Oncology Clinic-Based Hereditary Cancer Genetic Testing in a Population-Based Health Care System
Abstract
1. Introduction
2. Results
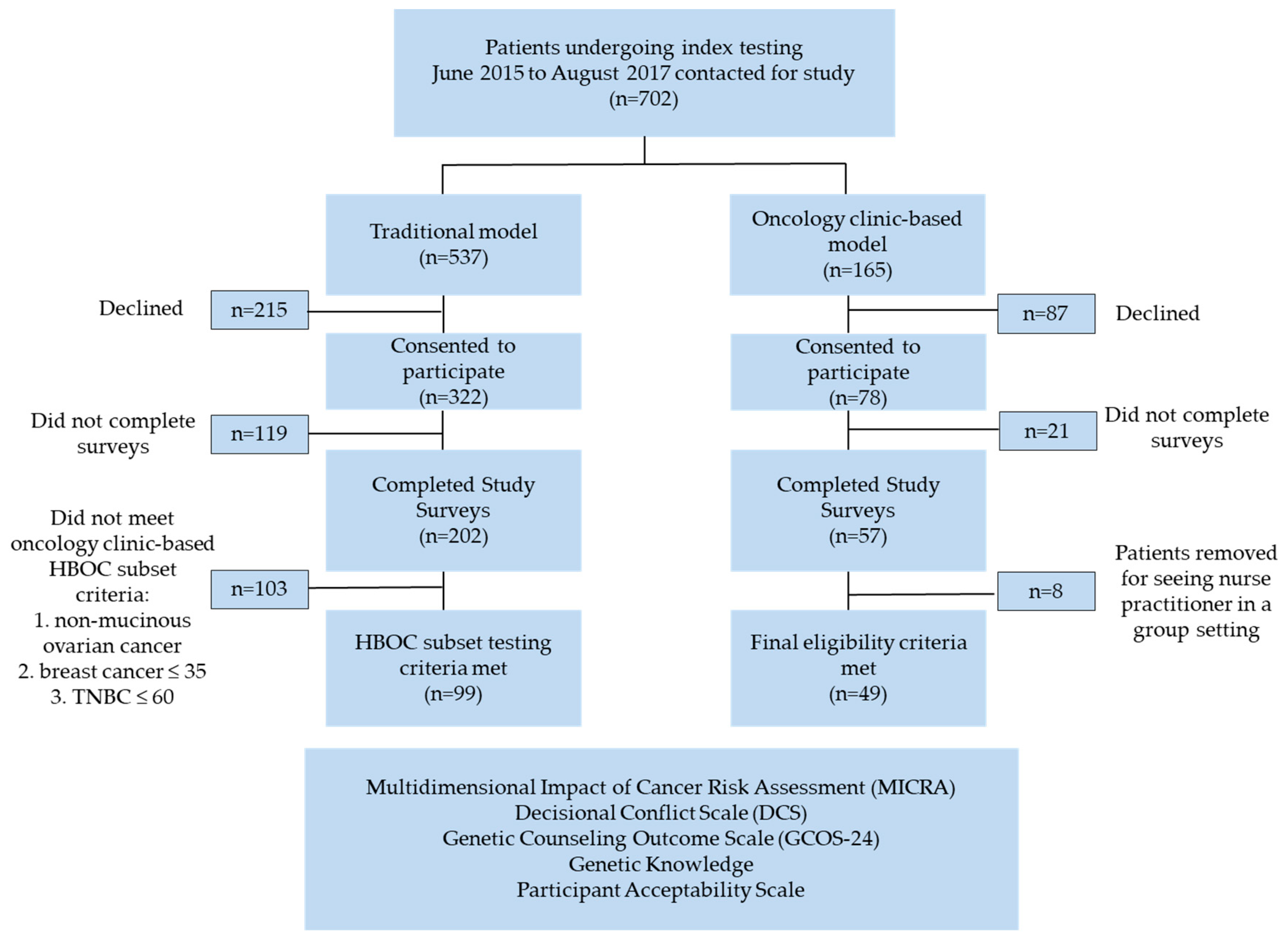
2.1. Study Population
2.2. Wait Times
2.3. Patient Demographics
2.4. Genetic Testing
2.5. Patient Reported Outcome Measures
2.5.1. Knowledge Questionnaire
2.5.2. Multidimensional Impact of Cancer Risk Assessment Survey
2.5.3. The Decisional Conflict Scale
2.5.4. Patient Acceptability Scale
2.5.5. The Genetic Counselling Outcome Scale
2.6. Oncologist and Genetic Counsellor Experieince
3. Discussion
4. Materials and Methods
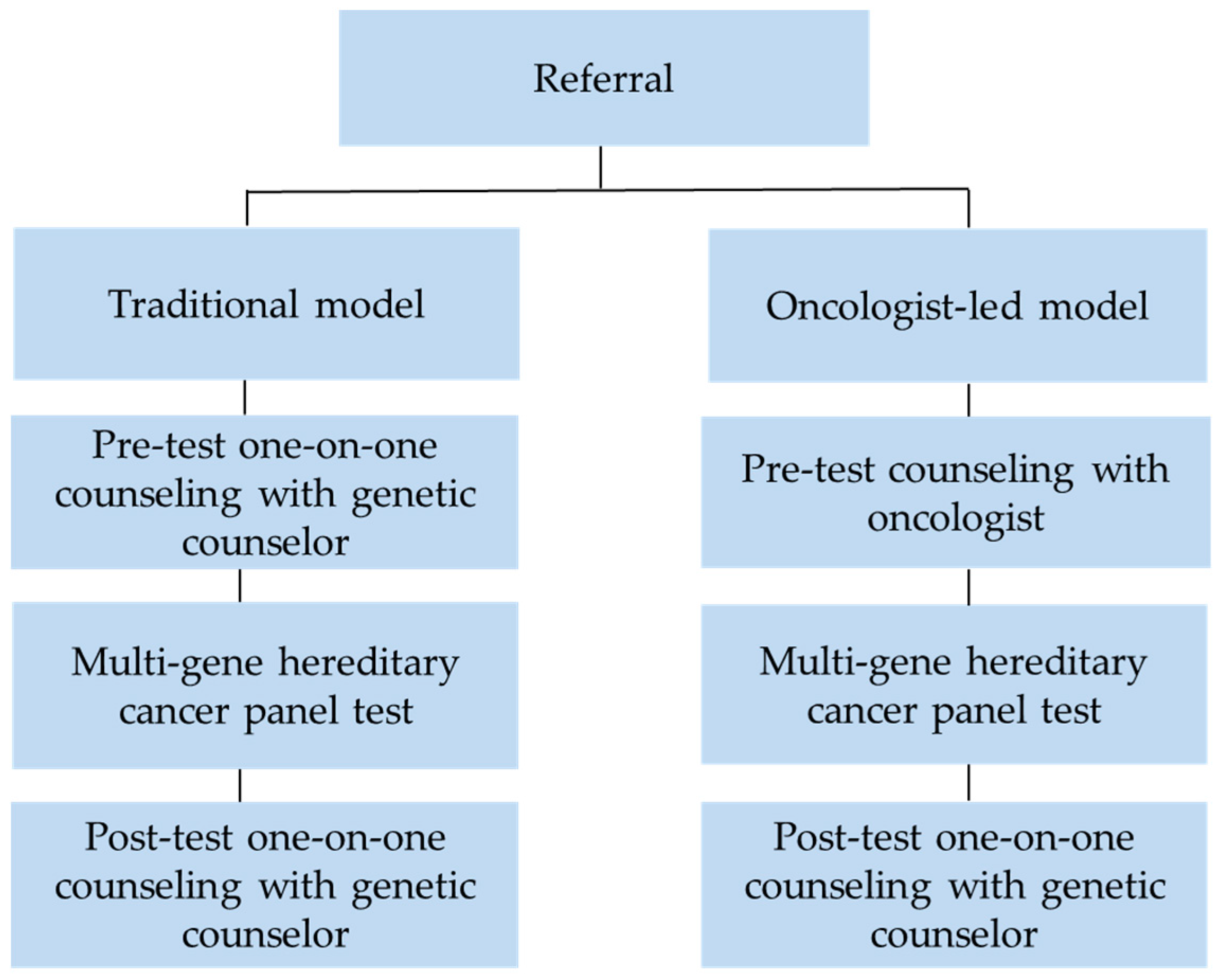
4.1. Clinical Models
4.2. Genetic Testing
4.3. Study Questionnaires
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Evans, D.G.; Barwell, J.; Eccles, D.M.; Collins, A.; Izatt, L.; Jacobs, C.; Donaldson, A.; Brady, A.F.; Cuthbert, A.; Harrison, R.; et al. The Angelina Jolie effect: How high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Res. 2014, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.L.; Vadaparampil, S.T.; Breen, N.; McNeel, T.S.; Wideroff, L.; Graubard, B.I. Awareness of cancer susceptibility genetic testing: The 2000, 2005, and 2010 national health interview surveys. Am. J. Prev. Med. 2014, 46, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C.M.; Adam, S.; Wiggins, S.; Theilmann, J.L.; Copley, T.T.; Bloch, M.; Squitieri, F.; McKellin, W.; Cox, S.; Brown, S.A. Proceed with care: Direct predictive testing for Huntington disease. Am. J. Hum. Genet. 1994, 55, 606–617. [Google Scholar]
- Ertmanski, S.; Metcalfe, K.; Trempala, J.; Glowacka, M.D.; Lubinski, J.; Narod, S.A.; Gronwald, J. Identification of patients at high risk of psychological distress after BRCA1 & BRCA2 genetic testing. Genet. Test Mol. Biomarkers 2009, 13, 325–330. [Google Scholar]
- Hamilton, J.G.; Lobel, M.; Moyer, A. emotional distress following genetic testing for hereditary breast and ovarian cancer: A meta-analytic review. Health Psychol. 2009, 28, 510–518. [Google Scholar] [CrossRef]
- Pieterse, A.H.; Ausems, G.E.M.; Spreeuwenberg, P.; van Dulmen, S. Longer-term influence of breast cancer genetic counseling on cognitions and distress: Smaller benefits for affected versus unaffected women. Patient Educ. Couns. 2011, 85, 425–431. [Google Scholar] [CrossRef]
