Multistate Markov Model to Predict the Prognosis of High-Risk Human Papillomavirus-Related Cervical Lesions
Abstract
1. Introduction
2. Results
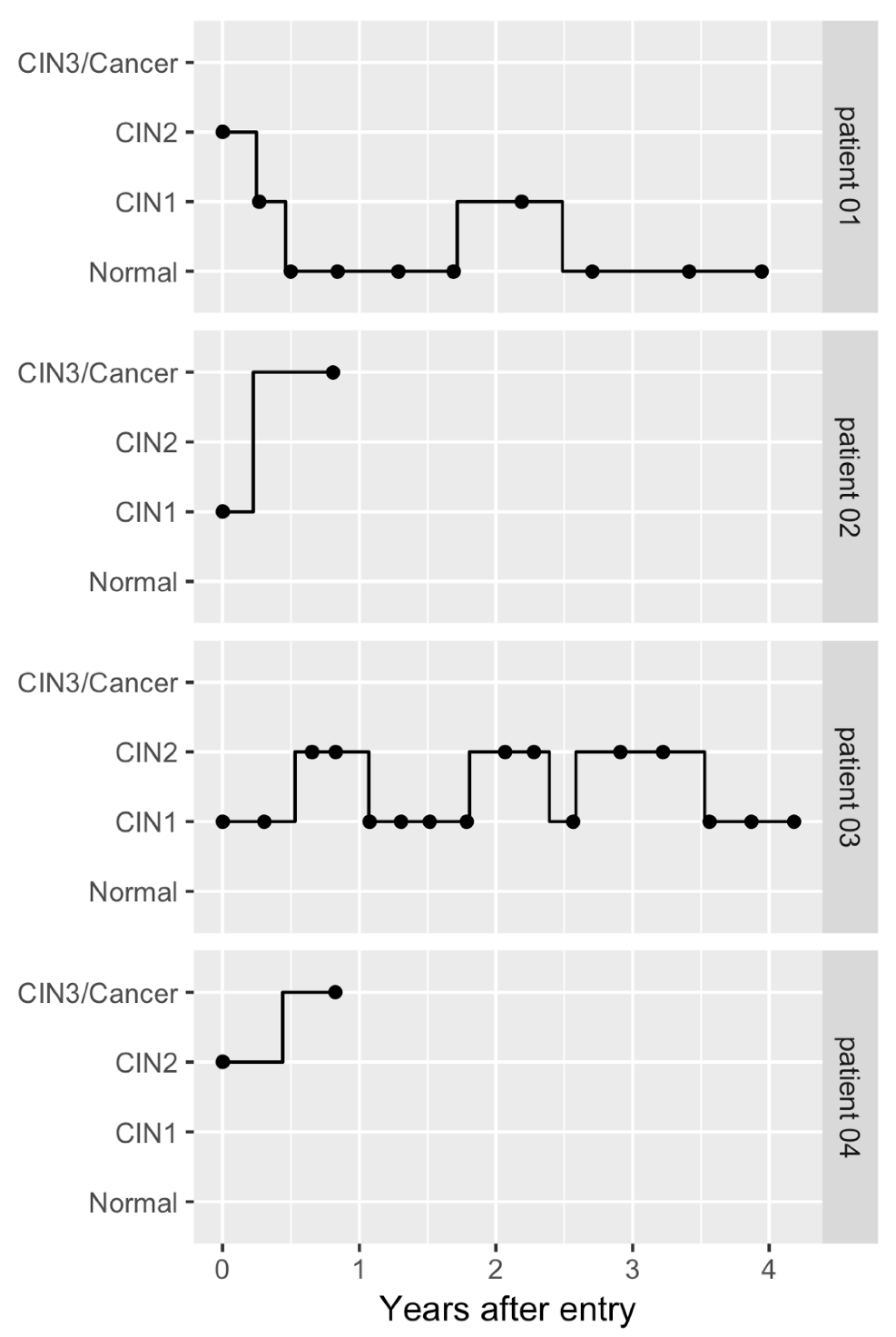
2.1. Patients
2.2. Prognosis of HPV-Infected Cervical Lesions Estimated Using the Markov Model
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Variables
4.3. DNA Extraction and HPV Genotyping
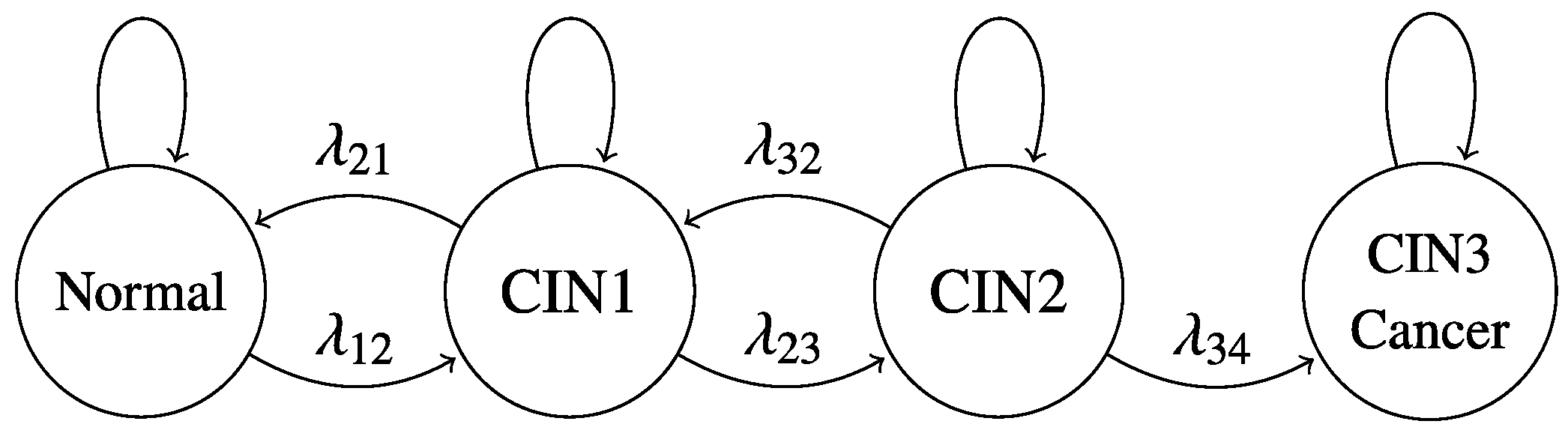
4.4. Continuous-Time Multistate Markov Model
4.5. Dataset Construction
4.6. Statistical Analysis
4.7. Sensitivity Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Ca Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- National Cancer Center. National Estimates of Cancer Incidence based on Cancer Registries in Japan (1975–2013). Available online: https://ganjoho.jp/en/professional/statistics/table_download.html (accessed on 1 January 2019).
- National Cancer Center. Cancer mortality from Vital Statistics in Japan (1958–2016). Available online: https://ganjoho.jp/en/professional/statistics/table_download.html (accessed on 1 January 2019).
- Lowy, D.R.; Schiller, J.T. Reducing HPV-Associated Cancer Globally. Cancer Prev. Res. 2012, 5, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Viens, L.J.; Henley, S.J.; Watson, M.; Markowitz, L.E.; Thomas, C.C.; Thompson, T.D.; Razzaghi, H.; Saraiya, M. Human Papillomavirus–Associated Cancers—United States, 2008–2012. Mmwr. Morb. Mortal. Wkly. Rep. 2016, 65, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.; Park, J. The Role of HPV E6 and E7 Oncoproteins in HPV-associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319. [Google Scholar] [CrossRef] [PubMed]
- Bzhalava, D.; Guan, P.; Franceschi, S.; Dillner, J.; Clifford, G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 2013, 445, 224–231. [Google Scholar] [CrossRef]
- Jeon, S.; Allen-Hoffmann, B.L.; Lambert, P.F. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J. Virol. 1995, 69, 2989–2997. [Google Scholar] [CrossRef]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. Plos Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef]
- Wright, T.C.; Massad, L.S.; Dunton, C.J.; Spitzer, M.; Wilkinson, E.J.; Solomon, D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am. J. Obs. Gynecol. 2007, 197, 340–345. [Google Scholar] [CrossRef]
- Wright, T.C.; Massad, L.S.; Dunton, C.J.; Spitzer, M.; Wilkinson, E.J.; Solomon, D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am. J. Obs. Gynecol. 2007, 197, 346–355. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oki, A.; Furuta, R.; Maeda, H.; Yasugi, T.; Takatsuka, N.; Mitsuhashi, A.; Fujii, T.; Hirai, Y.; Iwasaka, T.; et al. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: A prospective cohort study. Int. J. Cancer 2011, 128, 2898–2910. [Google Scholar] [CrossRef]
