Inhibition of Wnt/β-Catenin Signaling in Neuroendocrine Tumors In Vitro: Antitumoral Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Culture, and Treatment
2.2. Cell Viability Assay and Population Doubling Time
2.3. Flow Cytometric Cell Cycle Distribution Assay
2.4. Caspase-3/7 Activity Assay
2.5. Wound Healing Assay
2.6. Protein Extraction and Western Blotting
2.7. siRNA and Cell Transfection
2.8. Statistical Analysis
3. Results
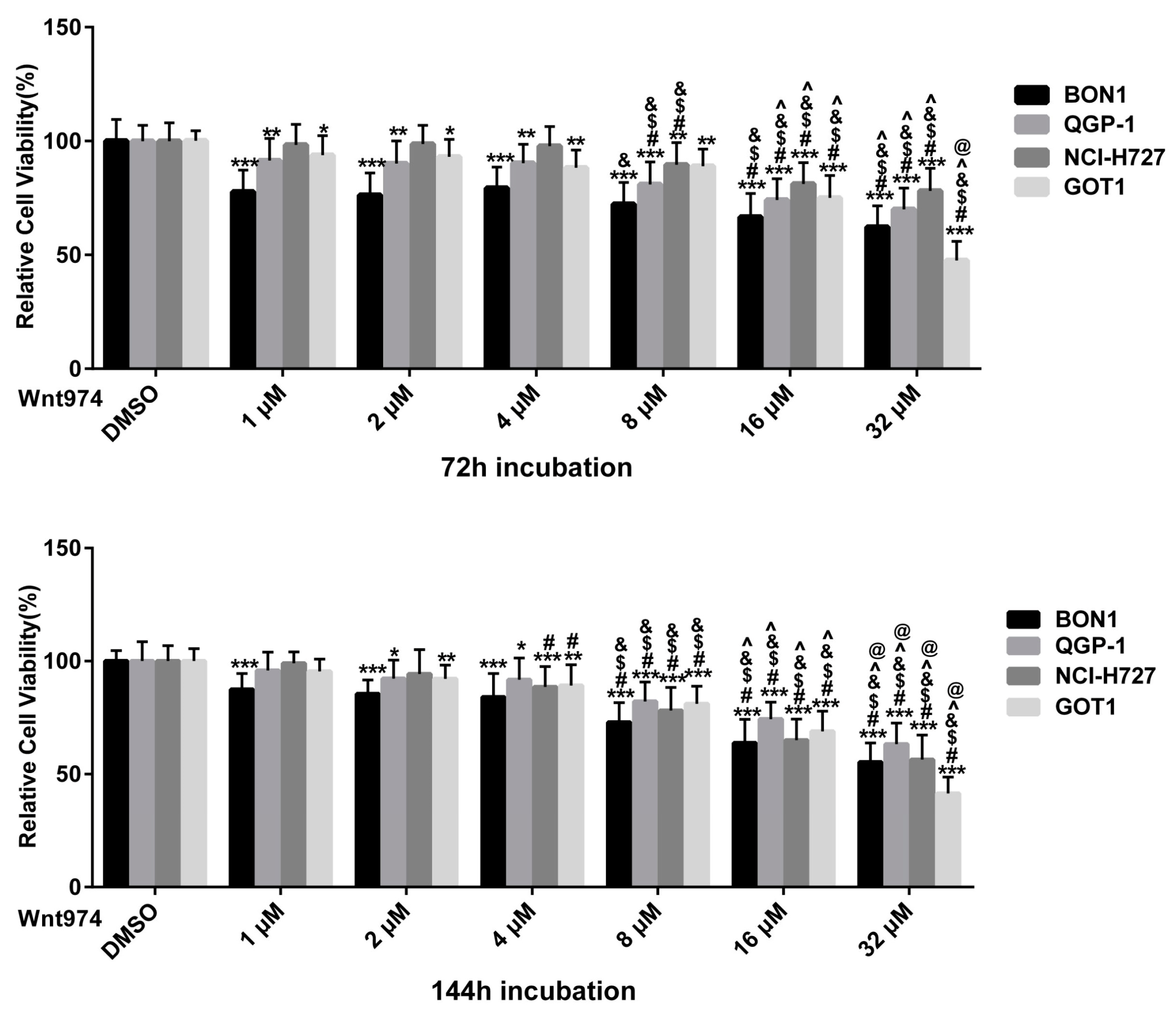
3.1. WNT974 Reduces NET Cell Viability in a Dose- and Time-Dependent Manner
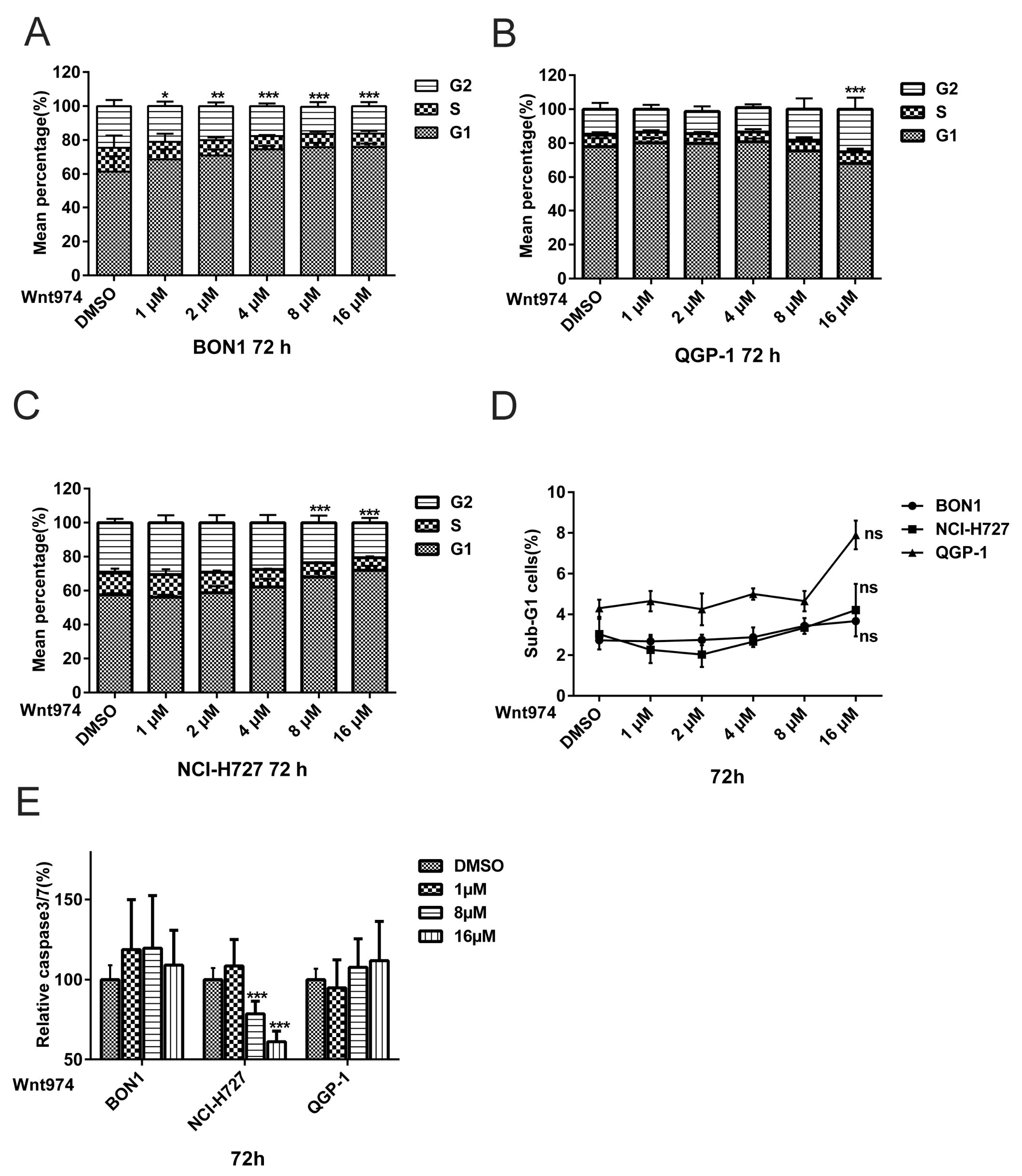
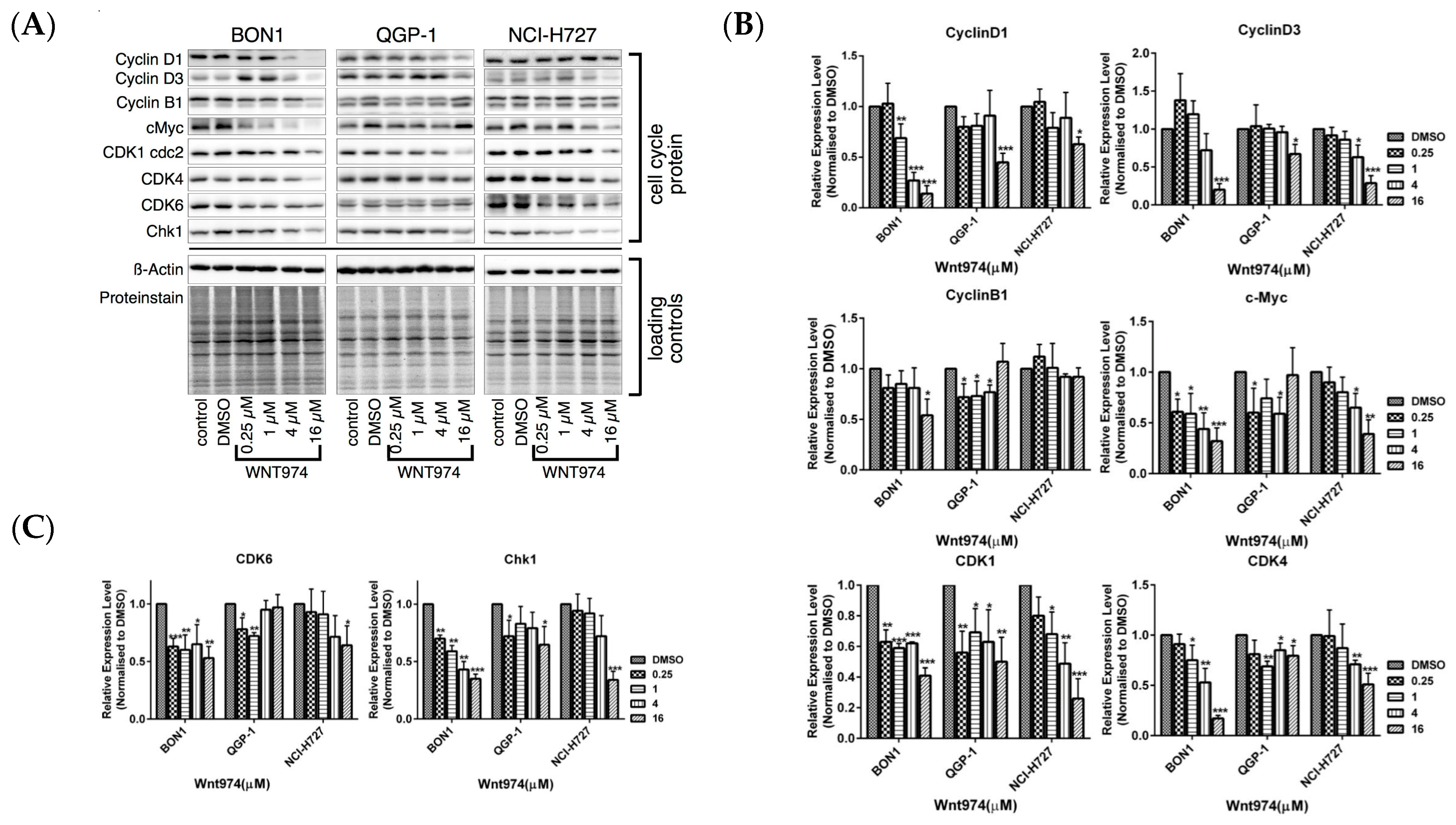
3.2. WNT974 induces NET Cell Cycle Arrest at the G0/G1 Phase and G2 Phase, but does not Cause Apoptosis
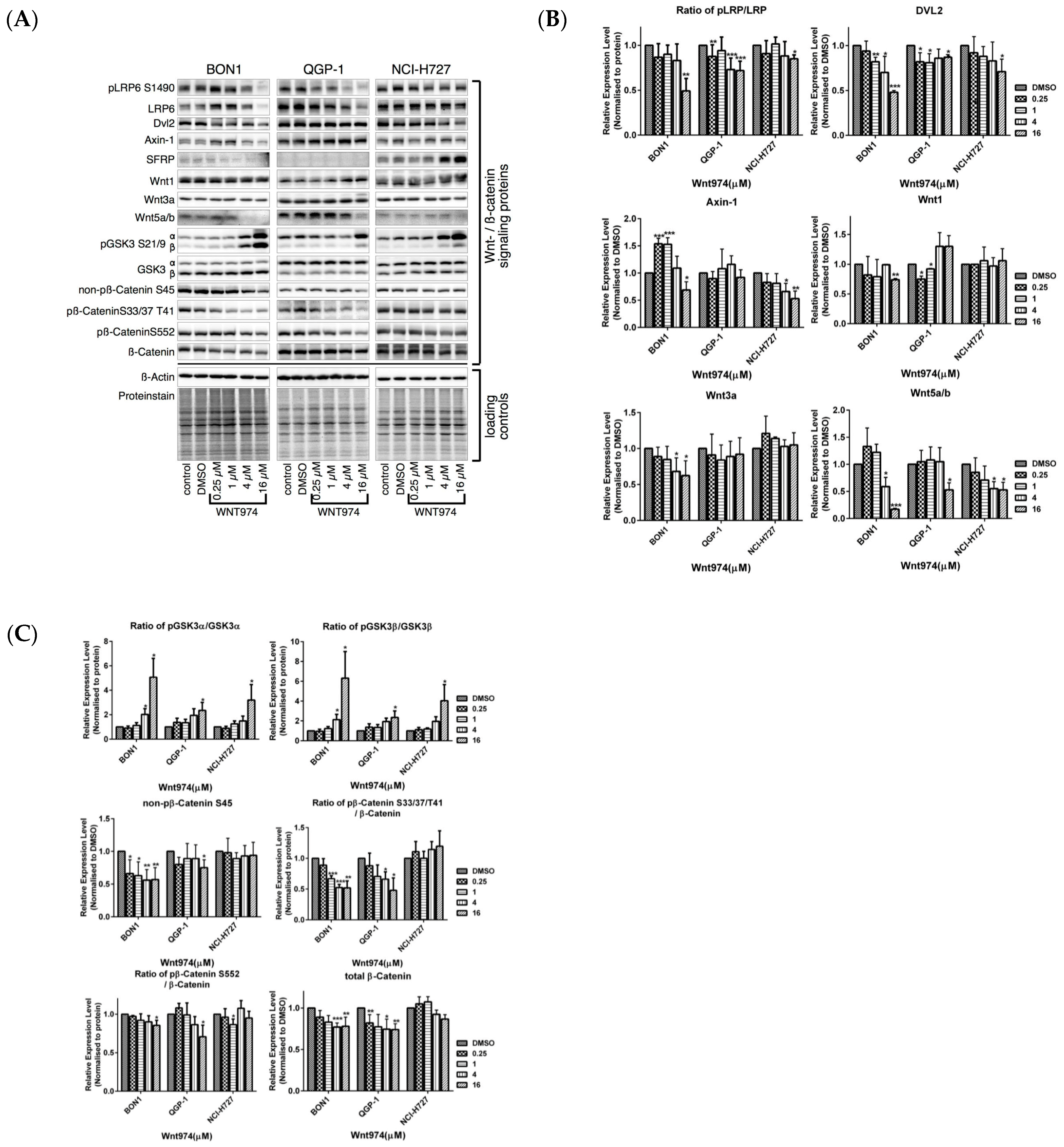
3.3. Effects of WNT974 on the Inhibition of Wnt/β-Catenin Signaling in NET Cells
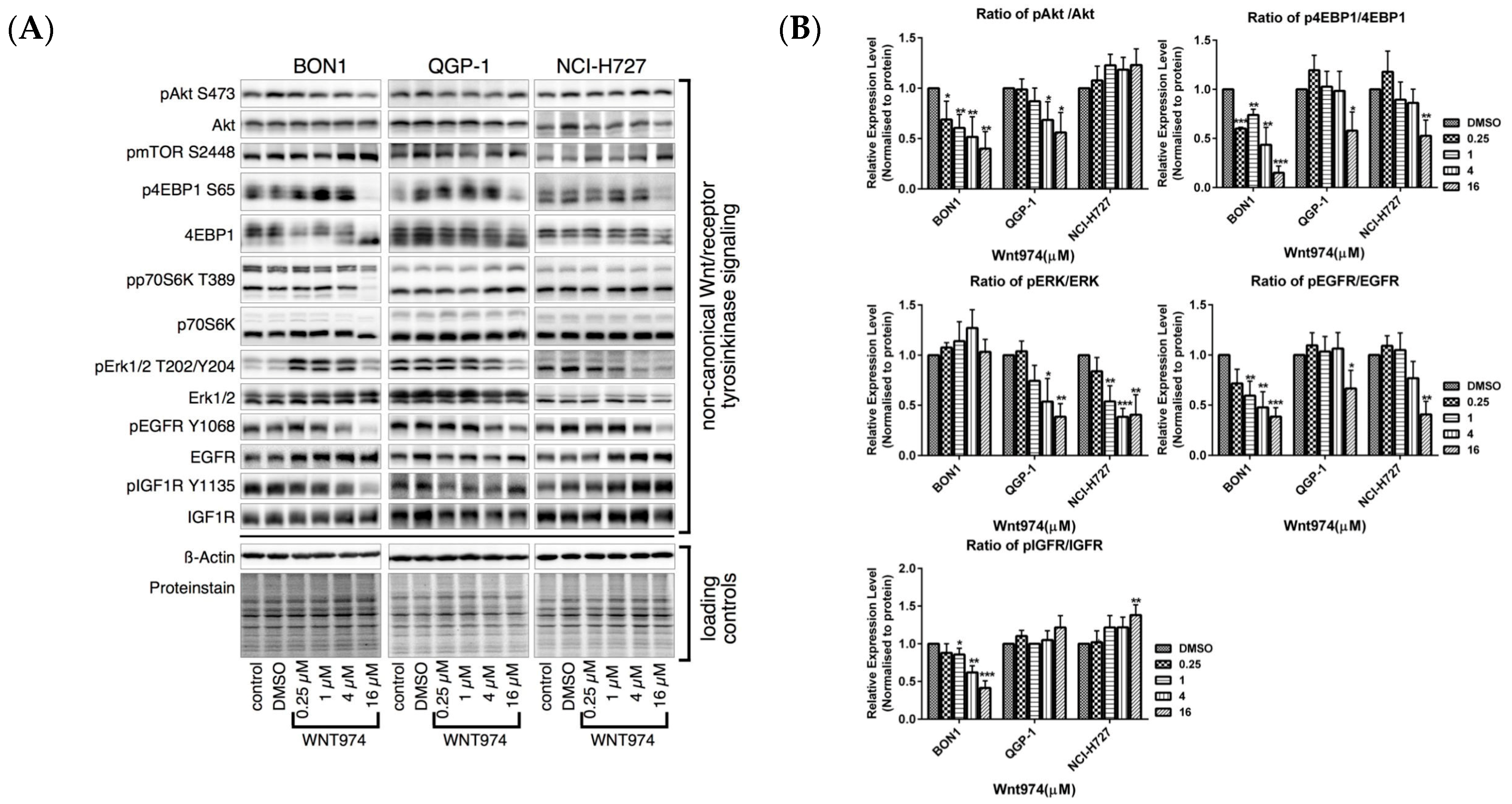
3.4. Effects of WNT974 on the Inhibition of the pAKT/mTOR, MAPK/ERK, pEGFR and pIGFR Pathways in NET Cells
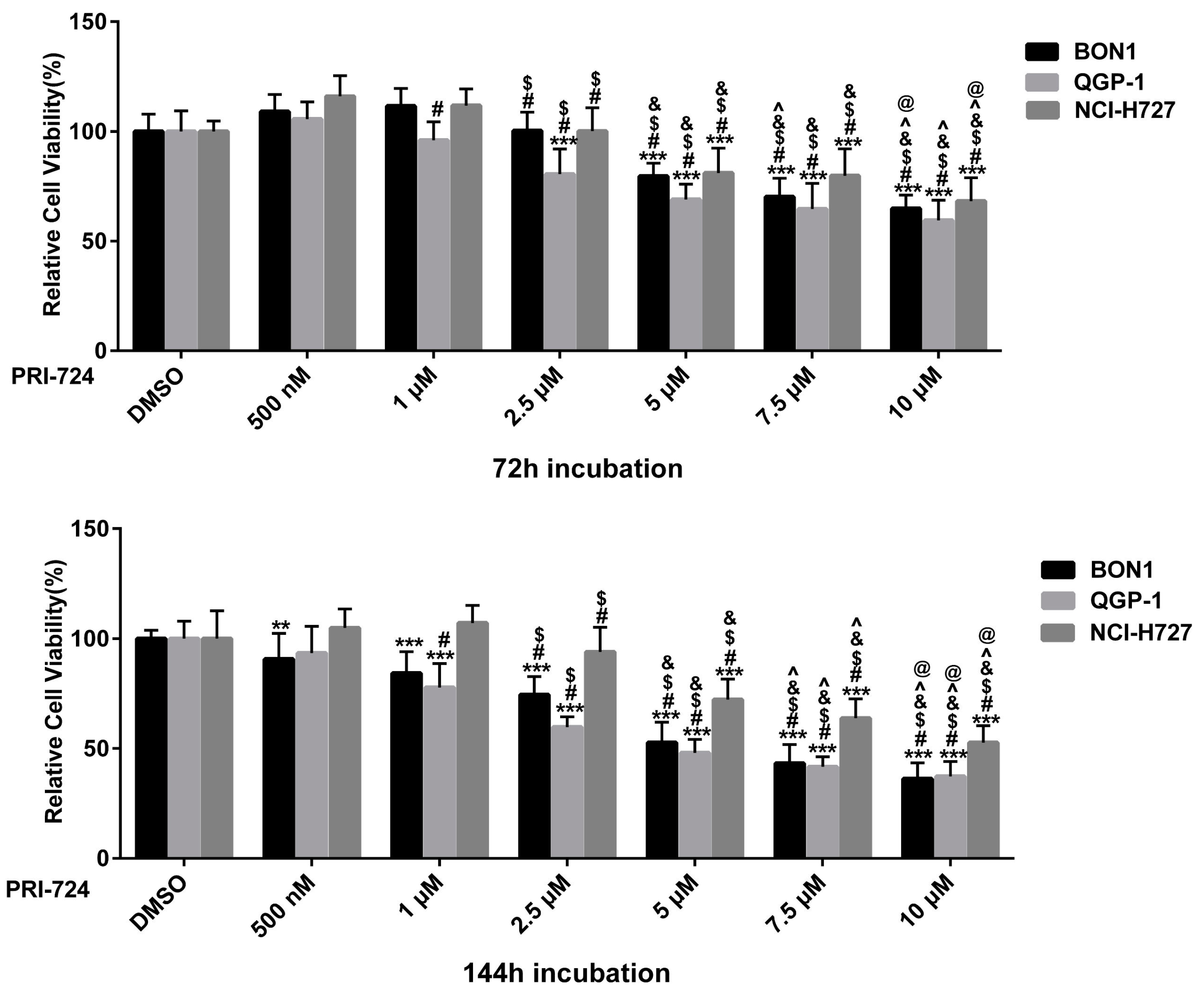
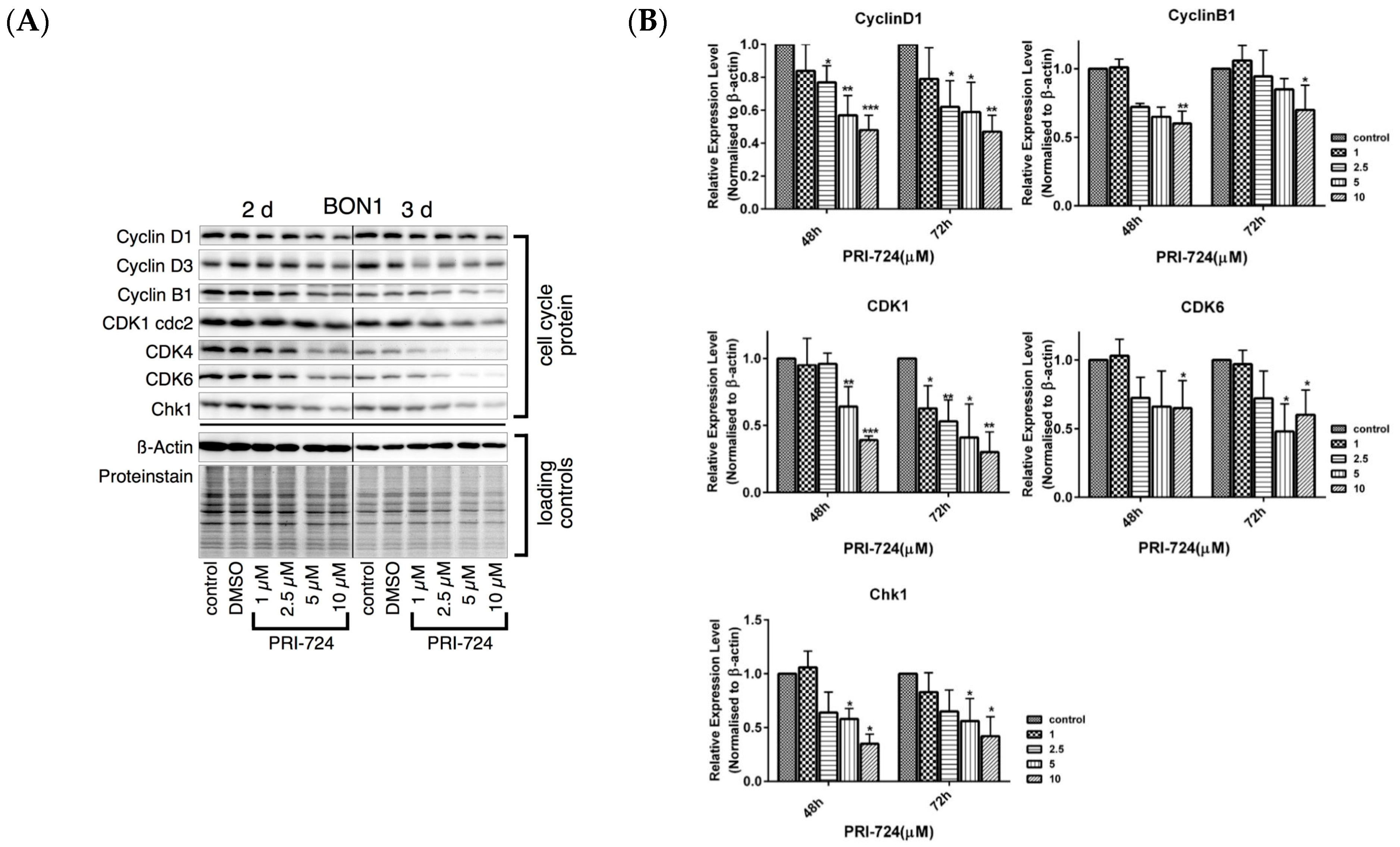
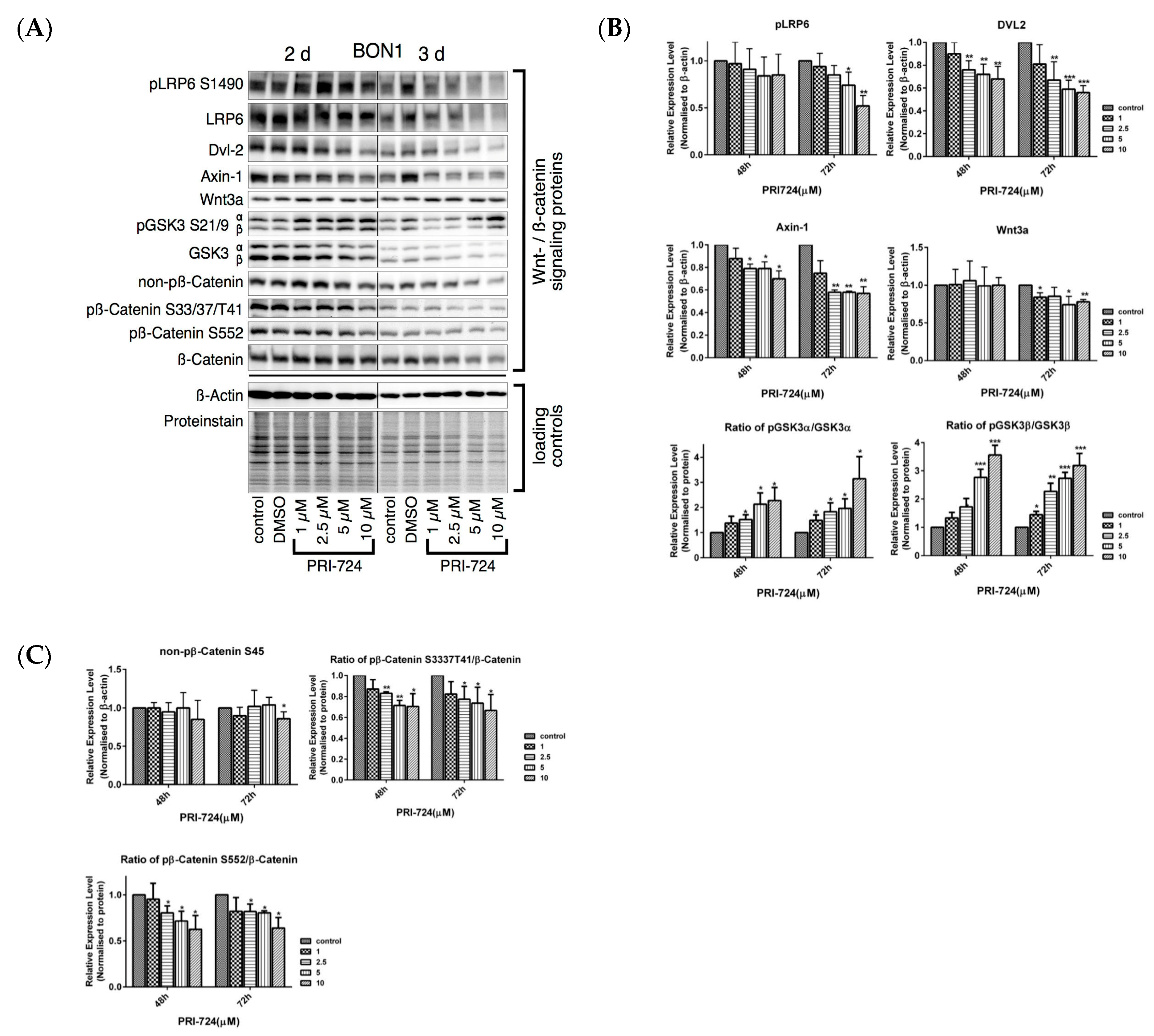
3.5. Effects of the Selective β-Catenin Inhibitor PRI-724 on NET Cell Viability and Protein Expression
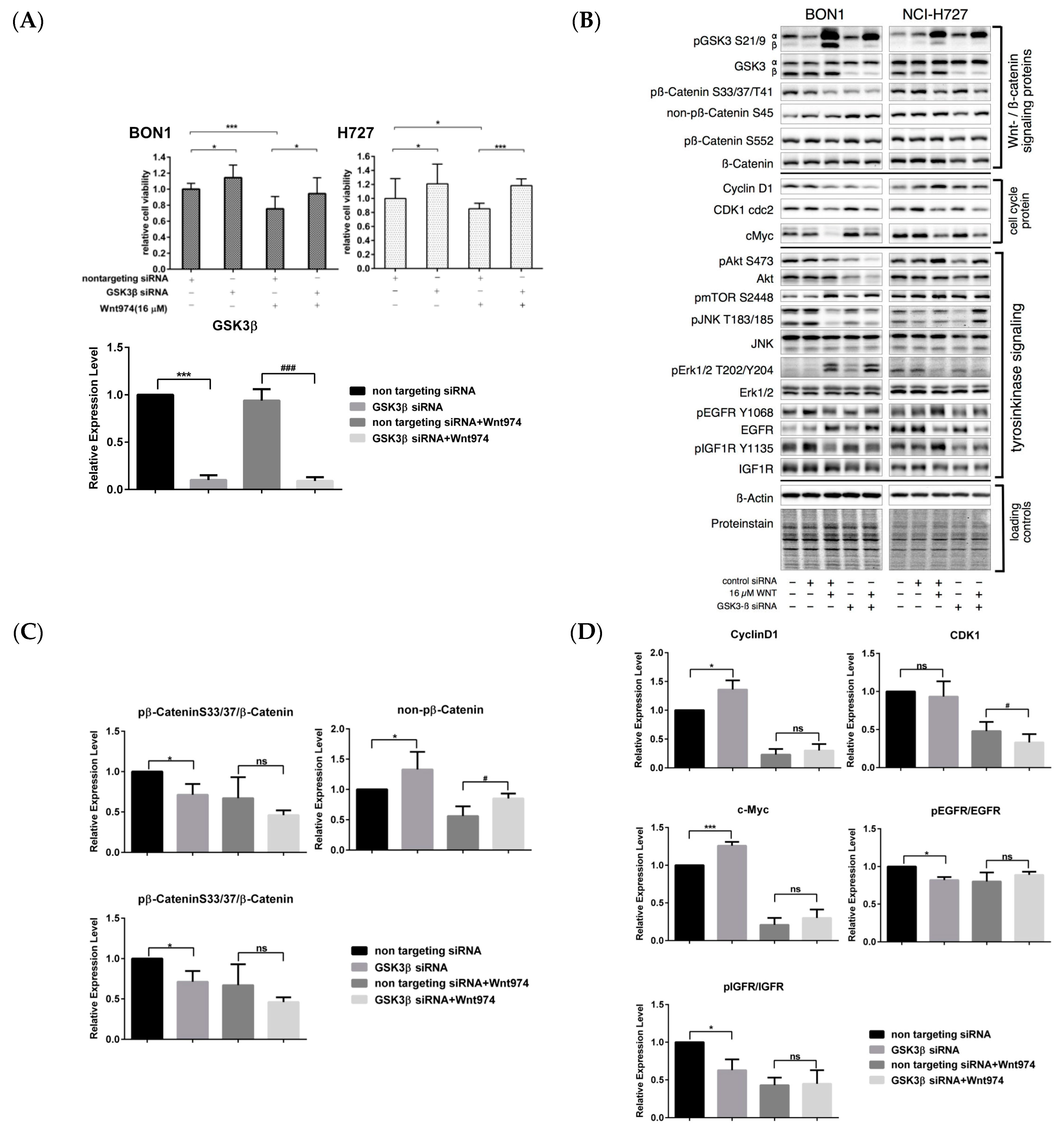
3.6. Effects of β-Catenin siRNA on the Regulation of NET Cell Viability and Protein Expression
3.7. WNT974 Regulation of p21 and p53 Expression
3.8. WNT974 Regulation of Neurotensin and Menin Expression
3.9. WNT974 Suppression of NET Cell Migration and Expression of the EMT Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with NeuroendocrineTumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; La Rosa, S.; Volante, M.; Papotti, M. Immunohistochemical biomarkers of gastrointestinal, pancreatic, pulmonary, and thymic neuroendocrine neoplasms. Endocr. Pathol. 2018, 29, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Auernhammer, C.J.; Spitzweg, C.; Angele, M.K.; Boeck, S.; Grossman, A.; Nölting, S.; Ilhan, H.; Knösel, T.; Mayerle, J.; Reincke, M.; et al. Advanced neuroendocrine tumours of the small intestine and pancreas: Clinical developments, controversies, and future strategies. Lancet Diabetes Endocrinol. 2018, 6, 404–415. [Google Scholar] [CrossRef]