- Scheuer, L.; Kauff, N.; Robson, M.; Kelly, B.; Barakat, R.; Satagopan, J.; Ellis, N.; Hensley, M.; Boyd, J.; Borgen, P.; et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J. Clin. Oncol. 2002, 20, 1260–1268. [Google Scholar] [CrossRef]
- Sie, A.S.; Spruijt, L.; van Zelst-Stams, W.A.G.; Mensenkamp, A.R.; Ligtenberg, M.J.L.; Brunner, H.G.; Prins, J.B.; Hoogerbrugge, N. High satisfaction and low distress in breast cancer patients one year after BRCA-mutation testing without prior face-to-face genetic counseling. J. Genet. Couns. 2016, 25, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Bedard, A.; Nuk, J.; Lawrence, C.; Sophie, S.; Schrader, K.A. DNA-Direct: Increasing efficiency for pre-test genetic counseling in multigene panel testing. In Proceedings of the Seventh International Symposium on Hereditary Breast and Ovarian Cancer—BRCA: From the Personal to the Population, Montreal, BC, Canada, 8–11 May 2018. [Google Scholar] [CrossRef]
- D’Agincourt-Canning, L.; McGillivray, B.; Panabaker, K.; Scott, J.; Pearn, A.; Ridge, Y.; Portigal-Todd, C. Evaluation of genetic counseling for hereditary cancer by videoconference in British Columbia. BC Med. J. 2008, 50, 554–559. [Google Scholar]
- Ridge, Y.; Panabaker, K.; McCullum, M.; Portigal-Todd, C.; Scott, J.; McGillivray, B. Evaluation of group genetic counseling for hereditary breast and ovarian cancer. J. Genet. Couns. 2009, 18, 87–100. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Riddell, D.; Seal, S.; Talukdar, S.; Mahamdallie, S.; Ruark, E.; Cloke, V.; Slade, I.; Kemp, Z.; Gore, M.; et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Huang, G.; Scambia, G.; Chalas, E.; Pignata, S.; Fiorica, J.; Van Le, L.; Ghamande, S.; González-Santiago, S.; Bover, I.; et al. Evaluation of a streamlined oncologist-led BRCA mutation testing and counseling model for patients with ovarian cancer. J. Clin. Oncol. 2018, 36, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Kentwell, M.; Dow, E.; Antill, Y.; Wrede, C.D.; McNally, O.; Higgs, E.; Hamilton, A.; Ananda, S.; Lindeman, G.J.; Scott, C.L. Mainstreaming cancer genetics: A model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol. Oncol. 2017, 145, 130–136. