Etiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Current Directions in Research
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
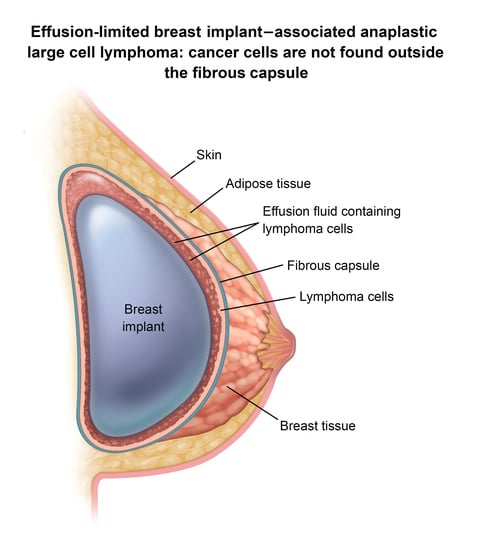
3. BIA-ALCL Immunological Characteristics
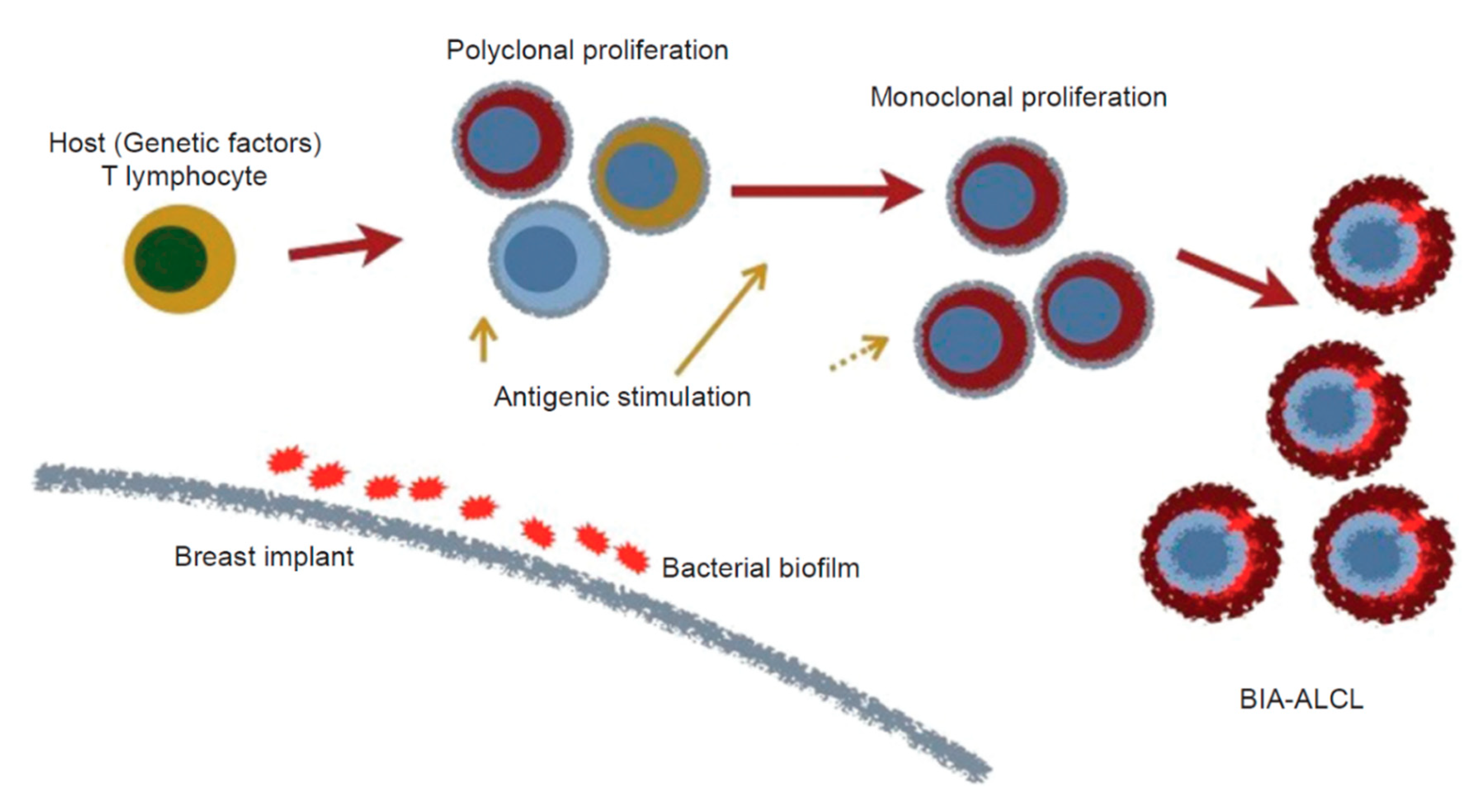
4. Etiology of BIA-ALCL
4.1. Mechanical Friction
4.2. Silicone Implant Shell Particulates
4.3. Leachables
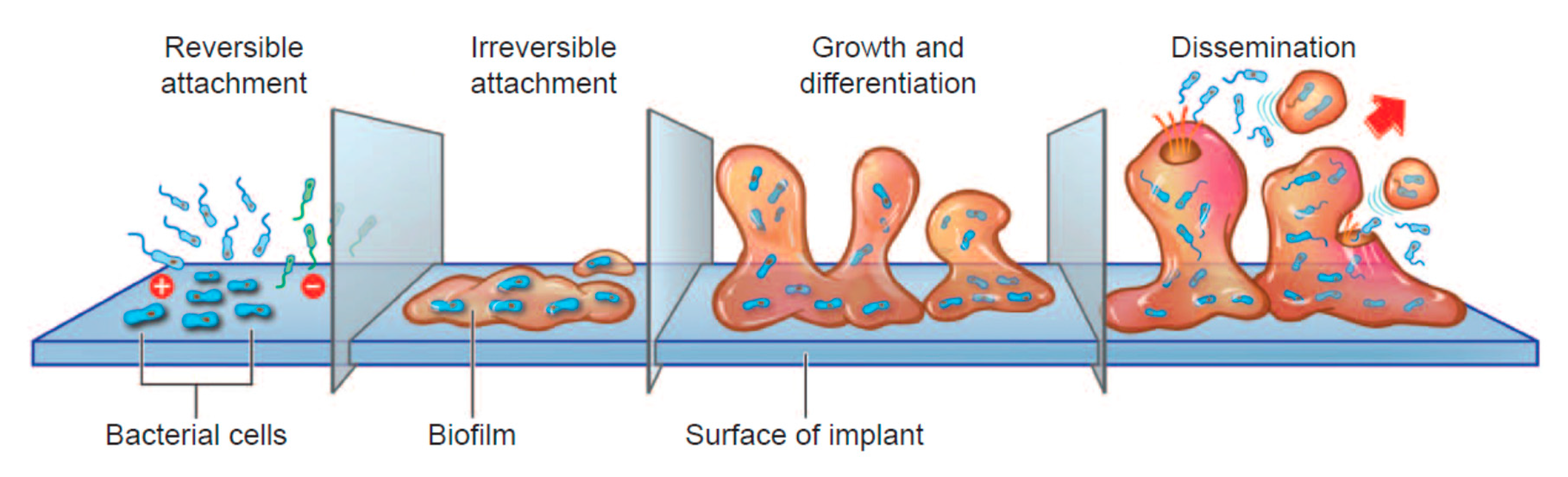
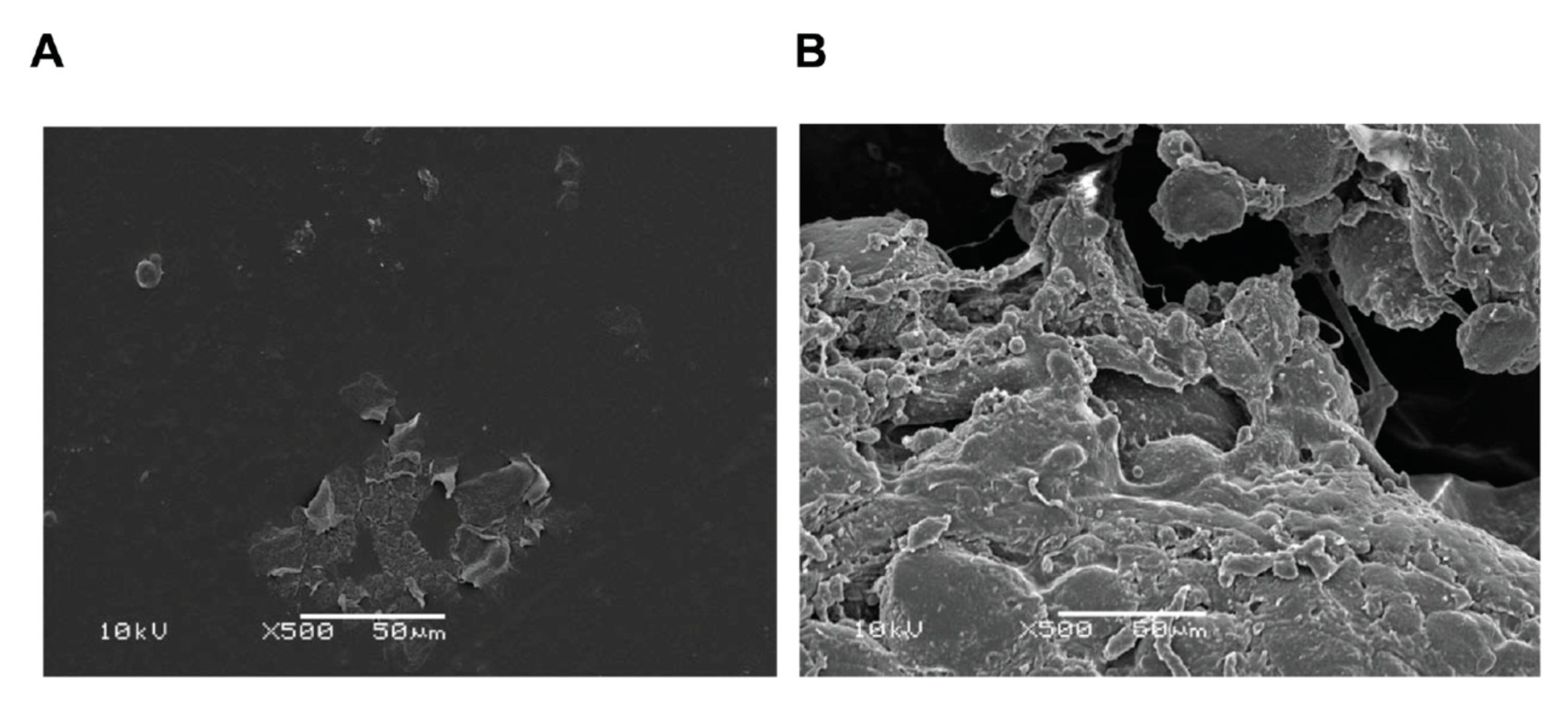
4.4. Bacteria/Biofilms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keech, J.A., Jr.; Creech, B.J. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast. Reconstr. Surg. 1997, 100, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, D.; Agostini, T.; Bocci, G.; Giannotti, G.; Fanelli, G.; Naccarato, A.G.; Danesi, R.; Tuccori, M.; Pantaloni, M.; D’Aniello, C. ALK-1-negative anaplastic large cell lymphoma associated with breast implants: A new clinical entity. Clin. Breast Cancer 2011, 11, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, D.R.; Miranda, R.N.; Kaura, A.; Francis, A.M.; Campanale, A.; Boldrini, R.; Alexander, J.; Deva, A.; Gravina, P.; Medeiros, L.J.; et al. Global adverse event reports of breast implant-associated ALCL: An international review of 40 government authority databases. Plast. Reconstr. Surg. 2017, 139, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Doren, E.L.; Miranda, R.N.; Selber, J.C.; Garvey, P.B.; Liu, J.; Medeiros, L.J.; Butler, C.E.; Clemens, M.W. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast. Reconstr. Surg. 2017, 139, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.N.; Aladily, T.N.; Prince, H.M.; Kanagal-Shamanna, R.; de Jong, D.; Fayad, L.E.; Amin, M.B.; Haideri, N.; Bhagat, G.; Brooks, G.S.; et al. Breast implant-associated anaplastic large-cell lymphoma: Long-term follow-up of 60 patients. J. Clin. Oncol. 2014, 32, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Maisel, W. Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL)—Letter to Health Care Providers. Available online: https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm630863.htm (accessed on 12 February 2019).