- Foster, D.S.; Jensen, R.; Norton, J.A. Management of Liver Neuroendocrine Tumors in 2018. JAMA Oncol. 2018, 4, 1605–1606. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.F.; Spampatti, M.P.; Spitzweg, C.; Auernhammer, C.J. Supportive therapy in gastroenteropancreatic neuroendocrine tumors: Often forgotten but important. Rev. Endocr. Metab. Disord. 2018, 19, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef]
- Di Domenico, A.; Wiedmer, T.; Marinoni, I.; Perren, A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr. Relat. Cancer 2017, 24, R315–R334. [Google Scholar] [CrossRef]
- Alvarez, M.J.; Subramaniam, P.S.; Tang, L.H.; Grunn, A.; Aburi, M.; Rieckhof, G.; Komissarova, E.V.; Hagan, E.A.; Bodei, L.; Clemons, P.A.; et al. A precision oncology approach to the pharmacological targeting of mechanistic dependencies in neuroendocrine tumors. Nat. Genet. 2018, 50, 979–989. [Google Scholar] [CrossRef]
- Aristizabal Prada, E.T.; Auernhammer, C.J. Targeted therapy of gastroenteropancreatic neuroendocrine tumours: Preclinical strategies and future targets. Endocr. Connect. 2018, 7, R1–R25. [Google Scholar] [CrossRef]
- Capdevila, J.; Bodei, L.; Davies, P.; Gorbounova, V.; Jensen, R.T.; Knigge, U.; Krejs, G.J.; Krenning, E.; O’Connor, J.M.; Peeters, M.; et al. Unmet medical needs in metastatic lung and digestive neuroendocrine neoplasms. Neuroendocrinology 2019, 108, 18–25. [Google Scholar] [CrossRef]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Canonical and non-canonical Wnt signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int. J. Oncol. 2017, 51, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/betaß-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef]
- Wang, B.; Tian, T.; Kalland, K.H.; Ke, X.; Qu, Y. Targeting Wnt/β-Catenin Signaling for Cancer Immunotherapy. Trends Pharmacol. Sci. 2018, 39, 648–658. [Google Scholar] [CrossRef]
- Frost, M.; Lines, K.E.; Thakker, R.V. Current and emerging therapies for PNETs in patients with or without MEN1. Nat. Rev. Endocrinol. 2018, 14, 216–227. [Google Scholar] [CrossRef]
- Kim, J.T.; Liu, C.; Zaytseva, Y.Y.; Weiss, H.L.; Townsend, C.M., Jr.; Evers, B.M. Neurotensin, a novel target of Wnt β-catenin pathway, promotes growth of neuroendocrine tumor cells. Int. J. Cancer 2015, 136, 1475–1481. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, Y.; Li, F.; Su, Y.; Li, Y.; Peng, Y.; Cheng, Y.; Zhang, C.; Wang, W.; Ning, G. Targeting β-catenin signaling for therapeutic intervention in MEN1-deficient pancreatic neuroendocrine tumours. Nat. Commun. 2014, 5, 5809. [Google Scholar] [CrossRef]