- Japan Society of Obstetrics and Gynecology; Japan Association of Obstetricians and Gynecologists (Eds.) Guideline for Gynecological Practice 2017; Japan Society of Obstetrics and Gynecology: Tokyo, Japan, 2017; pp. 53–57. (in Japanese) [Google Scholar]
- Kyrgiou, M.; Koliopoulos, G.; Martin-Hirsch, P.; Arbyn, M.; Prendiville, W.; Paraskevaidis, E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: Systematic review and meta-analysis. Lancet 2006, 367, 489–498. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Athanasiou, A.; Paraskevaidi, M.; Mitra, A.; Kalliala, I.; Martin-Hirsch, P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: Systematic review and meta-analysis. BMJ 2016, 71, i3633. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Matsumoto, K.; Oki, A.; Satoh, T.; Tsunoda, H.; Yasugi, T.; Taketani, Y.; Yoshikawa, H. Do we need a different strategy for HPV screening and vaccination in East Asia? Int. J. Cancer 2006, 119, 2713–3715. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, S.N.; Olawande, O.; Famooto, A.; Dareng, E.O.; Offiong, R.; Adebamowo, C.A. Persistent Low-Risk and High-Risk Human Papillomavirus Infections of the Uterine Cervix in HIV-Negative and HIV-Positive Women. Front. Public Heal. 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Wright, T.C.; Stoler, M.H.; Behrens, C.M.; Sharma, A.; Zhang, G.; Wright, T.L. Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test. Gynecol. Oncol. 2015, 136, 189–197. [Google Scholar] [CrossRef]
- Naucler, P.; Ryd, W.; Törnberg, S.; Strand, A.; Wadell, G.; Hansson, B.G.; Rylander, E.; Dillner, J. HPV type-specific risks of high-grade CIN during 4 years of follow-up: A population-based prospective study. Br. J. Cancer 2007, 97, 129–132. [Google Scholar] [CrossRef]
- Rositch, A.F.; Koshiol, J.; Hudgens, M.G.; Razzaghi, H.; Backes, D.M.; Pimenta, J.M.; Franco, E.L.; Poole, C.; Smith, J.S. Patterns of persistent genital human papillomavirus infection among women worldwide: A literature review and meta-analysis. Int. J. Cancer 2013, 133, 1271–1285. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Schiffman, M.; Herrero, R.; Wacholder, S.; Hildesheim, A.; Castle, P.E.; Solomon, D.; Burk, R. Rapid Clearance of Human Papillomavirus and Implications for Clinical Focus on Persistent Infections. Jnci. J. Natl. Cancer Inst. 2008, 100, 513–517. [Google Scholar] [CrossRef]
