Trans-Mucosal Efficacy of Non-Thermal Plasma Treatment on Cervical Cancer Tissue and Human Cervix Uteri by a Next Generation Electrosurgical Argon Plasma Device
Abstract
1. Introduction
2. Results
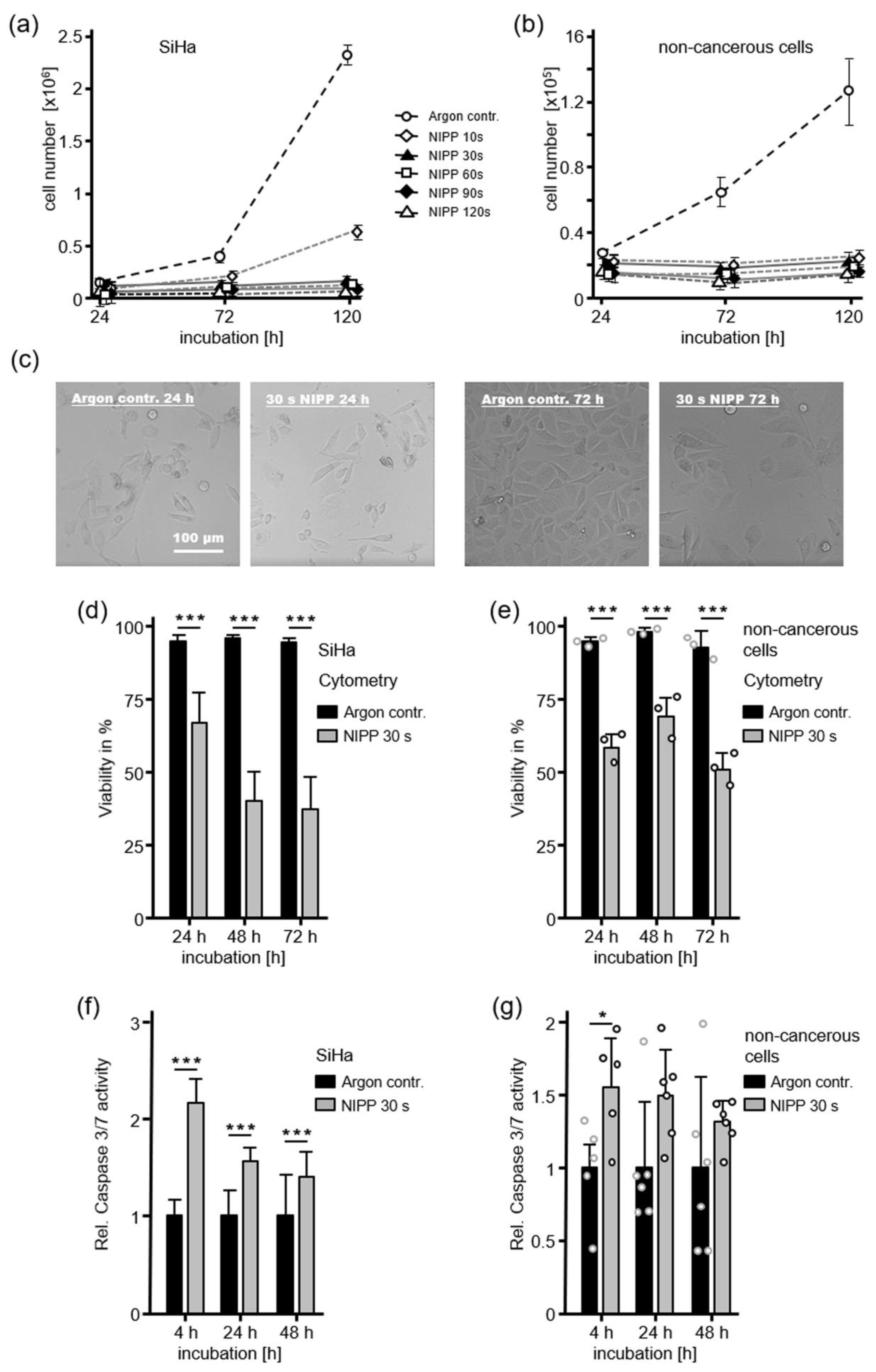
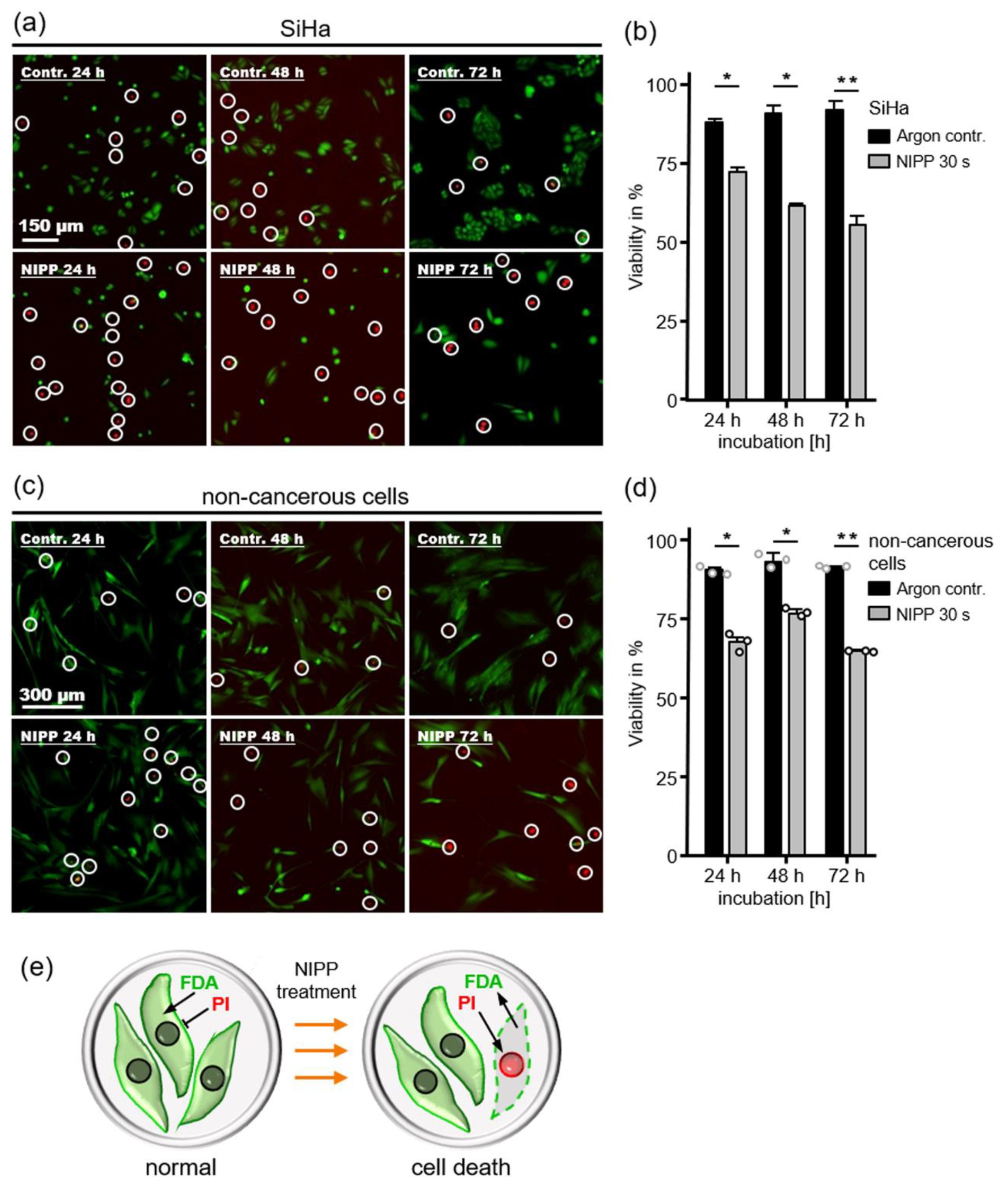
2.1. Antiproliferative Cell Response after NIPP Treatment
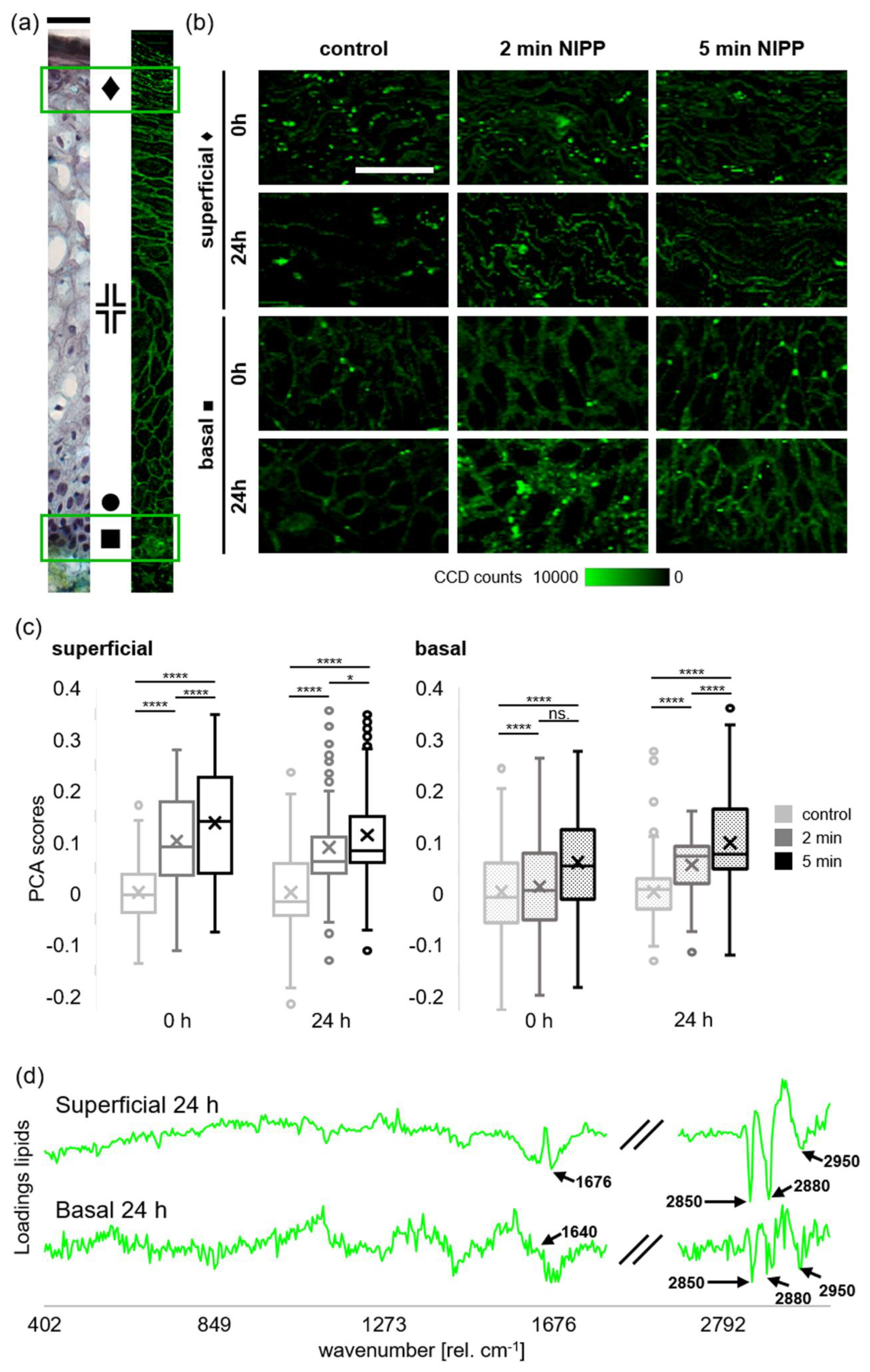
2.2. NIPP Induced Changes of Lipids in Human Cervical Mucosa Identified by Raman Imaging
3. Discussion
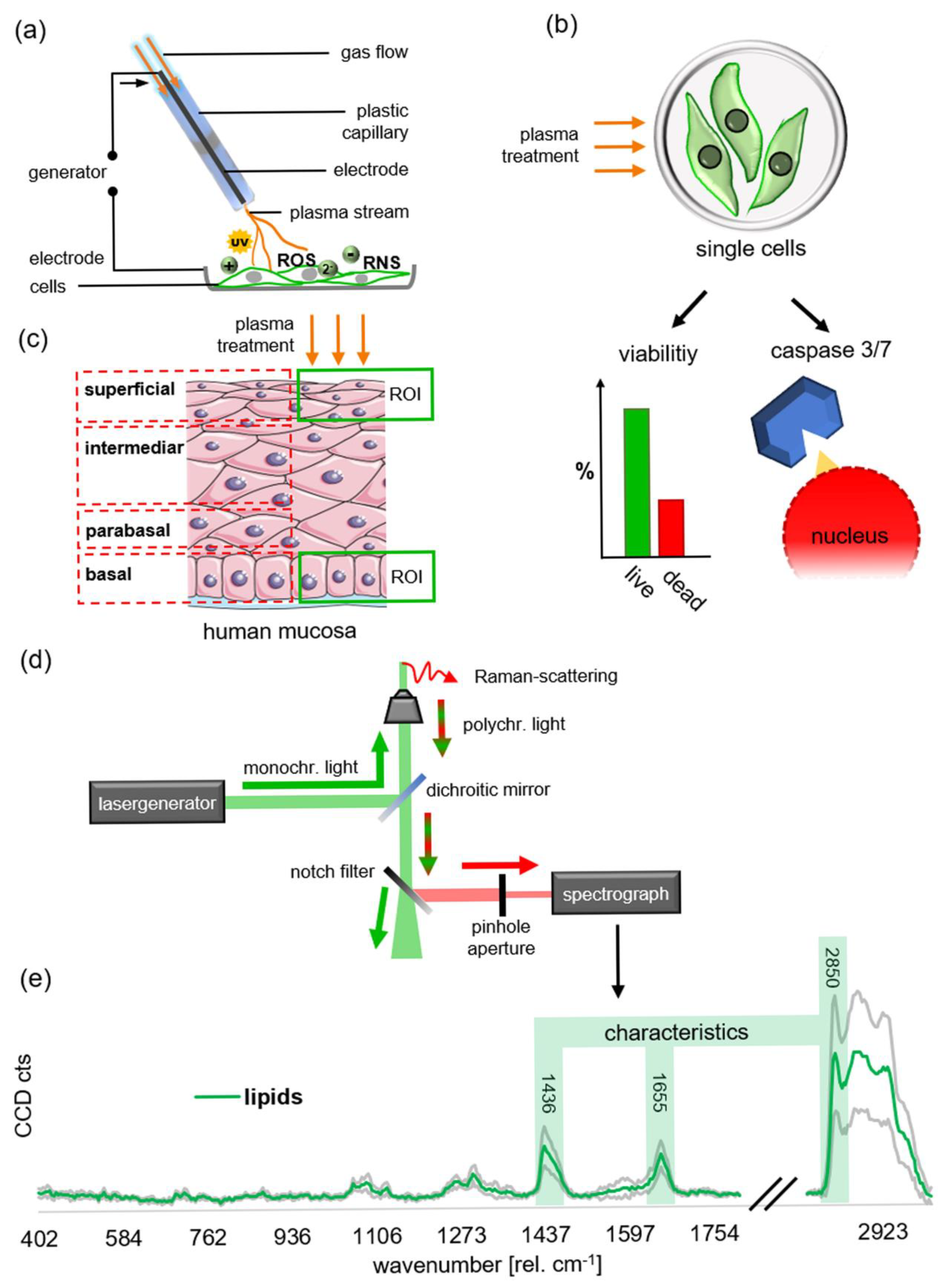
4. Materials and Methods
4.1. Cell Culture
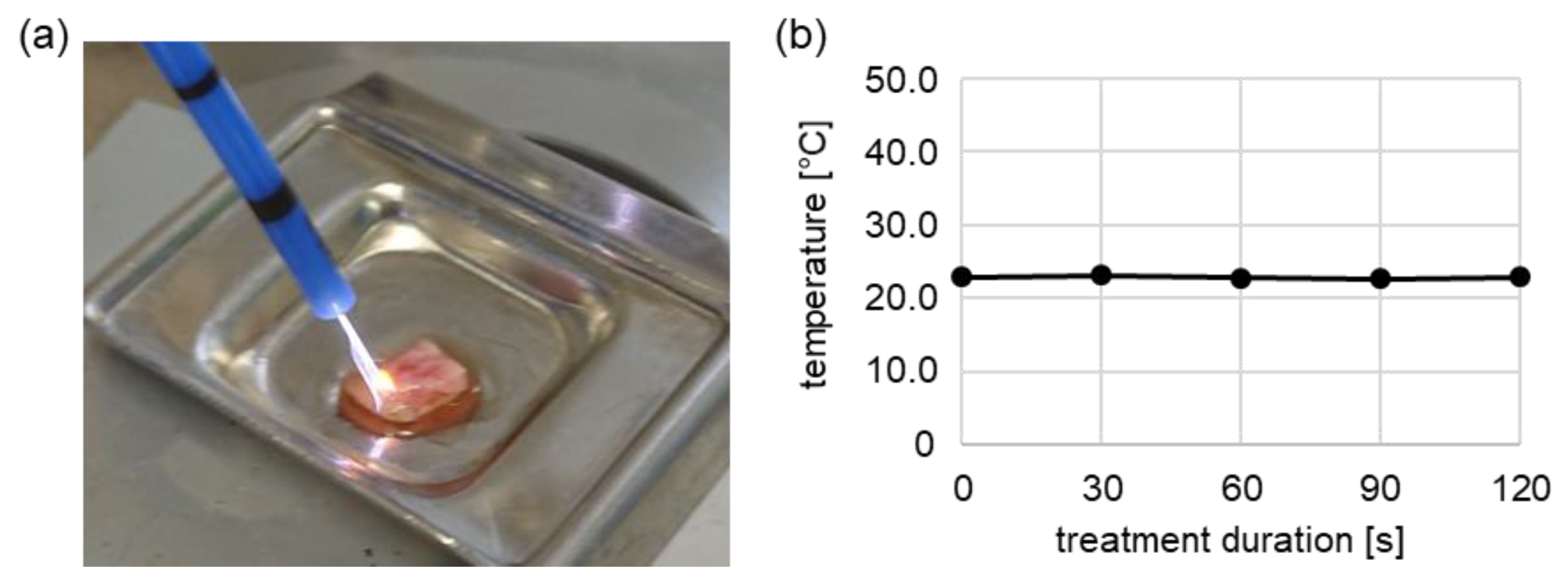
4.2. Plasma Treatment
4.3. Live/Dead: PI/FDA Staining
4.4. Live/Dead: Guava ViaCount Assay
4.5. Apoptosis: Caspase-Glo 3/7 Assay
4.6. Proliferation Assay
4.7. Human Tissue Samples
4.8. Raman Imaging
4.9. Raman Image Analysis
4.10. Principal Component Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arndt, S.; Wacker, E.; Li, Y.-F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.-K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Köritzer, J.; Boxhammer, V.; Schafer, A.; Shimizu, T.; Klämpfl, T.G.; Li, Y.-F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of Sensitivity in Chemo—Resistant Glioma Cells by Cold Atmospheric Plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef] [PubMed]
- Koensgen, D.; Besic, I.; Gümbel, D.; Kaul, A.; Weiss, M.; Diesing, K.; Kramer, A.; Bekeschus, S.; Mustea, A.; Stope, M.B. Cold Atmospheric Plasma (CAP) and CAP-Stimulated Cell Culture Media Suppress Ovarian Cancer Cell Growth – A Putative Treatment Option in Ovarian Cancer Therapy. Anticancer Res. 2017, 37, 6739–6744. [Google Scholar] [PubMed]