- Kim, J.T.; Li, J.; Jang, E.R.; Gulhati, P.; Rychahou, P.G.; Napier, D.L.; Wang, C.; Weiss, H.L.; Lee, E.Y.; Anthony, L.; et al. Deregulation of Wnt/β-catenin signaling through genetic or epigenetic alterations in human neuroendocrine tumors. Carcinogenesis 2013, 34, 953–961. [Google Scholar] [CrossRef]
- Wei, Y.L.; Hua, J.; Liu, X.Y.; Hua, X.M.; Sun, C.; Bai, J.A.; Tang, Q.Y. LncNEN885 inhibits epithelial-mesenchymal transition by partially regulation of Wnt/β-catenin signalling ingastroenteropancreatic neuroendocrine neoplasms. Cancer Sci. 2018, 109, 3139–3148. [Google Scholar] [CrossRef]
- Lines, K.E.; Stevenson, M.; Filippakopoulos, P.; Müller, S.; Lockstone, H.E.; Wright, B.; Grozinsky-Glasberg, S.; Grossman, A.B.; Knapp, S.; Buck, D.; et al. Epigenetic pathway inhibitors represent potential drugs for treating pancreatic and bronchial neuroendocrine tumors. Oncogenesis 2017, 6, e332. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Barbi, S.; Fassan, M.; Mafficini, A.; Ali, G.; Vicentini, C.; Sperandio, N.; Corbo, V.; Rusev, B.; Mastracci, L.; et al. Gene Expression Profiling of Lung Atypical Carcinoids and Large Cell Neuroendocrine Carcinomas Identifies Three Transcriptomic Subtypes with Specific Genomic Alterations. J. Thorac. Oncol. 2019, 14, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Veschi, S.; Lattanzio, R.; Aceto, G.M.; Curia, M.C.; Magnasco, S.; Angelucci, D.; Cama, A.; Piantelli, M.; Battista, P. Alterations of MEN1 and E-cadherin/β-catenin complex in sporadic pulmonary carcinoids. Int. J. Oncol. 2012, 41, 1221–1228. [Google Scholar] [PubMed]
- Vollbrecht, C.; Werner, R.; Walter, R.F.; Christoph, D.C.; Heukamp, L.C.; Peifer, M.; Hirsch, B.; Burbat, L.; Mairinger, T.; Schmid, K.W.; et al. Mutational analysis of pulmonary tumours with neuroendocrine features using targeted massive parallel sequencing: A comparison of a neglected tumour group. Br. J. Cancer 2015, 113, 1704–1711. [Google Scholar] [CrossRef]
- Simbolo, M.; Vicentini, C.; Mafficini, A.; Fassan, M.; Pedron, S.; Corbo, V.; Mastracci, L.; Rusev, B.; Pedrazzani, C.; Landoni, L.; et al. Mutational and copy number asset of primary sporadic neuroendocrine tumors of the small intestine. Virchows. Arch. 2018, 473, 709–717. [Google Scholar] [CrossRef]
