Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1)
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
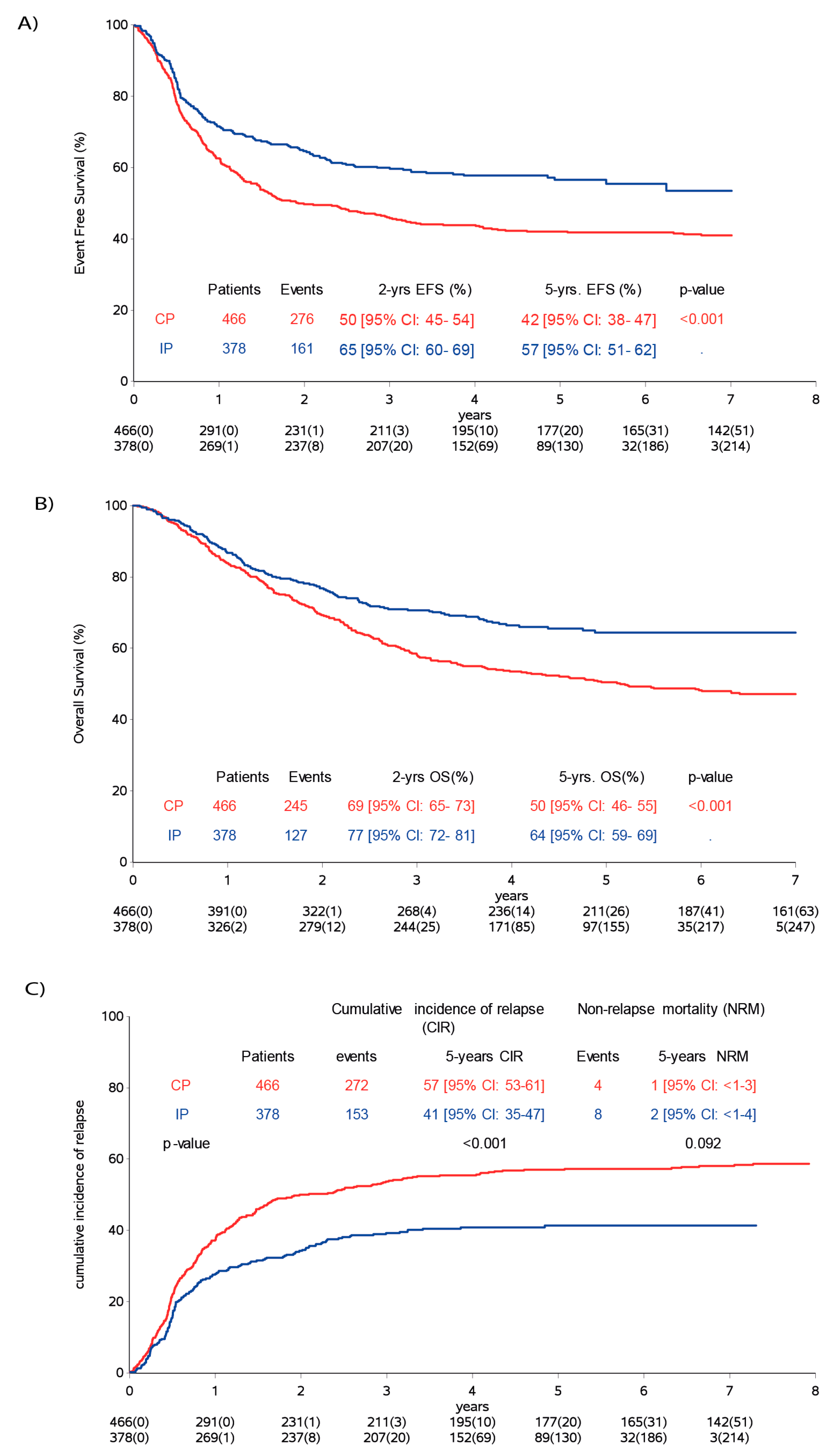
2.2. Survival
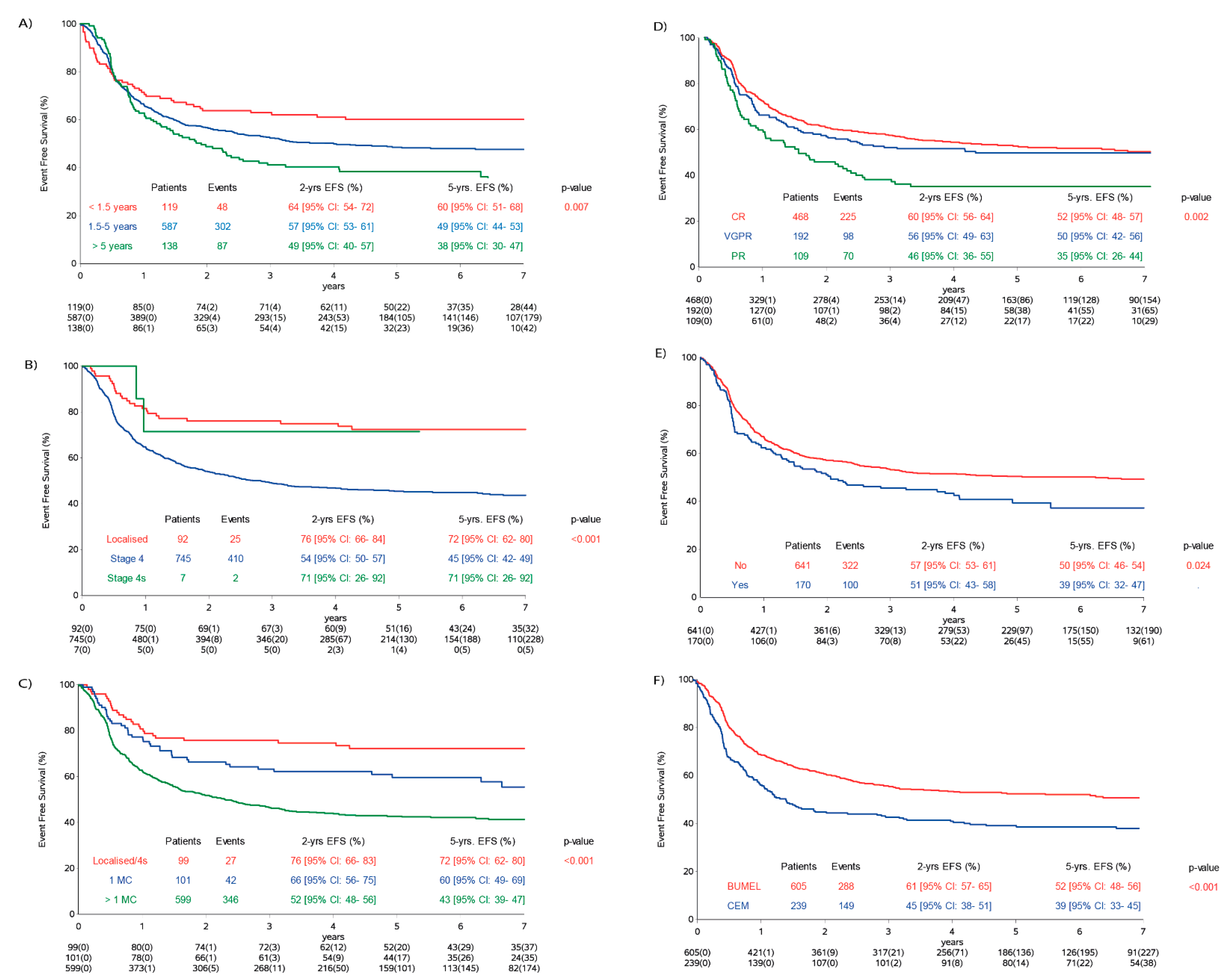
2.3. Influence of Risk Factors
2.4. Multivariate Analysis on Analysis Cohort
2.5. Response to Maintenance Treatments
2.6. Adverse Events and Toxicity
3. Discussion
4. Materials and Methods
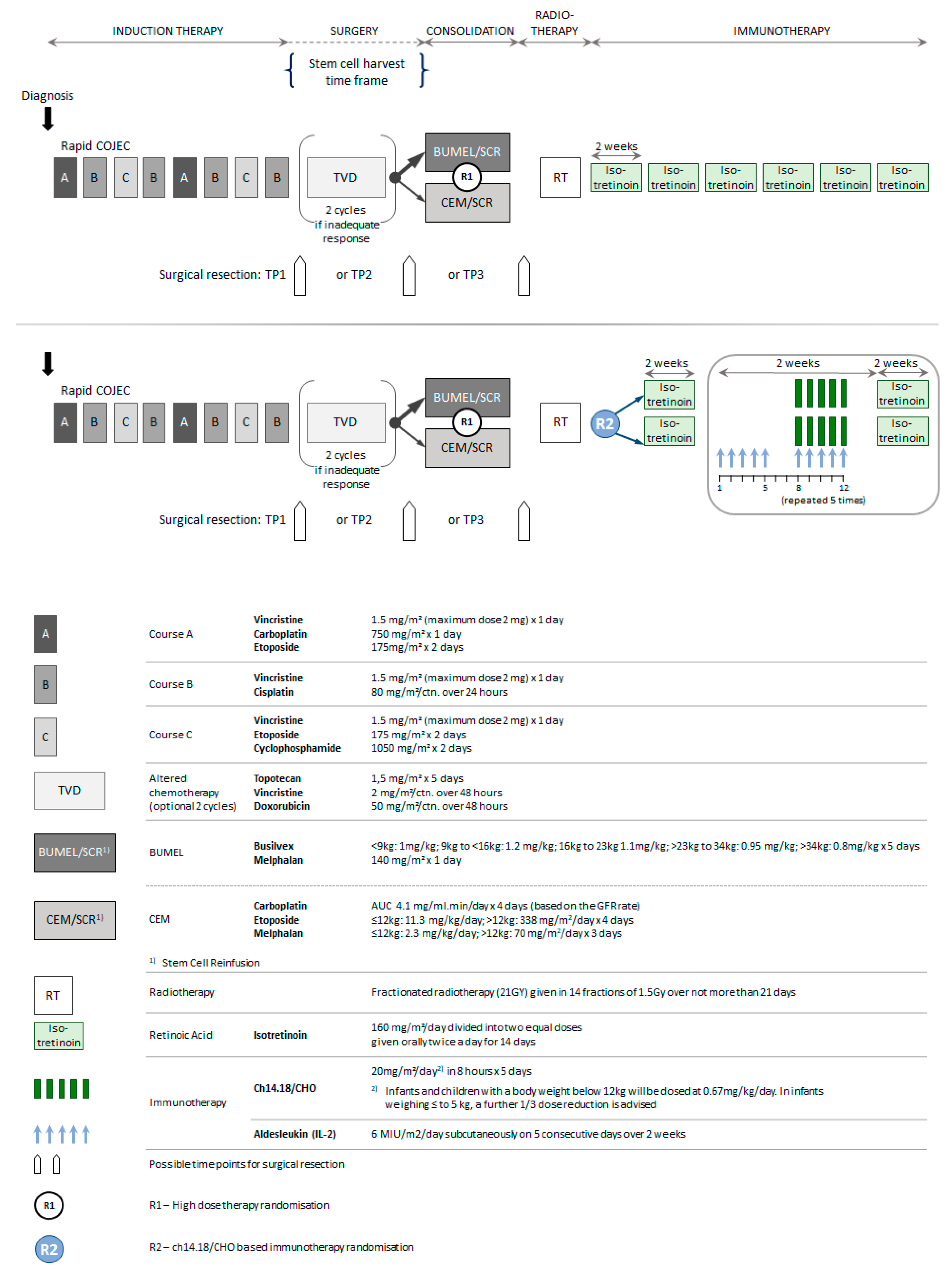
4.1. Trial Eligibility
4.2. Eligibiliy for the Analysis Cohort and Treatments Given
4.3. Statistical Analysis
4.3.1. Establishment of the Analysis Cohort
4.3.2. Outcome Parameters
4.3.3. Multivariate Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- London, W.B.; Castleberry, R.P.; Matthay, K.K.; Look, A.T.; Seeger, R.C.; Shimada, H.; Thorner, P.; Brodeur, G.; Maris, J.M.; Reynolds, C.P.; et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J. Clin. Oncol. 2005, 23, 6459–6465. [Google Scholar] [CrossRef] [PubMed]
- Valteau-Couanet, D.; Le Deley, M.-C.; Bergeron, C.; Ducassou, S.; Michon, J.; Rubie, H.; Le Teuff, G.; Coze, C.; Plantaz, D.; Sirvent, N.; et al. Long-term results of the combination of the N7 induction chemotherapy and the busulfan-melphalan high dose chemotherapy. Pediatr. Blood Cancer 2014, 61, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009, 27, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, A.D.J.; Pinkerton, C.R.; Lewis, I.J.; Imeson, J.; Ellershaw, C.; Machin, D. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet. Oncol. 2008, 9, 247–256. [Google Scholar] [CrossRef]
- Ladenstein, R.; Valteau-Couanet, D.; Brock, P.; Yaniv, I.; Castel, V.; Laureys, G.; Malis, J.; Papadakis, V.; Lacerda, A.; Ruud, E.; et al. Randomized Trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: The European HR-NBL1/SIOPEN study. J. Clin. Oncol. 2010, 28, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Pötschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 500–514. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [Green Version]
- Cheung, N.-K.V.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Fest, S.; Kunert, R.; Katinger, H.; Pistoia, V.; Michon, J.; Lewis, G.; Ladenstein, R.; Lode, H.N. Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol. Immunol. 2005, 42, 1311–1319. [Google Scholar] [CrossRef]