- Weiss, M.; Gümbel, D.; Gelbrich, N.; Brandenburg, L.-O.; Mandelkow, R.; Zimmermann, U.; Ziegler, P.; Burchardt, M.; Stope, M.B. Inhibition of Cell Growth of the Prostate Cancer Cell Model LNCaP by Cold Atmospheric Plasma. In Vivo 2015, 29, 611–616. [Google Scholar]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doering, F.; Normann, L.; Diedrich, S.; Weiss, F.-U.; Evert, M.; Huebner, N.O.; Guenther, C.; et al. Tissue Tolerable Plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef]
- Weiss, M.; Gümbel, D.; Hanschmann, E.-M.; Mandelkow, R.; Gelbrich, N.; Zimmermann, U.; Walther, R.; Ekkernkamp, A.; Sckell, A.; Kramer, A.; et al. Cold Atmospheric Plasma Treatment Induces Anti-Proliferative Effects in Prostate Cancer Cells by Redox and Apoptotic Signaling Pathways. PLoS ONE 2015, 10, e0130350. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.; Laroussi, M.; Reuter, S.; Graves, D.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Ann Stepp, M.; Srinivasan, P.; Sandler, A.; Trink, B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Weiss, M.; Utz, R.; Ackermann, M.; Taran, F.-A.; Krämer, B.; Hahn, M.; Wallwiener, D.; Brucker, S.; Haupt, M.; Barz, J.; et al. Characterization of a non-thermally operated electrosurgical argon plasma source by electron spin resonance spectroscopy. Plasma Process. Polym. 2019, 16, 1800150. [Google Scholar] [CrossRef]