- Bottarelli, L.; Azzoni, C.; Pizzi, S.; D’Adda, T.; Silini, E.M.; Bordi, C.; Rindi, G. Adenomatous polyposis coli gene involvement in ileal enterochromaffin cell neuroendocrine neoplasms. Hum. Pathol. 2013, 44, 2736–2742. [Google Scholar] [CrossRef]
- Weiss, V.; Dueber, J.; Wright, J.P.; Cates, J.; Revetta, F.; Parikh, A.A.; Merchant, N.B.; Shi, C. Immunohistochemical analysis of the Wnt/β-catenin signaling pathway in pancreatic neuroendocrine neoplasms. World J. Gastrointest. Oncol. 2016, 8, 615–622. [Google Scholar] [CrossRef]
- Galván, J.A.; Astudillo, A.; Vallina, A.; Crespo, G.; Folgueras, M.V.; González, M.V. Prognostic and diagnostic value of epithelial to mesenchymal transition markers in pulmonary neuroendocrine tumors. BMC Cancer 2014, 14, 855. [Google Scholar] [CrossRef]
- Benoit, Y.D.; Guezguez, B.; Boyd, A.L.; Bhatia, M. Molecular pathways: Epigenetic modulation of Wnt-glycogen synthase kinase-3 signaling to target human cancer stem cells. Clin. Cancer Res. 2014, 20, 5372–5378. [Google Scholar] [CrossRef][Green Version]
- Katoh, M.; Katoh, M. Molecular genetics and targeted therapy of WNT-related human diseases. Int. J. Mol. Med. 2017, 40, 587–606. [Google Scholar] [CrossRef]
- Asciolla, J.J.; Miele, M.M.; Hendrickson, R.C.; Resh, M.D. An in vitro fatty acylation assay reveals a mechanism for Wnt recognition by the acyltransferase Porcupine. J. Biol. Chem. 2017, 292, 13507–13513. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef] [PubMed]
- Rudy, S.F.; Brenner, J.C.; Harris, J.L.; Liu, J.; Che, J.; Scott, M.V.; Owen, J.H.; Komarck, C.M.; Graham, M.P.; Bellile, E.L.; et al. In vivo Wnt pathway inhibition of human squamous cell carcinoma growth and metastasis in the chick chorioallantoic model. J. Otolaryngol. Head Neck Surg. 2016, 45, 26. [Google Scholar]
- Boone, J.D.; Arend, R.C.; Johnston, B.E.; Cooper, S.J.; Gilchrist, S.A.; Oelschlager, D.K.; Grizzle, W.E.; McGwin, G., Jr.; Gangrade, A.; Straughn, J.M., Jr.; et al. Targeting the Wnt/β-catenin pathway in primary ovarian cancer with the porcupine inhibitor WNT974. Lab. Invest. 2016, 96, 249–259. [Google Scholar] [CrossRef]