- Ladenstein, R.; Weixler, S.; Baykan, B.; Bleeke, M.; Kunert, R.; Katinger, D.; Pribill, I.; Glander, P.; Bauer, S.; Pistoia, V.; et al. Ch14.18 antibody produced in CHO cells in relapsed or refractory Stage 4 neuroblastoma patients: A SIOPEN Phase 1 study. MAbs 2013, 5, 801–809. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Siabalis, D.; Garaventa, A.; Bergeron, C.; Lewis, I.; Stein, J.; Kohler, J.; Shaw, P.J.; Holter, W.; et al. Dose Finding Study for the Use of Subcutaneous Recombinant Interleukin-2 to Augment Natural Killer Cell Numbers in an Outpatient Setting for Stage 4 Neuroblastoma After Megatherapy and Autologous Stem-Cell Reinfusion. J. Clin. Oncol. 2011, 29, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M.; et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.; Naranjo, A.; Van Ryn, C.; Yu, A.L.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.-E.-N.; Diccianni, M.B.; Sondel, P.M.; et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 946–957. [Google Scholar] [CrossRef] [Green Version]
- Furman, W.L.; Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Davidoff, A.M.; Krasin, M.J.; Sahr, N.; Sykes, A.; Wu, J.; Brennan, R.C.; et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-Risk Neuroblastoma. Clin. Cancer Res. 2019, 25, 6320–6328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef]
- Ambros, I.M.; Benard, J.; Boavida, M.; Bown, N.; Caron, H.; Combaret, V.; Couturier, J.; Darnfors, C.; Delattre, O.; Freeman-Edward, J.; et al. Quality Assessment of Genetic Markers Used for Therapy Stratification. J. Clin. Oncol. 2003, 21, 2077–2084. [Google Scholar] [CrossRef]
- Amoroso, L.; Erminio, G.; Makin, G.; Pearson, A.D.; Brock, P.; Valteau-Couanet, D.; Castel, V.; Pasquet, M.; Laureys, G.; Thomas, C.; et al. Topotecan-Vincristine-Doxorubicin in Stage 4 High Risk Neuroblastoma Patients Failing to Achieve a Complete Metastatic Response to Rapid COJEC-a SIOPEN Study. Cancer Res. Treat. 2018, 50, e148. [Google Scholar] [CrossRef]
- Peto, R.; Pike, M.C.; Armitage, P.; Breslow, N.E.; Cox, D.R.; Howard, S.V.; Mantel, N.; McPherson, K.; Peto, J.; Smith, P.G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br. J. Cancer 1977, 35, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002; Chapter 8.3.3; pp. 251–254. ISBN 0-471-36357-X. [Google Scholar]
- Gray, R.J. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Andersen, P.K.; Perme, M.P. Pseudo-observations in survival analysis. Stat. Methods Med. Res. 2010, 19, 71–99. [Google Scholar] [CrossRef]