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.; Peart, T.M.; Foley, N.; Bertrand, M.; Prefontaine, M.; Sugimoto, A.; Ettler, H.; Welch, S.; Panabaker, K. Direct Genetics Referral Pathway for High-Grade Serous Ovarian Cancer Patients: The “Opt-Out” Process. J. Oncol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.T.; Bernhisel, R.; Brown, K.; Kidd, J.; Manly, S. Clinical testing with a panel of 25 genes associated with increased cancer risk results in a significant increase in clinically significant findings across a broad range of cancer histories. Cancer Genet. 2017, 218–219, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Susswein, L.R.; Marshall, M.L.; Nusbaum, R.; Vogel Postula, K.J.; Weissman, S.M.; Yackowski, L.; Vaccari, E.M.; Bissonnette, J.; Booker, J.K.; Cremona, M.L.; et al. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet. Med. 2016, 18, 823–832. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.; Wood, A.M.; Dunn, G.; Shiloh, S.; Todd, C. The Genetic Counseling Outcome Scale: A new patient-reported outcome measure for clinical genetics services. Clin. Genet. 2011, 79, 413–424. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.M. Validation of a Decisional Conflict Scale. Med. Decis. Mak. 1995, 15, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Hughes, C.; Peterman, A.; Chang, C.; Peshkin, B.N.; Schwartz, M.D.; Wenzel, L.; Lemke, A.; Marcus, A.C.; Lerman, C. A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002, 21, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Gomez-Caminero, A.; Benkendorf, J.; Kerner, J.; Isaacs, C.; Barter, J.; Lerman, C. Ethnic differences in knowledge and attitudes about BRCA1 testing in women at increased risk. Patient Educ. Couns. 1997, 32, 51–62. [Google Scholar] [CrossRef]
| Total Patient Population | Oncology Clinic-Based | Traditional | p-Values | |
|---|---|---|---|---|
| Wait Time (days) | p < 0.001 * | |||
| Referral to return of genetic test results (SD) | 191 (174) | 403 (312) | ||
| Age (years, SD) | 62.2 (12.0) | 56.7 (12.0) | p = 0.013 | |
| Gender (n, %) | 148 (100) | N/A | ||
| Female | 49 (100) | 99 (100) | ||
| Personal History (n, %) | p = 0.006 | |||