- Schiffman, M.; Herrero, R.; DeSalle, R.; Hildesheim, A.; Wacholder, S.; Cecilia Rodriguez, A.; Bratti, M.C.; Sherman, M.E.; Morales, J.; Guillen, D.; et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 2005, 337, 76–84. [Google Scholar] [CrossRef]
- Cox, D.R.; Miller, H.D. The Theory of Stochastic Processes, 1st ed.; Routledge: London, NY, USA; Chapman & Hall/CRC: London, UK, 1965. [Google Scholar]
- Kalbfleisch, J.D.; Lawless, J.F. The Analysis of Panel Data under a Markov Assumption. J. Am. Stat. Assoc. 1985, 80, 863–871. [Google Scholar] [CrossRef]
- Kay, R. A Markov Model for Analysing Cancer Markers and Disease States in Survival Studies. Biometrics 1986, 42, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Leurgans, S.E.; Yu, L.; Wilson, R.S.; Lim, A.S.; James, B.D.; Shulman, J.M.; Bennett, D.A. Incident parkinsonism in older adults without Parkinson disease. Neurology 2016, 87, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Buter, T.C.; van den Hout, A.; Matthews, F.E.; Larsen, J.P.; Brayne, C.; Aarsland, D. Dementia and survival in Parkinson disease: A 12-year population study. Neurology 2008, 70, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Dancourt, V.; Quantin, C.; Abrahamowicz, M.; Binquet, C.; Alioum, A.; Faivre, J. Modeling recurrence in colorectal cancer. J. Clin. Epidemiol. 2004, 57, 243–251. [Google Scholar] [CrossRef]
- Jack, C.R.; Therneau, T.M.; Wiste, H.J.; Weigand, S.D.; Knopman, D.S.; Lowe, V.J.; Mielke, M.M.; Vemuri, P.; Roberts, R.O.; Machulda, M.M.; et al. Transition rates between amyloid and neurodegeneration biomarker states and to dementia: A population-based, longitudinal cohort study. Lancet Neurol. 2016, 15, 56–64. [Google Scholar] [CrossRef]
- Pan, S.-L.; Lien, I.-N.; Yen, M.-F.; Lee, T.-K.; Chen, T.H.-H. Dynamic Aspect of Functional Recovery after Stroke Using a Multistate Model. Arch. Phys. Med. Rehabil. 2008, 89, 1054–1060. [Google Scholar] [CrossRef]
- Price, M.J.; Ades, A.E.; De Angelis, D.; Welton, N.J.; Macleod, J.; Soldan, K.; Simms, I.; Turner, K.; Horner, P.J. Risk of Pelvic Inflammatory Disease Following Chlamydia trachomatis Infection: Analysis of Prospective Studies With a Multistate Model. Am. J. Epidemiol. 2013, 178, 484–492. [Google Scholar] [CrossRef]
- Sharples, L.D.; Jackson, C.H.; Parameshwar, J.; Wallwork, J.; Large, S.R. Diagnostic accuracy of coronary angiography and risk factors for post-heart-transplant cardiac allograft vasculopathy. Transplantation 2003, 76, 679–682. [Google Scholar] [CrossRef]