- Zimmerli, D.; Cecconi, V.; Valenta, T.; Hausmann, G.; Cantù, C.; Restivo, G.; Hafner, J.; Basler, K.; van den Broek, M. WNT ligands control initiation and progression of human papillomavirus-driven squamous cell carcinoma. Oncogene 2018, 37, 3753–3762. [Google Scholar] [CrossRef]
- Hayashi, M.; Baker, A.; Goldstein, S.D.; Albert, C.M.; Jackson, K.W.; McCarty, G.; Kahlert, U.D.; Loeb, D.M. Inhibition of porcupine prolongs metastasis free survival in a mouse xenograft model of Ewing sarcoma. Oncotarget 2017, 8, 78265–78276. [Google Scholar] [CrossRef]
- Suwala, A.K.; Koch, K.; Rios, D.H.; Aretz, P.; Uhlmann, C.; Ogorek, I.; Felsberg, J.; Reifenberger, G.; Köhrer, K.; Deenen, R.; et al. Inhibition of Wnt/beta-catenin signaling downregulates expression of aldehyde dehydrogenase isoform 3A1 (ALDH3A1) to reduce resistance against temozolomide in glioblastoma in vitro. Oncotarget 2018, 9, 22703–22716. [Google Scholar] [CrossRef]
- Tian, D.; Shi, Y.; Chen, D.; Liu, Q.; Fan, F. The Wnt inhibitor LGK-974 enhances radiosensitivity of HepG2 cells by modulating Nrf2 signaling. Int. J. Oncol. 2017, 51, 545–554. [Google Scholar] [CrossRef]
- Lin, H.H.; Feng, W.C.; Lu, L.C.; Shao, Y.Y.; Hsu, C.H.; Cheng, A.L. Inhibition of the Wnt/β-catenin signaling pathway improves the anti-tumor effects of sorafenib against hepatocellular carcinoma. Cancer Lett. 2016, 381, 58–66. [Google Scholar] [CrossRef]
- Bahrami, A.; Amerizadeh, F.; ShahidSales, S.; Khazaei, M.; Ghayour-Mobarhan, M.; Sadeghnia, H.R.; Maftouh, M.; Hassanian, S.M.; Avan, A. Therapeutic potential of targeting wnt/β-catenin pathway in treatment of colorectal cancer: Rational and progress. J. Cell Biochem. 2017, 118, 1979–1983. [Google Scholar] [CrossRef]
- Jiang, X.; Mak, P.Y.; Mu, H.; Tao, W.; Mak, D.H.; Kornblau, S.; Zhang, Q.; Ruvolo, P.; Burks, J.K.; Zhang, W.; et al. Disruption of Wnt/β-Catenin Exerts Antileukemia Activity and Synergizes with FLT3 Inhibition in FLT3-Mutant Acute Myeloid Leukemia. Clin. Cancer Res. 2018, 24, 2417–2429. [Google Scholar] [CrossRef]
- Manegold, P.; Lai, K.K.Y.; Wu, Y.; Teo, J.L.; Lenz, H.J.; Genyk, Y.S.; Pandol, S.J.; Wu, K.; Lin, D.P.; Chen, Y.; et al. Differentiation Therapy Targeting the β-Catenin/CBP Interaction in Pancreatic Cancer. Cancers (Basel) 2018, 29, 95. [Google Scholar] [CrossRef] [PubMed]