- Campanale, A.; Boldrini, R.; Marletta, M. 22 cases of breast implant-associated ALCL: Awareness and outcome tracking from the Italian Ministry of Health. Plast. Reconstr. Surg. 2018, 141, 11e–19e. [Google Scholar] [CrossRef]
- Cordeiro, P.G.; Ghione, P.; Ni, A.; Hu, Q.; Ganesan, N.; Galasso, N.; Dogan, A.; Horwitz, S.M. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 841–846. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, R.A.; Holland, M.; Sieber, D.A.; Wen, K.W.; Rugo, H.S.; Kadin, M.E.; Bean, G.R. Synchronous breast implant-associated anaplastic large cell lymphoma and invasive carcinoma: Genomic profiling and management implications. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2188. [Google Scholar] [CrossRef]
- De Boer, M.; van Leeuwen, F.E.; Hauptmann, M.; Overbeek, L.I.H.; de Boer, J.P.; Hijmering, N.J.; Sernee, A.; Klazen, C.A.H.; Lobbes, M.B.I.; van der Hulst, R.; et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018, 4, 335–341. [Google Scholar] [CrossRef]
- Oliveira, A.C.P.; Maino, M.; Zanin, E.M.; de Carli, L.; Duarte, D.W.; Collares, M.V.M. Breast implants follow-up: Results of a cross-sectional study on patients submitted to MRI breast examinations. Aesthetic Plast. Surg. 2020. [Google Scholar] [CrossRef]
- Leberfinger, A.N.; Behar, B.J.; Williams, N.C.; Rakszawski, K.L.; Potochny, J.D.; Mackay, D.R.; Ravnic, D.J. Breast implant-associated anaplastic large cell lymphoma: A systematic review. JAMA Surg. 2017, 152, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- International Research Collaborations on Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) Initiated by a Scientific Meeting Organised by RIVM in Amsterdam on November the 19th, 2018. Available online: https://www.rivm.nl/en/medical-devices/silicone-breast-implants/international-meeting-on-bia-alcl (accessed on 12 December 2018).
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, R.N.; Feldman, A.; Soares, F. WHO Classification of Tumours: Breast Tumours, 5th ed.; IARC: Lyon, France, 2019; Volume 2. [Google Scholar]
- Jaffe, E.S.; Ashar, B.S.; Clemens, M.W.; Feldman, A.L.; Gaulard, P.; Miranda, R.N.; Sohani, A.R.; Stenzel, T.; Yoon, S.W. Best practices guideline for the pathologic diagnosis of breast implant-associated anaplastic large-cell lymphoma. J. Clin. Oncol. 2020, 38, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Delas, A.; Gaulard, P.; Haioun, C.; Moreau, A.; Xerri, L.; Traverse-Glehen, A.; Rousset, T.; Quintin-Roue, I.; Petrella, T.; et al. Breast implant-associated anaplastic large cell lymphoma: Two distinct clinicopathological variants with different outcomes. Ann. Oncol. 2016, 27, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.E.; Medeiros, L.J.; Clemens, M.W.; Ferrufino-Schmidt, M.C.; Pina-Oviedo, S.; Miranda, R.N. Breast implant-associated anaplastic large cell lymphoma: A review. Mod. Pathol. 2018, 32, 166–188. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, A.; Pepe, G.; Giarnieri, E.; Cippitelli, C.; Bonifacino, A.; Mattei, M.; Martelli, M.; Falasca, C.; Cox, M.C.; Santino, I.; et al. Cytological diagnostic features of late breast implant seromas: From reactive to anaplastic large cell lymphoma. PLoS ONE 2017, 12, e0181097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Story, S.K.; Schowalter, M.K.; Geskin, L.J. Breast implant-associated ALCL: A unique entity in the spectrum of CD30+ lymphoproliferative disorders. Oncologist 2013, 18, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadin, M.E.; Adams, W.P., Jr.; Inghirami, G.; Di Napoli, A. Does breast implant-associated ALCL begin as a lymphoproliferative disorder? Plast. Reconstr. Surg. 2020, 145, 30e–38e. [Google Scholar] [CrossRef] [PubMed]
- Sieber, D.A.; Adams, W.P., Jr. What’s your micromort? A patient-oriented analysis of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthetic Surg. J. 2017, 37, 887–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, M.W.; Horwitz, S.M. NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthetic Surg. J. 2017, 37, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Clemens, M.W.; Medeiros, L.J.; Butler, C.E.; Hunt, K.K.; Fanale, M.A.; Horwitz, S.; Weisenburger, D.D.; Liu, J.; Morgan, E.A.; Kanagal-Shamanna, R.; et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J. Clin. Oncol. 