- Skogvoll, E.; Eftestøl, T.; Gundersen, K.; Kvaløy, J.T.; Kramer-Johansen, J.; Olasveengen, T.M.; Steen, P.A. Dynamics and state transitions during resuscitation in out-of-hospital cardiac arrest. Resuscitation 2008, 78, 30–37. [Google Scholar] [CrossRef]
- Sweeting, M.J.; De Angelis, D.; Neal, K.R.; Ramsay, M.E.; Irving, W.L.; Wright, M.; Brant, L.; Harris, H.E. Estimated progression rates in three United Kingdom hepatitis C cohorts differed according to method of recruitment. J. Clin. Epidemiol. 2006, 59, 144–152. [Google Scholar] [CrossRef]
- Bulk, S.; Berkhof, J.; Rozendaal, L.; Fransen Daalmeijer, N.C.; Gök, M.; de Schipper, F.A.; van Kemenade, F.J.; Snijders, P.J.; Meijer, C.J. The contribution of HPV18 to cervical cancer is underestimated using high-grade CIN as a measure of screening efficiency. Br. J. Cancer 2007, 96, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, T.D.; Bontkes, H.J.; Walboomers, J.M.M.; Stukart, M.J.; Doekhie, F.S.; Remmink, A.J.; Helmerhorst, T.J.M.; Verheijen, R.H.M.; Duggan-Keen, M.F.; Stern, P.L.; et al. Differential T helper cell responses to human papillomavirus type 16 E7 related to viral clearance or persistence in patients with cervical neoplasia: A longitudinal study. Cancer Res. 1998, 58, 1700–1706. [Google Scholar] [PubMed]
- Ahdieh, L.; Munoz, A.; Vlahov, D.; Trimble, C.L.; Timpson, L.A.; Shah, K. Cervical Neoplasia and Repeated Positivity of Human Papillomavirus Infection In Human Immunodeficiency Virus-seropositive and -seronegative Women. Am. J. Epidemiol. 2000, 151, 1148–1157. [Google Scholar] [CrossRef][Green Version]
- Brennan, D.C.; Aguado, J.M.; Potena, L.; Jardine, A.G.; Legendre, C.; Säemann, M.D.; Mueller, N.J.; Merville, P.; Emery, V.; Nashan, B. Effect of maintenance immunosuppressive drugs on virus pathobiology: Evidence and potential mechanisms. Rev. Med. Virol. 2013, 23, 97–125. [Google Scholar] [CrossRef]
- De Sanjose, S.; Quint, W.G.V.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Brown, D.R.; Shew, M.L.; Qadadri, B.; Neptune, N.; Vargas, M.; Tu, W.; Juliar, B.E.; Breen, T.E.; Fortenberry, J.D. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J. Infect. Dis. 2005, 191, 182–192. [Google Scholar] [CrossRef]
