.M.; Sideras, K.; Wang, W.; Li, J.; Cao, W.; Bots, S.J.; Kodach, L.L.; de Man, R.A.; et al. SMAD4 exerts a tumor-promoting role in hepatocellular carcinoma. Oncogene 2015, 34, 5055–5068. [Google Scholar] [CrossRef]
- Moles, A.; Tarrats, N.; Fernández-Checa, J.C.; Marí, M. Cathepsins B and D drive hepatic stellate cell proliferation and promote their fibrogenic potential. Hepatology 2009, 49, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, M.; Das, P.; Gahlot, G.P.S.; Singh, R.; Roeb, E.; Roderfeld, M.; Datta Gupta, S.; Saraya, A.; Pandey, R.M.; Chauhan, S.S. Cathepsin L and B as Potential Markers for Liver Fibrosis: Insights from Patients and Experimental Models. Clin. Transl. Gastroenterol. 2017, 8, e99. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Kakinuma, S.; Kamiya, A.; Tsunoda, T.; Tsuchiya, J.; Sato, A.; Kaneko, S.; Nitta, S.; Kawai-Kitahata, F.; Murakawa, M.; et al. LIM homeobox 2 promotes interaction between human iPS-derived hepatic progenitors and iPS-derived hepatic stellate-like cells. Sci Rep. 2019, 9, 2072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Pan, L.H.; Pang, Y.; Yang, J.X.; Lv, M.J.; Liu, F.; Qu, X.F.; Chen, X.X.; Gong, H.J.; Liu, D.; et al. GDF11/BMP11 as a novel tumor marker for liver cancer. Exp. Ther. Med. 2018, 15, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- Plouhinec, J.L.; Zakin, L.; de Robertis, E.M. Systems control of BMP morphogen flow in vertebrate embryos. Curr. Opin. Genet. Dev. 2011, 21, 696–703. [Google Scholar] [CrossRef]
- Mueller, T.D.; Nickel, J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012, 586, 1846–1859. [Google Scholar] [CrossRef]
- Deng, T.; Lin, D.; Zhang, M.; Zhao, Q.; Li, W.; Zhong, B.; Deng, Y.; Fu, X. Differential expression of bone morphogenetic protein 5 in human lung squamous cell carcinoma and adenocarcinoma (non-small-cell lung cancer (NSCLC)). Acta Biochim. Biophys. Sin. (Shanghai) 2015, 47, 557–563. [Google Scholar] [CrossRef]
- Romagnoli, M.; Belguise, K.; Yu, Z.; Wang, X.; Landesman-Bollag, E.; Seldin, D.C.; Chalbos, D.; Barillé-Nion, S.; Jézéquel, P.; Seldin, M.L.; et al. Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated by Blimp-1-dependent repression of BMP-5. Cancer Res. 2012, 72, 6268–6278. [Google Scholar] [CrossRef]
- Tang, C.S.; Jin, Y.; Cheng, K.I.; Yu, K.L.; Suwa, F.; Nakatsuji, I.; Makigusa, K.; Fang, Y.R. Transcriptional mRNA of bone morphogenetic proteins 2, 3, 4, and 5 in trigeminal nerve, benign and malignant peripheral nerve sheath tumors. Kaohsiung J. Med. Sci. 2001, 17, 16–24. [Google Scholar]
- Johnsen, I.K.; Kappler, R.; Auernhammer, C.J.; Beuschlein, F. Bone morphogenetic proteins 2 and 5 are down-regulated in adrenocortical carcinoma and modulate adrenal cell proliferation and steroidogenesis. Cancer Res. 2009, 69, 5784–5792. [Google Scholar] [CrossRef]
- Chen, E.; Yang, F.; He, H.; Li, Q.; Zhang, W.; Xing, J.; Zhu, Z.; Jiang, J.; Wang, H.; Zhao, X.; et al. Alteration of tumor suppressor BMP5 in sporadic colorectal cancer: A genomic and transcriptomic profiling based study. Mol. Cancer 2018, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Li, Q.; Wang, H.; Zhang, P.; Zhao, X.; Yang, F.; Yang, J. MiR-32 promotes tumorigenesis of colorectal cancer by targeting BMP5. Biomed. Pharmacother. 2018, 106, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.; Alarmo, E.L.; Sandström, S.; Ampuja, M.; Kallioniemi, A. Bone morphogenetic protein -4 and -5 in pancreatic cancer—Novel bidirectional players. Exp. Cell Res. 2011, 317, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Sanchez, A.; Fabregat, I. BMPs and liver: More questions than answers. Curr. Pharm. Des. 2012, 18, 4114–4125. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tipoe, G.L.; Liong, E.C.; Lau, T.Y.; Fung, P.C.; Leung, K.M. Overexpression of BMP-2/4, -5 and BMPR-IA associated with malignancy of oral epithelium. Oral Oncol. 2001, 37, 225–233. [Google Scholar] [CrossRef]
- Chen, J.; Gingold, J.A.; Su, X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol. Med. 2019. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).