| Ovarian cancer Breast cancer ≤ 35 Triple negative breast cancer ≤ 60 Breast ≤ 35 and ovarian cancer | 86 (58.1) 15 (10.1) 46 (31.1) 1 (0.7) | 38 (80.7) 3 (5.3) 8 (14) 0 | 48 (48.0) 12 (13.0 38 (38.0) 1 (1.0) | |
| Family History of Breast/Ovarian Cancer (n, %) | p = 1 | |||
| No Yes | 89 (60.1) 59 (39.9) | 29 (59.2) 20 (40.8) | 60 (60.6) 39 (39.4) | |
| Pre-Test Appointment Type (n, %) | p < 0.001 * | |||
| In-person Telephone Videoconference | 90 (60.8) 24 (16.2) 34 (23.0) | 49 (100) 0 0 | 41 (41.4) 24 (24.2) 34 (34.3) | |
| Results Appointment Type(n, %) | p < 0.001 * | |||
| In-person Telephone Videoconference | 32 (21.6) 95 (64.2) 21 (14.2) | 24 (48.9) 4 (8.2) 21 (42.9) | 8 (8.1) 91 (91.9) 0 | |
| Genetic Testing (n, %) | p = 0.015 | |||
| 14-gene panel 17-gene panel Prior BRCA1 and BRCA2 uninformative Other | 55 (37.2) 77 (52.0) 3 (2.0) 13 (8.8) | 21 (42.9) 28 (57.1) 0 0 | 34 (34.3) 49 (49.5) 3 (3.0) 13 (13.1) | |
| Additional Expanded Panel Testing (n, %) | p = 0.086 | |||
| No Yes | 116 (78.4) 32 (21.6) | 43 (87.78) 6 (12.2) | 73 (73.7) 26 (26.3) | |
| Genetic Test Results 1 (n, %) | p = 0.470 | |||
| Pathogenic or likely pathogenic Pathogenic monoallelic MUTYH 2 Variant of uncertain significance Likely benign Uninformative | 18 (12.2) 3 (2.0) 31 (20.9) 14 (9.5) 82 (55.4) | 6 (12.2) 1 (2.0) 12 (24.5) 7 (14.3) 23 (46.9) | 12 (12.1) 2 (2.0) 19 (19.2) 7 (7.1) 59 (59.6) | |
| Pathogenic BRCA Variant (n, %) | p = 0.507 | |||
| No Yes | 136 (91.9) 12 (8.1) | 44 (89.8) 5 (10.2) | 92 (92.9) 7 (7.1) |
| Gene | Syndrome |
|---|---|
| BRCA1, BRCA2 | Hereditary breast and ovarian cancer |
| PALB2 * | Hereditary breast and pancreatic cancer |
| TP53 | Li Fraumeni syndrome |
| PTEN | PTEN hamartoma tumor syndrome (Cowden Syndrome) |
| CDH1 | Hereditary diffuse gastric and lobular breast cancer |
| MLH1, MSH2, MSH6, PMS2 | Lynch syndrome |
| MUTYH | MUTYH-associated polyposis (MAP) |
| APC | Familial adenomatous polyposis (FAP) |
| POLE * | Hereditary colorectal cancer and colonic polyposis |
| POLD1 * | Hereditary colorectal and uterine cancer; colonic polyposis |
| STK11 | Peutz–Jeghers syndrome |
| SMAD4, BMPR1A | Juvenile polyposis syndrome |
| Survey | Oncology Clinic-Based Model | Traditional Model | Population Total | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Genetic Knowledge | 49 | 8.46 (1.79) | 99 | 8.67 (1.52) | 148 | 8.60 (1.59) |