- Simon, T.; Hero, B.; Faldum, A.; Handgretinger, R.; Schrappe, M.; Niethammer, D.; Berthold, F. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J. Clin. Oncol. 2004, 22, 3549–3557. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Hero, B.; Faldum, A.; Handgretinger, R.; Schrappe, M.; Niethammer, D.; Berthold, F. Infants with Stage 4 Neuroblastoma: The Impact of the Chimeric Anti-GD2-Antibody ch14.18 Consolidation Therapy. Klin. Pädiatrie 2005, 217, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Hero, B.; Faldum, A.; Handgretinger, R.; Schrappe, M.; Klingebiel, T.; Berthold, F. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer 2011, 11, e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Control Population | Immunotherapy Population | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | number | 466 | 378 | ||
| Sex | Female | 180 | 39% | 140 | 37% |
| Male | 286 | 61% | 238 | 63% | |
| Age | <1.5 years | 64 | 13% | 55 | 14% |
| 1.5-<5 years | 333 | 71% | 254 | 67% | |
| ≥5 years | 69 | 15% | 69 | 18% | |
| Median | 2.70 | 2.87 | |||
| Stage | Localised | 60 | 13% | 32 | 8% |
| Stage 4 | 406 | 87% | 339 | 90% | |
| Stage 4s | 0 | 0% | 7 | 2% | |
| MYCN Stage 4 | MNA NR | 27 | 6% | 16 | 4% |
| MNA no | 217 | 57% | 197 | 61% | |
| MNA yes | 162 | 43% | 126 | 39% | |
| MC | NR | 23 | 5% | 23 | 6% |
| 0 | 60 | 14% | 32 | 9% | |
| 1 | 70 | 16% | 35 | 10% | |
| 2 | 136 | 31% | 112 | 32% | |
| 3 | 120 | 27% | 112 | 32% | |
| >3 | 57 | 13% | 64 | 18% | |
| TVD given | NR | 23 | 5% | 10 | 3% |
| No | 391 | 88% | 250 | 68% | |
| Yes | 52 | 12% | 118 | 32% | |
| Surgery | CME | 318 | 76% | 261 | 75% |
| IME | 101 | 24% | 87 | 25% | |
| Status prior HDT | NR | 25 | 5% | 33 | 9% |
| CR | 174 | 39% | 116 | 34% | |
| VGPR | 159 | 36% | 149 | 43% | |
| PR | 108 | 24% | 80 | 23% | |
| HDT | BuMel | 257 | 55% | 348 | 92% |
| CEM | 209 | 45% | 30 | 8% | |
| Status prior Maintenance | NR | 58 | 12% | 17 | 4% |
| CR | 258 | 63% | 210 | 58% | |
| VGPR | 93 | 23% | 99 | 27% | |
| PR | 57 | 14% | 52 | 14% | |
| Characteristics | Total Population | Control Group | Immunotherapy Group | p-Value B | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (A) Event Free Survival | Events/Pts | 5-years EFS (95% CI) | p-value A | Events/Pts | 5-years EFS (95% CI) | Events/Pts | 5-years EFS (95% CI) | |||
| Total | 844 Pts | 466 Pts | 378 Pts | |||||||
| Sex | female | 164/320 | 49 (44–55) | 0.803 | 105/180 | 43 (36–50) | 59/140 | 57 (49–65) | 0.938 | |
| male | 273/524 | 48 (44–52) | 171/286 | 41 (36–47) | 102/238 | 56 (49–62) | ||||