- Farin, G.; Grund, K.E. Technology of argon plasma coagulation with particular regard to endoscopic applications. Endosc. Surg. Allied Technol. 1994, 2, 71–77. [Google Scholar] [PubMed]
- Kähler, G.F.; Szyrach, M.N.; Hieronymus, A.; Grobholz, R.; Enderle, M.D. Investigation of the thermal tissue effects of the argon plasma coagulation modes “pulsed” and “precise” on the porcine esophagus, ex vivo and in vivo. Gastrointest. Endosc. 2009, 70, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Erbe Elektromedizin GmbH APC 3: Power your VIO®3. Available online: https://de.erbe-med.com/index.php?eID=dumpFile&t=f&f=5769&token=9d3ed1a2c30728a45a2d6e1d72346a68bd5ea99e (accessed on 23 November 2019).
- Raizer, Y.P. Physics of Gas Discharge; Allen, J.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; ISBN 978-3-642-64760-4. [Google Scholar]
- Fridman, A.; Chirokov, A.; Gutsol, A. Non-thermal atmospheric pressure discharges. J. Phys. D Appl. Phys. 2005, 38, R1–R24. [Google Scholar] [CrossRef]
- Zenker, M. Argon plasma coagulation. GMS Krankenhhyg. Interdiszip. 2008, 3, Doc15. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- De Rosa, N.; Lavitola, G.; Giampaolino, P.; Morra, I.; Nappi, C.; Bifulco, G. Impact of Ospemifene on Quality of Life and Sexual Function in Young Survivors of Cervical Cancer: A Prospective Study. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Hillemanns, P.; Friese, K.; Dannecker, C.; Klug, S.; Seifert, U.; Iftner, T.; Hädicke, J.; Löning, T.; Horn, L.; Schmidt, D.; et al. Prevention of Cervical Cancer. Geburtshilfe Frauenheilkd. 2019, 79, 148–159. [Google Scholar]