- Cho, H.W.; So, K.A.; Lee, J.K.; Hong, J.H. Type-specific persistence or regression of human papillomavirus genotypes in women with cervical intraepithelial neoplasia 1: A prospective cohort study. Obstet. Gynecol. Sci. 2015, 58, 40–45. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schiffman, M.; Dunn, T.; Zuna, R.E.; Gold, M.A.; Allen, R.A.; Zhang, R.; Sherman, M.E.; Wacholder, S.; Walker, J.; et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int. J. Cancer 2009, 125, 2151–2158. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Katki, H.A.; Hildesheim, A.; Rodríguez, A.C.; Quint, W.; Schiffman, M.; Van Doorn, L.-J.; Porras, C.; Wacholder, S.; Gonzalez, P.; et al. Human Papillomavirus Infection with Multiple Types: Pattern of Coinfection and Risk of Cervical Disease. J. Infect. Dis. 2011, 203, 910–920. [Google Scholar] [CrossRef]
- Kojima, S.; Kawana, K.; Tomio, K.; Yamashita, A.; Taguchi, A.; Miura, S.; Adachi, K.; Nagamatsu, T.; Nagasaka, K.; Matsumoto, Y.; et al. The Prevalence of Cervical Regulatory T Cells in HPV-Related Cervical Intraepithelial Neoplasia (CIN) Correlates Inversely with Spontaneous Regression of CIN. Am. J. Reprod Immunol. 2013, 69, 134–141. [Google Scholar] [CrossRef]
- Jackson, C.H. Multi-State Models for Panel Data: The msm Package for R. J. Stat. Softw. 2011, 38, 1–29. [Google Scholar] [CrossRef]

| Diagnosis at the Time of Entry | HPV 16 | HPV 18 | HPV 52 | HPV 58 | Other hrHPVs | No hrHPVs | All | |
|---|---|---|---|---|---|---|---|---|
| Normal | N | 13 | 11 | 17 | 13 | 30 | 122 | 195 |
| Age at the time of entry (years), mean (SD) | 42.4 (13.8) | 39.5 (15.0) | 36.7 (10.0) | 41.3 (16.8) | 42.5 (16.9) | 41.2 (10.5) | 41.3 (12.1) | |
| Number of visits, mean (SD) | 5.1 (4.8) | 7.2 (3.4) | 7.5 (5.7) | 6.8 (4.6) | 7.0 (4.8) | 6.5 (3.9) | 6.8 (4.3) | |
| Follow-up interval (years), mean (SD) | 0.49 (0.37) | 0.46 (0.27) | 0.50 (0.35) | 0.49 (0.42) | 0.47 (0.27) | 0.53 (0.40) | 0.51 (0.37) | |
| Follow-up period (years), mean (SD) | 2.1 (2.5) | 2.9 (1.6) | 3.7 (3.1) | 2.9 (2.3) | 2.9 (2.2) | 3.1 (2.1) | 3.1 (2.2) | |
| CIN1 | N | 23 | 11 | 38 | 24 | 79 | 111 | 259 |
| Age at the time of entry (years), mean (SD) | 34.6 (8.2) | 33.0 (10.0) | 36.6 (8.6) | 36.1 (7.7) | 34.5 (7.2) | 39.0 (10.3) | 36.9 (9.2) | |
| Number of visits, mean (SD) | 7.6 (5.3) | 7.6 (4.7) | 10.7 (5.9) | 10.5 (6.4) | 9.0 (4.9) | 9.3 (5.2) | 9.3 (5.2) | |
| Follow-up interval (years), mean (SD) | 0.42 (0.43) | 0.51 (0.53) | 0.38 (0.18) | 0.45 (0.42) | 0.39 (0.22) | 0.42 (0.31) | 0.41 (0.29) | |
| Follow-up period (years), mean (SD) | 3.2 (2.6) | 3.2 (2.2) | 4.0 (2.4) | 3.9 (2.6) | 3.4 (2.2) | 3.9 (2.3) | 3.7 (2.3) | |
| CIN2 | N | 67 | 15 | 65 | 57 | 67 | 54 | 283 |
| Age at the time of entry (years), mean (SD) | 37.6 (7.9) | 42.0 (5.7) | 41.2 (8.2) | 39.7 (8.5) | 37.9 (8.5) | 36.6 (9.2) | 39.1 (8.4) | |