| Age | <1.5 years | 48/119 | 60 (51–68) | 0.007 | 27/64 | 59 (46–70) | 21/55 | 61 (47–73) | 0.161 | |
| 1.5–<5 years | 302/587 | 49 (44–53) | 194/333 | 42 (37–47) | 108/254 | 57 (50–63) | ||||
| ≥5 years | 87/138 | 38 (30–47) | 55/69 | 25 (16–36) | 32/69 | 53 (40–64) | ||||
| Stage | Localised | 25/92 | 72 (62–80) | <0.001 | 14/60 | 76 (63–85) | 11/32 | 66 (47–79) | 0.007 | |
| Stage 4 | 410/745 | 45 (42–49) | 262/406 | 37 (32–42) | 148/339 | 56 (50–61) | ||||
| Stage 4s | 2/7 | 71 (26–92) | – | – | 2/7 | 71 (26–92) | ||||
| Stage 4 MNA no– | 236/414 | 43 (38–48) | 0.819 | 147/217 | 33 (27–39) | 89/197 | 54 (47–61) | 0.25 | ||
| Stage 4 MNA yes+ | 154/288 | 47 (41–53) | 98/162 | 42 (34–49) | 56/126 | 55 (45–63) | ||||
| MC | 0 | 25/92 | 72 (62–80) | <0.001 | 14/60 | 67 (63–85) | 11/32 | 66 (47–79) | 0.025 | |
| 1 | 43/105 | 60 (50–69) | 34/70 | 54 (42–65) | 9/35 | 71 (50–85) | ||||
| 2 | 136/248 | 46 (39–52) | 85/136 | 39 (31–47) | 51/112 | 55 (45–63) | ||||
| 3 | 134/232 | 43 (36–49) | 89/120 | 27 (20–36) | 45/112 | 60 (50–69) | ||||
| >3 | 76/121 | 37 (28–46) | 42/57 | 28 (17–40) | 34/64 | 45 (32–57) | ||||
| TVD | no | 322/641 | 50 (46–54) | 0.024 | 225/391 | 44 (39–49) | 97/250 | 61 (54–67) | 0.732 | |
| yes | 100/170 | 39 (32–47) | 38/52 | 27 (16–39) | 62/118 | 45 (34–55) | ||||
| Surgery | CME | 288/578 | 50 (46–54) | 0.123 | 183/318 | 43 (38–49) | 105/260 | 59 (52–65) | 0.946 | |
| IME | 106/188 | 45 (38–52) | 66/101 | 38 (29–48) | 40/87 | 53 (42–63) | ||||
| Status Prior HDT | ||||||||||
| CR | 134/290 | 54 (48–59) | 0.022 | 86/174 | 51 (43–58) | 48/116 | 57 (47–66) | 0.294 | ||
| VGPR | 171/308 | 45 (39–51) | 104/159 | 36 (29–44) | 67/149 | 55 (46–63) | ||||
| PR | 104/188 | 45 (38–53) | 70/108 | 38 (29–47) | 34/80 | 57 (45–67) | ||||
| Characteristics | Total Population | Control Group | Immunotherapy Group | p–value B | ||||||
| (A) Event Free Survival | Events/Pts | 5–years EFS (95% CI) | p–value A | Events/Pts | 5–years EFS (95% CI) | Events/Pts | 5–years EFS (95% CI) | |||
| HDT | BuMel | 288/605 | 52 (48–56) | <0.001 | 137/257 | 48 (41–54) | 151/348 | 56 (50–61) | 0.055 | |
| CEM | 149/239 | 39 (33–45) | 139/209 | 35 (29–42) | 10/30 | 67 (47–80) | ||||
| Status Prior Maintenance | ||||||||||
| CR | 225/468 | 52 (48–47) | 0.002 | 144/258 | 46 (39–52) | 81/210 | 61 (53–67) | 0.84 | ||
| VGPR | 98/192 | 50 (42–56) | 55/93 | 43 (33–53) | 43/99 | 56 (46–66) | ||||
| PR | 70/109 | 35 (26–44) | 42/57 | 26 (15–33) | 28/52 | 45 (32–58) | ||||