- Cubal, A.F.R.; Carvalho, J.I.F.; Costa, M.F.M.; Branco, A.P.T. Fertility-Sparing Surgery for Early-Stage Cervical Cancer. Int. J. Surg. Oncol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Brucker, S.Y.; Taran, F.-A.; Bogdanyova, S.; Ebersoll, S.; Wallwiener, C.W.; Schönfisch, B.; Krämer, B.; Abele, H.; Neis, F.; Sohn, C.; et al. Patient-reported quality-of-life and sexual-function outcomes after laparoscopic supracervical hysterectomy (LSH) versus total laparoscopic hysterectomy (TLH): A prospective, questionnaire-based follow-up study in 915 patients. Arch. Gynecol. Obstet. 2014, 290, 1141–1149. [Google Scholar] [CrossRef]
- Sadler, L.; Saftlas, A.; Wang, W.; Exeter, M.; Whittaker, J.; McCowan, L. Treatment for Cervical Intraepithelial Neoplasia and Risk of Preterm Delivery. JAMA 2004, 291, 2100. [Google Scholar] [CrossRef] [PubMed]
- Henk, H.J.; Insinga, R.P.; Singhal, P.K.; Darkow, T. Incidence and Costs of Cervical Intraepithelial Neoplasia in a US Commercially Insured Population. J. Low. Genit. Tract Dis. 2010, 14, 29–36. [Google Scholar] [CrossRef]
- Gümbel, D.; Suchy, B.; Wien, L.; Gelbrich, N.; Napp, M.; Kramer, A.; Ekkernkamp, A.; Daeschlein, G.; Stope, M.B. Comparison of Cold Atmospheric Plasma Devices’ Efficacy on Osteosarcoma and Fibroblastic In Vitro Cell Models. Anticancer Res. 2017, 37, 5407–5414. [Google Scholar] [PubMed]