| Number of visits, mean (SD) | 6.8 (5.4) | 7.0 (4.4) | 7.2 (5.4) | 8.9 (5.8) | 8.6 (5.6) | 8.6 (5.8) | 8.0 (5.5) | |
| Follow-up interval (years), mean (SD) | 0.35 (0.15) | 0.34 (0.14) | 0.38 (0.24) | 0.38 (0.24) | 0.39 (0.33) | 0.38 (0.33) | 0.37 (0.26) | |
| Follow-up period (years), mean (SD) | 2.2 (2.2) | 2.1 (1.6) | 2.5 (2.2) | 3.5 (2.6) | 3.2 (2.3) | 3.3 (2.5) | 2.9 (2.3) |
| Diagnosis at tth Visit | ||||||
|---|---|---|---|---|---|---|
| Diagnosis at (t-1)th Visit | HPV Category | Normal | CIN1 | CIN2 | CIN3 | Cancer |
| Normal | HPV 16 | 206 (84.4) | 13 (5.3) | 21 (8.6) | 4 (1.6) | 0 (0.0) |
| HPV 18 | 89 (81.6) | 12 (11.0) | 8 (7.3) | 0 (0.0) | 0 (0.0) | |
| HPV 52 | 277 (75.8) | 54 (14.7) | 32 (8.7) | 2 (0.5) | 0 (0.0) | |
| HPV 58 | 230 (80.4) | 33 (11.5) | 21 (7.3) | 2 (0.6) | 0 (0.0) | |
| Other hrHPVs | 611 (86.1) | 72 (10.1) | 23 (3.2) | 3 (0.4) | 0 (0.0) | |
| No hrHPVs | 1289 (90.2) | 109 (7.6) | 26 (1.8) | 3 (0.2) | 1 (0.0) | |
| CIN1 | HPV 16 | 29 (28.9) | 34 (34.0) | 35 (35.0) | 2 (2.0) | 0 (0.0) |
| HPV 18 | 18 (38.2) | 19 (40.4) | 8 (17.0) | 2 (4.2) | 0 (0.0) | |
| HPV 52 | 80 (35.0) | 90 (39.4) | 53 (23.2) | 5 (2.1) | 0 (0.0) | |
| HPV 58 | 51 (31.6) | 68 (42.2) | 40 (24.8) | 2 (1.2) | 0 (0.0) | |
| Other hrHPVs | 132 (40.7) | 143 (44.1) | 45 (13.8) | 4 (1.2) | 0 (0.0) | |
| No hrHPVs | 203 (54.5) | 132 (35.4) | 34 (9.1) | 3 (0.8) | 0 (0.0) | |
| CIN2 | HPV 16 | 31 (12.1) | 37 (14.4) | 147 (57.4) | 40 (15.6) | 1 (0.3) |
| HPV 18 | 10 (12.9) | 8 (10.3) | 51 (66.2) | 8 (10.3) | 0 (0.0) | |
| HPV 52 | 41 (13.8) | 53 (17.9) | 168 (56.9) | 33 (11.1) | 0 (0.0) | |
| HPV 58 | 32 (10.2) | 45 (14.4) | 210 (67.5) | 24 (7.7) | 0 (0.0) | |
| Other hrHPVs | 49 (16.7) | 52 (17.8) | 166 (56.8) | 25 (8.5) | 0 (0.0) | |
| No hrHPVs | 58 (27.2) | 31 (14.5) | 114 (53.5) | 10 (4.6) | 0 (0.0) | |
| Current State | State after 2 Years | ||||
|---|---|---|---|---|---|
| HPV Category | Normal | CIN1 | CIN2 | CIN3/Cancer | |
| Normal | HPV 16 | 0.598 (0.506–0.684) | 0.099 (0.074–0.128) | 0.169 (0.127–0.215) | 0.132 (0.090–0.183) |
| HPV 18 | 0.610 (0.479–0.719) | 0.156 (0.109–0.215) | 0.156 (0.093–0.230) | 0.076 (0.033–0.148) | |
| HPV 52 | 0.533 (0.474–0.593) | 0.189 (0.162–0.219) | 0.180 (0.146–0.216) | 0.096 (0.070–0.130) | |
| HPV 58 | 0.559 (0.484–0.627) | 0.171 (0.140–0.205) | 0.206 (0.162–0.255) | 0.062 (0.041–0.089) | |
| Other hrHPVs | 0.723 (0.676–0.766) | 0.155 (0.132–0.182) | 0.085 (0.066–0.108) | 0.034 (0.023–0.050) | |
| No hrHPVs | 0.838 (0.814–0.861) | 0.105 (0.090–0.121) | 0.042 (0.032–0.054) | 0.012 (0.007–0.020) | |