| (B) Overall Survival | Events/ Pts | 5-year OS (95% CI) | p-value A | Events/Pts | 5-year OS (95% CI) | Events/Pts | 5-year OS (95% CI) | |||
| Sex | female | 138/320 | 59 (53–64) | 0.536 | 95/180 | 53 (45–60) | 43/140 | 59 (53–64) | 0.488 | |
| male | 234/524 | 55 (50–59) | 150/286 | 49 (43–55) | 84/238 | 55 (50–59) | ||||
| Age | <1.5 years | 45/119 | 62 (52–70) | 0.445 | 26/64 | 61 (48–72) | 19/55 | 62 (52–70) | 0.409 | |
| 1.5–<5 years | 259/587 | 57 (53–61) | 177/333 | 50 (45–55) | 82/254 | 57 (53–61) | ||||
| ≥5 years | 68/138 | 49 (40–58) | 42/69 | 42 (30–54) | 26/69 | 49 (40–58) | ||||
| Stage | localised | 24/92 | 76 (66–83) | 0.001 | 13/60 | 82 (69–89) | 11/32 | 76 (66–83) | 0.003 | |
| Stage 4 | 347/745 | 54 (50–57) | 232/406 | 46 (41–51) | 115/339 | 54 (50–57) | ||||
| Stage 4s | 0/7 | 83 (27–97) | 0/0 | 1/7 | ||||||
| Stage 4 MNA no- | 193/414 | 53 (47–58) | 0.235 | 129/217 | 43 (37–50) | 64/197 | 53 (47–58) | 0.150 | ||
| Stage 4 MNA yes+ | 137/288 | 54 (48–60) | 88/162 | 49 (41–56) | 49/126 | 54 (48–60) | ||||
| MC | 0 | 24/92 | 76 (66–83) | <0.001 | 13/60 | 82 (69–89) | 11/32 | 76 (66–83) | 0.013 | |
| 1 | 36/105 | 68 (58–76) | 29/70 | 62 (50–73) | 7/35 | 68 (58–76) | ||||
| 2 | 109/248 | 56 (50–62) | 71/136 | 51 (42–59) | 38/112 | 56 (50–62) | ||||
| 3 | 117/232 | 49 (42–56) | 81/120 | 36 (27–44) | 36/112 | 49 (42–56) | ||||
| >3 | 70/121 | 43 (34–52) | 41/57 | 32 (20–44) | 29/64 | 43 (34–52) | ||||
| TVD | no | 281/641 | 57 (53–61) | 0.224 | 200/391 | 52 (47–57) | 81/250 | 57 (53–61) | 0.441 | |
| yes | 78/170 | 52 (44–60) | 34/52 | 36 (23–49) | 44/118 | 52 (44–60) | ||||
| Surgery | CME | 248/578 | 57 (53–61) | 0.218 | 165/318 | 51 (45–56) | 83/260 | 57 (53–61) | 0.765 | |
| IME | 91/188 | 52 (45–59) | 58/101 | 46 (36–55) | 33/87 | 52 (45–59) | ||||
| Status Prior HDT | ||||||||||
| CR | 116/290 | 60 (54–66) | 0.089 | 80/174 | 56 (48–63) | 36/116 | 60 (54–66) | 0.522 | ||
| VGPR | 140/308 | 55 (49–61) | 86/159 | 50 (42–58) | 54/149 | 55 (49–61) | ||||
| PR | 92/188 | 52 (45–59) | 65/108 | 44 (34–53) | 27/80 | 52 (45–59) | ||||
| HDT | BUMEL | 238/605 | 60 (56–64) | <0.001 | 121/257 | 56 (50–62) | 117/348 | 60 (56–64) | 0.267 | |
| CEM | 134/239 | 46 (40–53) | 124/209 | 44 (37–50) | 10/30 | 46 (40–53) | ||||
| Characteristics | Total Population | Control Group | Immunotherapy Group | p–value B | ||||||
| (B) Overall Survival | Events/ Pts | 5-year OS (95% CI) | p-value A | Events/Pts | 5-year OS (95% CI) | Events/Pts | 5-year OS (95% CI) | |||