- Kolby, L.; Bernhardt, P.; Ahlman, H.; Wängberg, B.; Johanson, V.; Wigander, A.; Forssell-Aronsson, E.; Karlsson, S.; Ahrén, B.; Stenman, G.; et al. A transplantable human carcinoid as model for somatostatin receptor-mediated and amine transporter-mediated radionuclide uptake. Am. J. Pathol. 2001, 158, 745–755. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Proffitt, R.T.; Reynolds, C.P. A microcomputer program for calculating cell population doubling time in vitro and in vivo. Cancer Chemother. Pharmacol. 1996, 37, 203–210. [Google Scholar] [CrossRef]
- Hofving, T.; Arvidsson, Y.; Almobarak, B.; Inge, L.; Pfragner, R.; Persson, M.; Stenman, G.; Kristiansson, E.; Johanson, V.; Nilsson, O. The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines. Endocr. Relat. Cancer 2018, 25, 367–380. [Google Scholar] [CrossRef]
- Chen, X.; Wang, R.; Liu, X.; Wu, Y.; Zhou, T.; Yang, Y.; Perez, A.; Chen, Y.C.; Hu, L.; Chadarevian, J.P.; et al. A Chemical-Genetic Approach Reveals the Distinct Roles of GSK3α and GSK3β in Regulating Embryonic Stem Cell Fate. Dev. Cell 2017, 43, 563–576. [Google Scholar]
- Shimozaki, S.; Yamamoto, N.; Domoto, T.; Nishida, H.; Hayashi, K.; Kimura, H.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Kato, T.; et al. Efficacy of glycogen synthase kinase-3β targeting against osteosarcoma via activation of β-catenin. Oncotarget 2016, 7, 77038–77051. [Google Scholar] [CrossRef][Green Version]
- Aristizabal Prada, E.T.; Spöttl, G.; Maurer, J.; Lauseker, M.; Lauseker, M.; Koziolek, E.J.; Schrader, J.; Grossman, A.; Pacak, K.; Beuschlein, F.; et al. The role of GSK3 and its reversal with GSK3 antagonism in everolimus resistance. The role of GSK3 and its reversal with GSK3 antagonism in everolimus resistance. Endocr Relat. Cancer 2018, 25, 893–908. [Google Scholar] [CrossRef]
- Aristizabal Prada, E.T.; Weis, C.; Orth, M.; Lauseker, M.; Spöttl, G.; Maurer, J.; Grabowski, P.; Grossman, A.; Auernhammer, C.J.; Nölting, S. GSK3α/β: A Novel Therapeutic Target for Neuroendocrine Tumors. Neuroendocrinology 2018, 106, 335–351. [Google Scholar] [CrossRef]
- Vlotides, G.; Tanyeri, A.; Spampatti, M.; Zitzmann, K.; Chourdakis, M.; Spttl, C.; Maurer, J.; Nölting, S.; Göke, B.; Auernhammer, C.J. Anticancer effects of metformin on neuroendocrine tumor cells in vitro. Hormones (Athens) 2014, 13, 498–508. [Google Scholar] [CrossRef]