- Wenzel, T.; Berrio, D.A.C.; Daum, R.; Reisenauer, C.; Weltmann, K.-D.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.-M.; Weiss, M. Molecular Effects and Tissue Penetration Depth of Physical Plasma in Human Mucosa Analyzed by Contact- and Marker-Independent Raman Microspectroscopy. ACS Appl. Mater. Interfaces 2019, 11, 42885–42895. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K. Conformation-dependent features in the raman spectra of simple lipids. Chem. Phys. Lipids 1973, 10, 165–176. [Google Scholar] [CrossRef]
- Falamas, A.; Kalra, S.; Chis, V.; Notingher, I. Monitoring the RNA distribution in human embryonic stem cells using Raman micro-spectroscopy and fluorescence imaging. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2013; Volume 1565, pp. 43–47. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Devpura, S.; Thakur, J.S.; Sethi, S.; Naik, V.M.; Naik, R. Diagnosis of head and neck squamous cell carcinoma using Raman spectroscopy: Tongue tissues. J. Raman Spectrosc. 2012, 43, 490–496. [Google Scholar] [CrossRef]
- Jangir, D.K.; Dey, S.K.; Kundu, S.; Mehrotra, R. Assessment of amsacrine binding with DNA using UV–visible, circular dichroism and Raman spectroscopic techniques. J. Photochem. Photobiol. B Boil. 2012, 114, 38–43. [Google Scholar] [CrossRef]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef]
- Weiss, M.; Stope, M.B. Physical plasma: A new treatment option in gynecological oncology. Arch. Gynecol. Obstet. 2018, 298, 853–855. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Barz, J.; Ackermann, M.; Utz, R.; Ghoul, A.; Weltmann, K.