| Patient Acceptability Scale | 49 | 4.54 (0.71) | 92 | 4.52 (0.69) | 141 | 4.53 (0.70) |
| Decision Conflict Scale | ||||||
| Uncertainty Informed Values Clarity | 48 48 48 | 22.57 (19.52) 19.71 (14.04) 24.13 (17.04) | 98 97 97 | 23.36 (21.25) 18.04 (17.38) 24.22 (19.73) | 146 145 145 | 23.10 (20.63) 18.59 (16.32) 24.19 (18.82) |
| Support | 48 | 25.18 (18.23) | 97 | 26.61 (20.94) | 145 | 26.13 (20.03) |
| Effective Decision | 48 | 13.16 (14.32) | 97 | 15.21 (19.43) | 145 | 14.53 (17.88) |
| Multidimensional Impact of Cancer Risk Assessment | ||||||
| Distress | 49 | 4.53 (5.65) | 99 | 3.37 (5.24) | 148 | 3.75 (5.39) |
| Uncertainty | 49 | 9.51 (8.19) | 99 | 10.02 (6.88) | 148 | 9.85 (7.32) |
| Positive experience | 49 | 6.00 (5.78) | 99 | 4.45 (4.66) | 148 | 4.96 (5.09) |
| Genetic Counselling Outcome Scale | 49 | 120.17 (16.78) | 98 | 120.93 (15.15) | 147 | 120.67 (15.66) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, M.; Min, H.J.; Hong, Q.; Compton, K.; Mung, S.W.; Lohn, Z.; Nuk, J.; McCullum, M.; Portigal-Todd, C.; Karsan, A.; et al. Oncology Clinic-Based Hereditary Cancer Genetic Testing in a Population-Based Health Care System. Cancers 2020, 12, 338. https://doi.org/10.3390/cancers12020338
Richardson M, Min HJ, Hong Q, Compton K, Mung SW, Lohn Z, Nuk J, McCullum M, Portigal-Todd C, Karsan A, et al. Oncology Clinic-Based Hereditary Cancer Genetic Testing in a Population-Based Health Care System. Cancers. 2020; 12(2):338. https://doi.org/10.3390/cancers12020338
Chicago/Turabian StyleRichardson, Matthew, Hae Jung Min, Quan Hong, Katie Compton, Sze Wing Mung, Zoe Lohn, Jennifer Nuk, Mary McCullum, Cheryl Portigal-Todd, Aly Karsan, and et al. 2020. "Oncology Clinic-Based Hereditary Cancer Genetic Testing in a Population-Based Health Care System" Cancers 12, no. 2: 338. https://doi.org/10.3390/cancers12020338
APA StyleRichardson, M., Min, H. J., Hong, Q., Compton, K., Mung, S. W., Lohn, Z., Nuk, J., McCullum, M., Portigal-Todd, C., Karsan, A., Regier, D., Brotto, L. A., Sun, S., & Schrader, K. A. (2020). Oncology Clinic-Based Hereditary Cancer Genetic Testing in a Population-Based Health Care System. Cancers, 12(2), 338. https://doi.org/10.3390/cancers12020338