- Spampatti, M.; Vlotides, G.; Spöttl, G.; Maurer, J.; Göke, B.; Auernhammer, C.J. Aspirin inhibits cell viability and mTOR downstream signaling in gastroenteropancreatic and bronchopulmonary neuroendocrine tumor cells. World J. Gastroenterol. 2014, 20, 10038–10049. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Napier, D.L.; Weiss, H.L.; Lee, E.Y.; Townsend, C.M., Jr.; Evers, B.M. Neurotensin Receptor 3/Sortilin Contributes to tumorigenesis of neuroendocrine tumors through augmentation of cell adhesion and migration. Neoplasia 2018, 20, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Li, J.; Song, J.; Lee, E.Y.; Weiss, H.L.; Townsend, C.M., Jr.; Evers, B.M. Differential expression and tumorigenic function of neurotensin receptor 1 in neuroendocrine tumor cells. Oncotarget 2015, 6, 26960–26970. [Google Scholar] [PubMed]
- Marini, F.; Giusti, F.; Fossi, C.; Cioppi, F.; Cianferotti, L.; Masi, L.; Boaretto, F.; Zovato, S.; Cetani, F.; Colao, A.; et al. Multiple endocrine neoplasia type 1: Analysis of germline MEN1 mutations in the Italian multicenter MEN1 patient database. Endocrine 2018, 62, 215–233. [Google Scholar] [CrossRef]
- Boora, G.K.; Kanwar, R.; Kulkarni, A.A.; Pleticha, J.; Ames, M.; Schroth, G.; Beutler, A.S.; Banck, M.S. Exome-level comparison of primary well-differentiated neuroendocrine tumors and their cell lines. Cancer Genet. 2015, 208, 374–381. [Google Scholar] [CrossRef]
- Kaldis, P.; Pagano, M. Wnt signaling in mitosis. Dev. Cell 2019, 17, 749–750. [Google Scholar] [CrossRef]
- Willert, K.; Jones, K.A. Wnt signaling: Is the party in the nucleus? Gene Devel. 2006, 20, 1394–1404. [Google Scholar] [CrossRef]
- Reuther, C.; Heinzle, V.; Nölting, S.; Herterich, S.; Hahner, S.; Halilovic, E.; Jeay, S.; Wuerthner, J.U.; Aristizabal Prada, E.T.; Spöttl, G.; et al. The HDM2 (MDM2) Inhibitor NVP-CGM097 Inhibits Tumor Cell Proliferation and Shows Additive Effects with 5-Fluorouracil on the p53-p21-Rb-E2F1 Cascade in the p53wild type Neuroendocrine Tumor Cell Line GOT1. Neuroendocrinology 2018, 106, 1–19. [Google Scholar] [CrossRef]
- Benard, O.; Qian, X.; Liang, H.; Ren, Z.; Suyama, K.; Norton, L.; Hazan, R.B. p21CIP1 Promotes Mammary Cancer-Initiating Cells via Activation of Wnt/TCF1/CyclinD1 Signaling. Mol. Cancer Res. 2019, 17, 1571–1581. [Google Scholar] [CrossRef]