-D.; Stope, M.B.; Wallwiener, D.; Schenke-Layland, K.; Oehr, C.; et al. Dose-Dependent Tissue-Level Characterization of a Medical Atmospheric Pressure Argon Plasma Jet. ACS Appl. Mater. Interfaces 2019, 11, 19841–19853. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, K.R.; Diedrich, S.; Pati, O.; Freund, E.; Flieger, R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Cold Physical Plasma Selectively Elicits Apoptosis in Murine Pancreatic Cancer Cells In Vitro and In Ovo. Anticancer. Res. 2018, 38, 5655–5663. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal Dielectric-Barrier Discharge Plasma-Induced Inactivation Involves Oxidative DNA Damage and Membrane Lipid Peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, M.; Van Der Paal, J.; Neyts, E.; Bogaerts, A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 839–847. [Google Scholar] [CrossRef]
- Brauchle, E.; Thude, S.; Brucker, S.Y.; Schenke-Layland, K. Cell death stages in single apoptotic and necrotic cells monitored by Raman microspectroscopy. Sci. Rep. 2015, 4, 4698. [Google Scholar] [CrossRef]
- Brauchle, E.; Kasper, J.; Daum, R.; Schierbaum, N.; Falch, C.; Kirschniak, A.; Schäffer, T.E.; Schenke-Layland, K. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Boil. 2018, 68–69, 180–193. [Google Scholar] [CrossRef]
- Marzi, J.; Brauchle, E.M.; Schenke-Layland, K.; Rolle, M.W. Non-invasive functional molecular phenotyping of human smooth muscle cells utilized in cardiovascular tissue engineering. Acta Biomater. 2019, 89, 193–205. [Google Scholar] [CrossRef]