| Status Prior Maintenance | ||||||||||
| CR | 190/468 | 60 (55–65) | 0.006 | 129/258 | 54 (47–59) | 61/210 | 60 (55–65) | 0.640 | ||
| VGPR | 83/192 | 57 (49–64) | 47/93 | 52 (42–62) | 36/99 | 57 (49–64) | ||||
| PR | 61/109 | 43 (33–53) | 38/57 | 36 (23–48) | 23/52 | 50 (33–65) | ||||
| Risk Factor | Characteristics | Pseudo Values for 5-Years EFS | ||
|---|---|---|---|---|
| cHR (95% CI) | p-Value | |||
| (A) | Multivariate Analysis | |||
| Immunotherapy vs. Control Cohort | 1.75 (1.36–2.25) | <0.0001 | ||
| Age (vs. <1.5 yrs) | 0.0931 * | |||
| 1.5–5 years | 1.31 (0.92–1.87) | 0.1384 | ||
| >5years | 1.59 (1.05–2.42) | 0.0138 | ||
| Stage 4, 4s and Number of MC (vs. MNA stages 2, 3) | <0.0001 * | |||
| 1 MC | 1.38 (0.80–2.47) | 0.2493 | ||
| >1MC | 2.69 (1.74–4.15) | <0.0001 | ||
| TVD | 1.28 (0.97–1.69) | 0.2478 | ||
| Status Prior Maintenance (vs. CR) | 0.0363 * | |||
| VGPR | 1.06 (0.81–1.39) | 0.6416 | ||
| PR | 1.49 (1.10–2.02) | 0.0103 | ||
| HDT | CEM vs. BuMel | 1.32 (1.02–1.70) | 0.0345 | |
| (B) | Subgroup Analysis According to HDT(after adjustment for age, stage, MC, TVD and status prior maintenance treatment) | |||
| BUMEL | IP vs. CP | 1.6 (1.2–2.1) | 0.001 | |
| CEM | IP vs. CP | 3.0 (1.5–5.8) | 0.002 | |
| Response Status before Maintenance | Response Status after Maintenance | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Evaluable | CR | VGPR | PR | SD | PD | ||
| Immunotherapy and Isotretinoin | ||||||||
| CR | 210 | 188 | 151 | 0 | 0 | 0 | 37 | |
| <CR | Total | 151 | 130 | 52 | 43 | 8 | 0 | 27 |
| VGPR | 99 | 85 | 36 | 31 | 0 | 0 | 18 | |
| PR | 52 | 45 | 16 | 12 | 8 | 0 | 9 | |
| Isotretinoin | ||||||||
| CR | 258 | 204 | 163 | 0 | 0 | 0 | 40 | |
| <CR | Total | 150 | 108 | 35 | 27 | 18 | 1 | 27 |
| VGPR | 93 | 67 | 28 | 23 | 2 | 0 | 14 | |
| PR | 57 | 41 | 7 | 4 | 16 | 1 | 13 | |
| Toxicities | Control | Without IL2 | With IL2 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eval | All | All | ||||||||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | 3 + 4 | 0 | 1 | 2 | 3 | 4 | 3 + 4 | 0 | 1 | 2 | 3 | 4 | 3 + 4 | |||||||
| Non-Hem. Tox. | 317 | 113 | 42 | 116 | 41 | 5 | 46 | 15% | 186 | 5 | 6 | 53 | 105 | 17 | 122 | 66% | 192 | 4 | 2 | 20 | 113 | 53 | 166 | 86% |
| General Condition | 314 | 225 | 69 | 13 | 4 | 3 | 7 | 2% | 185 | 42 | 70 | 43 | 24 | 6 | 30 | 16% | 192 | 25 | 53 | 36 | 66 | 12 | 78 | 41% |
| Haemoglobin | 313 | 208 | 39 | 54 | 8 | 4 | 12 | 4% | 186 | 21 | 2 | 84 | 69 | 10 | 79 | 42% | 191 | 14 | 5 | 46 | 102 | 24 | 126 | 66% |