| CIN1 | HPV 16 | 0.434 (0.349–0.512) | 0.089 (0.067–0.115) | 0.175 (0.134–0.223) | 0.300 (0.225–0.378) |
| HPV 18 | 0.535 (0.396–0.652) | 0.146 (0.100–0.207) | 0.172 (0.102–0.257) | 0.146 (0.069–0.267) | |
| HPV 52 | 0.473 (0.413–0.529) | 0.178 (0.152–0.208) | 0.183 (0.150–0.221) | 0.164 (0.122–0.219) | |
| HPV 58 | 0.469 (0.399–0.535) | 0.165 (0.135–0.197) | 0.239 (0.192–0.291) | 0.126 (0.084–0.181) | |
| Other hrHPVs | 0.656 (0.606–0.702) | 0.156 (0.133–0.181) | 0.102 (0.079–0.128) | 0.084 (0.058–0.119) | |
| No hrHPVs | 0.808 (0.781–0.835) | 0.107 (0.091–0.123) | 0.049 (0.038–0.065) | 0.034 (0.021–0.054) | |
| CIN2 | HPV 16 | 0.335 (0.266–0.404) | 0.079 (0.059–0.101) | 0.165 (0.121–0.218) | 0.418 (0.330–0.512) |
| HPV 18 | 0.373 (0.245–0.501) | 0.119 (0.074–0.178) | 0.186 (0.099–0.302) | 0.320 (0.178–0.507) | |
| HPV 52 | 0.381 (0.324–0.434) | 0.156 (0.129–0.184) | 0.175 (0.138–0.216) | 0.286 (0.220–0.367) | |
| HPV 58 | 0.356 (0.291–0.419) | 0.150 (0.122–0.181) | 0.260 (0.209–0.319) | 0.232 (0.167–0.307) | |
| Other hrHPVs | 0.518 (0.453–0.571) | 0.146 (0.122–0.169) | 0.117 (0.089–0.148) | 0.218 (0.159–0.299) | |
| No hrHPVs | 0.706 (0.643–0.749) | 0.106 (0.090–0.123) | 0.063 (0.045–0.089) | 0.124 (0.079–0.191) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taguchi, A.; Hara, K.; Tomio, J.; Kawana, K.; Tanaka, T.; Baba, S.; Kawata, A.; Eguchi, S.; Tsuruga, T.; Mori, M.; et al. Multistate Markov Model to Predict the Prognosis of High-Risk Human Papillomavirus-Related Cervical Lesions. Cancers 2020, 12, 270. https://doi.org/10.3390/cancers12020270
Taguchi A, Hara K, Tomio J, Kawana K, Tanaka T, Baba S, Kawata A, Eguchi S, Tsuruga T, Mori M, et al. Multistate Markov Model to Predict the Prognosis of High-Risk Human Papillomavirus-Related Cervical Lesions. Cancers. 2020; 12(2):270. https://doi.org/10.3390/cancers12020270
Chicago/Turabian StyleTaguchi, Ayumi, Konan Hara, Jun Tomio, Kei Kawana, Tomoki Tanaka, Satoshi Baba, Akira Kawata, Satoko Eguchi, Tetsushi Tsuruga, Mayuyo Mori, and et al. 2020. "Multistate Markov Model to Predict the Prognosis of High-Risk Human Papillomavirus-Related Cervical Lesions" Cancers 12, no. 2: 270. https://doi.org/10.3390/cancers12020270
APA StyleTaguchi, A., Hara, K., Tomio, J., Kawana, K., Tanaka, T., Baba, S., Kawata, A., Eguchi, S., Tsuruga, T., Mori, M., Adachi, K., Nagamatsu, T., Oda, K., Yasugi, T., Osuga, Y., & Fujii, T. (2020). Multistate Markov Model to Predict the Prognosis of High-Risk Human Papillomavirus-Related Cervical Lesions. Cancers, 12(2), 270. https://doi.org/10.3390/cancers12020270