- Wellenstein, M.D.; Coffelt, S.B.; Duits, D.E.M.; van Miltenburg, M.H.; Slagter, M.; de Rink, I.; Henneman, L.; Kas, S.M.; Prekovic, S.; Hau, C.S.; et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature 2019, 572, 538–542. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends. Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bishop, W.R.; Liu, M. Differential effects of cell cycle regulatory protein p21(WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug Resist. Updat. 2003, 6, 183–195. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Xiang, R.; Sun, P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem. Sci. 2014, 39, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Janicke, R.U.; Sohn, D.; Essmann, F.; Schulze-Osthoff, K. The multiple battles fought by anti-apoptotic p21. Cell Cycle 2007, 6, 407–413. [Google Scholar] [CrossRef]
- Wu, D.; Pan, W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Vandamme, T.; Beyens, M.; Peeters, M.; Van Camp, G.; de Beeck, K.O. Next generation exome sequencing of pancreatic neuroendocrine tumor cell lines BON-1 and QGP-1 reveals different lineages. Cancer Genet. 2015, 208, 523. [Google Scholar] [CrossRef]
- Fankhauser, M.; Bechmann, N.; Lauseker, M.; Goncalves, J.; Favier, J.; Klink, B.; William, D.; Gieldon, L.; Maurer, J.; Spöttl, G.; et al. Synergistic Highly Potent Targeted Drug Combinations in different Pheochromocytoma Models including Human Tumor Cultures. Endocrinology 2019, 160, 2600–2617. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.-F.; Spöttl, G.; Maurer, J.; Nölting, S.; Auernhammer, C.J. Inhibition of Wnt/β-Catenin Signaling in Neuroendocrine Tumors In Vitro: Antitumoral Effects. Cancers 2020, 12, 345. https://doi.org/10.3390/cancers12020345
Jin X-F, Spöttl G, Maurer J, Nölting S, Auernhammer CJ. Inhibition of Wnt/β-Catenin Signaling in Neuroendocrine Tumors In Vitro: Antitumoral Effects. Cancers. 2020; 12(2):345. https://doi.org/10.3390/cancers12020345
Chicago/Turabian StyleJin, Xi-Feng, Gerald Spöttl, Julian Maurer, Svenja Nölting, and Christoph Josef Auernhammer. 2020. "Inhibition of Wnt/β-Catenin Signaling in Neuroendocrine Tumors In Vitro: Antitumoral Effects" Cancers 12, no. 2: 345. https://doi.org/10.3390/cancers12020345
APA StyleJin, X.-F., Spöttl, G., Maurer, J., Nölting, S., & Auernhammer, C. J. (2020). Inhibition of Wnt/β-Catenin Signaling in Neuroendocrine Tumors In Vitro: Antitumoral Effects. Cancers, 12(2), 345. https://doi.org/10.3390/cancers12020345