| WBC | 313 | 235 | 32 | 28 | 15 | 3 | 18 | 6% | 186 | 35 | 30 | 73 | 42 | 6 | 48 | 26% | 191 | 35 | 30 | 57 | 51 | 18 | 69 | 36% |
| Granulocytes | 313 | 244 | 23 | 25 | 16 | 5 | 21 | 7% | 186 | 44 | 26 | 54 | 43 | 19 | 62 | 33% | 191 | 34 | 12 | 34 | 69 | 42 | 111 | 58% |
| Platelets | 313 | 260 | 13 | 12 | 16 | 12 | 28 | 9% | 186 | 66 | 25 | 31 | 40 | 24 | 64 | 34% | 191 | 30 | 18 | 26 | 61 | 56 | 117 | 61% |
| Infection | 315 | 220 | 52 | 23 | 19 | 1 | 20 | 6% | 185 | 79 | 26 | 32 | 47 | 1 | 48 | 26% | 191 | 60 | 20 | 47 | 58 | 6 | 64 | 34% |
| Fever | 314 | 241 | 14 | 54 | 4 | 1 | 5 | 2% | 185 | 41 | 3 | 116 | 24 | 1 | 25 | 14% | 190 | 28 | 4 | 82 | 66 | 10 | 76 | 40% |
| Stomatitis | 312 | 293 | 10 | 7 | 2 | 0 | 2 | 1% | 185 | 156 | 18 | 8 | 0 | 3 | 3 | 2% | 191 | 149 | 27 | 12 | 2 | 1 | 3 | 2% |
| Nausea/Vomiting | 313 | 284 | 6 | 19 | 4 | 0 | 4 | 1% | 185 | 88 | 11 | 76 | 9 | 1 | 10 | 5% | 191 | 68 | 12 | 94 | 14 | 3 | 17 | 9% |
| Diarrhoea | 313 | 286 | 11 | 13 | 3 | 0 | 3 | 1% | 185 | 93 | 32 | 47 | 10 | 3 | 13 | 7% | 192 | 75 | 25 | 51 | 34 | 7 | 41 | 21% |
| Constipation | 312 | 302 | 7 | 3 | 0 | 0 | 0 | 0% | 185 | 110 | 43 | 32 | 0 | 0 | 0 | 0% | 191 | 142 | 21 | 21 | 4 | 3 | 7 | 4% |
| Skin | 315 | 181 | 50 | 73 | 10 | 1 | 11 | 3% | 185 | 65 | 46 | 65 | 9 | 0 | 9 | 5% | 192 | 48 | 48 | 77 | 19 | 0 | 19 | 10% |
| Allergy | 314 | 307 | 4 | 3 | 0 | 0 | 0 | 0% | 185 | 88 | 50 | 28 | 14 | 5 | 19 | 10% | 191 | 75 | 39 | 38 | 32 | 7 | 39 | 20% |
| Cardiac Function | 298 | 298 | 0 | 0 | 0 | 0 | 0 | 0% | 182 | 178 | 0 | 0 | 3 | 1 | 4 | 2% | 191 | 183 | 3 | 1 | 3 | 1 | 4 | 2% |
| Echo LV/SV | 298 | 298 | 0 | 0 | 0 | 0 | 0 | 0% | 182 | 181 | 0 | 0 | 0 | 1 | 1 | 1% | 189 | 181 | 5 | 2 | 0 | 1 | 1 | 1% |
| Hypotension | 298 | 296 | 2 | 0 | 0 | 0 | 0 | 0% | 182 | 139 | 22 | 8 | 12 | 1 | 13 | 7% | 191 | 119 | 23 | 17 | 25 | 7 | 32 | 17% |
| Hypertension | 298 | 298 | 0 | 0 | 0 | 0 | 0 | 0% | 182 | 162 | 10 | 3 | 7 | 0 | 7 | 4% | 190 | 177 | 4 | 6 | 3 | 0 | 3 | 2% |
| Creatinine | 312 | 301 | 9 | 2 | 0 | 0 | 0 | 0% | 185 | 167 | 14 | 1 | 3 | 0 | 3 | 2% | 192 | 159 | 20 | 11 | 2 | 0 | 2 | 1% |
| Proteinuria | 311 | 307 | 4 | 0 | 0 | 0 | 0 | 0% | 184 | 169 | 13 | 2 | 0 | 0 | 0 | 0% | 191 | 178 | 12 | 1 | 0 | 0 | 0 | 0% |
| Haematuria | 311 | 305 | 6 | 0 | 0 | 0 | 0 | 0% | 183 | 167 | 11 | 5 | 0 | 0 | 0 | 0% | 191 | 169 | 16 | 6 | 0 | 0 | 0 | 0% |