- Brauchle, E.; Schenke-Layland, K. Raman spectroscopy in biomedicine - non-invasive in vitro analysis of cells and extracellular matrix components in tissues. Biotechnol. J. 2013, 8, 288–297. [Google Scholar] [CrossRef]
- Kartaschew, K.; Mischo, M.; Baldus, S.; Bruendermann, E.; Awakowicz, P.; Havenith, M. Unraveling the interactions between cold atmospheric plasma and skin-components with vibrational microspectroscopy. Biointerphases 2015, 10, 29516. [Google Scholar] [CrossRef]
- Wang, S.; Doona, C.J.; Setlow, P.; Li, Y.-Q. Use of Raman Spectroscopy and Phase-Contrast Microscopy To Characterize Cold Atmospheric Plasma Inactivation of Individual Bacterial Spores. Appl. Environ. Microbiol. 2016, 82, 5775–5784. [Google Scholar] [CrossRef]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.; Klämpfl, T.; Steffes, B.; Thomas, H.; et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef]
| Peaks (rel. cm−1) | Found in | Assignment | Reference |
|---|---|---|---|
| 1169 | NIPP | C=C stretch lipids | [29,30] |
| 1306 | NIPP | CH3/CH2 twisting or bending mode of lipid/collagen; Lipid/protein | [29,30] |
| 1368 | NIPP | νs (CH3) (phospholipids) | [29,31] |
| 2850 | control | νs CH2, lipids, fatty acids, CH2 symmetric | [29] |
| 2880 | control | CH2 asymmetric stretch of lipids | [29] |
| 2910–2920 | NIPP | C-H vibrations in lipids νas CH2, lipids, fatty acids; saturated and unsaturated fatty acids | [29,32] |