| GFR | 310 | 302 | 5 | 3 | 0 | 0 | 0 | 0% | 183 | 172 | 6 | 2 | 3 | 0 | 3 | 2% | 190 | 179 | 9 | 1 | 1 | 0 | 1 | 1% |
| Central Neuro | 311 | 304 | 4 | 0 | 0 | 3 | 3 | 1% | 185 | 165 | 14 | 3 | 3 | 0 | 3 | 2% | 191 | 158 | 16 | 6 | 3 | 8 | 11 | 6% |
| Periph Neuro | 311 | 308 | 1 | 0 | 1 | 1 | 2 | 1% | 185 | 173 | 8 | 3 | 1 | 0 | 1 | 1% | 191 | 167 | 14 | 4 | 5 | 1 | 6 | 3% |
| Bilirubin | 309 | 301 | 4 | 2 | 2 | 0 | 2 | 1% | 185 | 169 | 1 | 10 | 4 | 1 | 5 | 3% | 192 | 159 | 5 | 21 | 6 | 1 | 7 | 4% |
| SGOT/SGPT | 311 | 218 | 68 | 19 | 6 | 0 | 6 | 2% | 185 | 68 | 43 | 43 | 30 | 1 | 31 | 17% | 192 | 68 | 40 | 40 | 43 | 1 | 44 | 23% |
| Dilated Pupils | 22 | 22 | 0 | 0 | 0 | 0 | 0 | 0% | 123 | 108 | 15 | 0 | 0 | 0 | 0 | 0% | 125 | 95 | 30 | 0 | 0 | 0 | 0 | 0% |
| Accommodation Defects | 22 | 22 | 0 | 0 | 0 | 0 | 0 | 0% | 121 | 115 | 6 | 0 | 0 | 0 | 0 | 0% | 125 | 111 | 14 | 0 | 0 | 0 | 0 | 0% |
| Capillary Leak Syndrome | 19 | 18 | 0 | 1 | 0 | 0 | 0 | 0% | 119 | 91 | 0 | 23 | 5 | 0 | 5 | 4% | 124 | 70 | 0 | 35 | 16 | 3 | 19 | 15% |
| Cytokine Release Syndrome | 19 | 18 | 1 | 0 | 0 | 0 | 0 | 0% | 118 | 95 | 8 | 10 | 5 | 0 | 5 | 4% | 123 | 85 | 12 | 17 | 9 | 0 | 9 | 7% |
| Pain related to ch14.18/CHO | 122 | 42 | 17 | 44 | 19 | 0 | 19 | 16% | 124 | 28 | 22 | 42 | 31 | 1 | 32 | 26% | ||||||||
| Papilloedema | 22 | 22 | 0 | 0 | 0 | 0 | 0 | 0% | 120 | 113 | 7 | 0 | 0 | 0 | 0 | 0% | 123 | 121 | 2 | 0 | 0 | 0 | 0 | 0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers 2020, 12, 309. https://doi.org/10.3390/cancers12020309
Ladenstein R, Pötschger U, Valteau-Couanet D, Luksch R, Castel V, Ash S, Laureys G, Brock P, Michon JM, Owens C, et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers. 2020; 12(2):309. https://doi.org/10.3390/cancers12020309
Chicago/Turabian StyleLadenstein, Ruth, Ulrike Pötschger, Dominique Valteau-Couanet, Roberto Luksch, Victoria Castel, Shifra Ash, Genevieve Laureys, Penelope Brock, Jean Marie Michon, Cormac Owens, and et al. 2020. "Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1)" Cancers 12, no. 2: 309. https://doi.org/10.3390/cancers12020309
APA StyleLadenstein, R., Pötschger, U., Valteau-Couanet, D., Luksch, R., Castel, V., Ash, S., Laureys, G., Brock, P., Michon, J. M., Owens, C., Trahair, T., Chi Fung Chan, G., Ruud, E., Schroeder, H., Beck-Popovic, M., Schreier, G., Loibner, H., Ambros, P., Holmes, K., ... Lode, H. N. (2020). Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers, 12(2), 309. https://doi.org/10.3390/cancers12020309