| 2950 | control | CH3 asymmetric stretch | [29] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenzel, T.; Carvajal Berrio, D.A.; Reisenauer, C.; Layland, S.; Koch, A.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.-M.; Weiss, M. Trans-Mucosal Efficacy of Non-Thermal Plasma Treatment on Cervical Cancer Tissue and Human Cervix Uteri by a Next Generation Electrosurgical Argon Plasma Device. Cancers 2020, 12, 267. https://doi.org/10.3390/cancers12020267
Wenzel T, Carvajal Berrio DA, Reisenauer C, Layland S, Koch A, Wallwiener D, Brucker SY, Schenke-Layland K, Brauchle E-M, Weiss M. Trans-Mucosal Efficacy of Non-Thermal Plasma Treatment on Cervical Cancer Tissue and Human Cervix Uteri by a Next Generation Electrosurgical Argon Plasma Device. Cancers. 2020; 12(2):267. https://doi.org/10.3390/cancers12020267
Chicago/Turabian StyleWenzel, Thomas, Daniel A. Carvajal Berrio, Christl Reisenauer, Shannon Layland, André Koch, Diethelm Wallwiener, Sara Y. Brucker, Katja Schenke-Layland, Eva-Maria Brauchle, and Martin Weiss. 2020. "Trans-Mucosal Efficacy of Non-Thermal Plasma Treatment on Cervical Cancer Tissue and Human Cervix Uteri by a Next Generation Electrosurgical Argon Plasma Device" Cancers 12, no. 2: 267. https://doi.org/10.3390/cancers12020267
APA StyleWenzel, T., Carvajal Berrio, D. A., Reisenauer, C., Layland, S., Koch, A., Wallwiener, D., Brucker, S. Y., Schenke-Layland, K., Brauchle, E.-M., & Weiss, M. (2020). Trans-Mucosal Efficacy of Non-Thermal Plasma Treatment on Cervical Cancer Tissue and Human Cervix Uteri by a Next Generation Electrosurgical Argon Plasma Device. Cancers, 12(2), 267. https://doi.org/10.3390/cancers12020267