2016, 34, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazenberg, M.D.; Spits, H. Human innate lymphoid cells. Blood 2014, 124, 700–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melvold, R.W.; Sticca, R.P. Basic and tumor immunology: A review. Surg. Oncol. Clin. N. Am. 2007, 16, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Warrington, R.; Watson, W.; Kim, H.L.; Antonetti, F.R. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2011, 7, S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aladily, T.N.; Medeiros, L.J.; Amin, M.B.; Haideri, N.; Ye, D.; Azevedo, S.J.; Jorgensen, J.L.; de Peralta-Venturina, M.; Mustafa, E.B.; Young, K.H.; et al. Anaplastic large cell lymphoma associated with breast implants: A report of 13 cases. Am. J. Surg. Pathol. 2012, 36, 1000–1008. [Google Scholar] [CrossRef]
- Lechner, M.G.; Megiel, C.; Church, C.H.; Angell, T.E.; Russell, S.M.; Sevell, R.B.; Jang, J.K.; Brody, G.S.; Epstein, A.L. Survival signals and targets for therapy in breast implant-associated ALK—Anaplastic large cell lymphoma. Clin. Cancer Res. 2012, 18, 4549–4559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberl, G.; Colonna, M.; Di Santo, J.P.; McKenzie, A.N. Innate lymphoid cells. Innate lymphoid cells: A new paradigm in immunology. Science 2015, 348, aaa6566. [Google Scholar] [CrossRef] [Green Version]
- Hawse, W.F.; Morel, P.A. An immunology primer for computational modelers. J. Pharmacokinet. Pharmacodyn. 2014, 41, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, D.; Rabensteiner, E.; Grundtman, C.; Bock, G.; Mayerl, C.; Parson, W.; Almanzar, G.; Hasenohrl, C.; Piza-Katzer, H.; Wick, G. T regulatory cells and TH17 cells in peri-silicone implant capsular fibrosis. Plast. Reconstr. Surg. 2012, 129, 327e–337e. [Google Scholar] [CrossRef]
- Kadin, M.E.; Morgan, J.; Xu, H.; Epstein, A.L.; Sieber, D.; Hubbard, B.A.; Adams, W.P., Jr.; Bacchi, C.E.; Goes, J.C.S.; Clemens, M.W.; et al. IL-13 is produced by tumor cells in breast implant associated anaplastic large cell lymphoma: Implications for pathogenesis. Hum. Pathol. 2018, 78, 54–62. [Google Scholar] [CrossRef]
- Kadin, M.E.; Morgan, J.; Kouttab, N.; Xu, H.; Adams, W.P.; Glicksman, C.; McGuire, P.; Sieber, D.; Epstein, A.L.; Miranda, R.N.; et al. Comparative analysis of cytokines of tumor cell lines, malignant and benign effusions around breast implants. Aesthetic Surg. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Chen, J. Disorders of the JAK/STAT pathway in T Cell lymphoma pathogenesis: Implications for immunotherapy. Annu. Rev. Immunol. 2017, 35, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Bollrath, J.; Phesse, T.J.; von Burstin, V.A.; Putoczki, T.; Bennecke, M.; Bateman, T.; Nebelsiek, T.; Lundgren-May, T.; Canli, O.; Schwitalla, S.; et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 2009, 15, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebouissou, S.; Amessou, M.; Couchy, G.; Poussin, K.; Imbeaud, S.; Pilati, C.; Izard, T.; Balabaud, C.; Bioulac-Sage, P.; Zucman-Rossi, J. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 2009, 457, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrogio, C.; Martinengo, C.; Voena, C.; Tondat, F.; Riera, L.; di Celle, P.F.; Inghirami, G.; Chiarle, R. NPM-ALK oncogenic tyrosine kinase controls T-cell identity by transcriptional regulation and epigenetic silencing in lymphoma cells. Cancer Res. 2009, 69, 8611–8619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, C.; Nicolae, A.; Laurent, C.; Le Bras, F.; Haioun, C.; Fataccioli, V.; Amara, N.; Adelaide, J.; Guille, A.; Schiano, J.M.; et al. Gene alterations in epigenetic modifiers and JAK-STAT signaling are frequent in breast implant-associated ALCL. Blood 2020, 135, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Petrus, M.N.; Xiao, W.; Nicolae, A.; Raffeld, M.; Pittaluga, S.; Bamford, R.N.; Nakagawa, M.; Ouyang, S.T.; et al. Cytokine receptor signaling is required for the survival of ALK- anaplastic large cell lymphoma, even in the presence of JAK1/STAT3 mutations. Proc. Natl. Acad. Sci. USA 2017, 114, 3975–3980. [Google Scholar] [CrossRef] [Green Version]
- Tevis, S.E.; Hunt, K.K.; Miranda, R.N.; Lange, C.; Butler, C.E.; Clemens, M.W. Differences in human leukocyte antigen expression between breast implant-associated anaplastic large cell lymphoma patients and the general population. Aesthetic Surg. J. 2019, 39, 1065–1070. [Google Scholar] [CrossRef]
- Montes-Mojarro, I.A.; Steinhilber, J.; Bonzheim, I.; Quintanilla-Martinez, L.; Fend, F. The pathological spectrum of systemic anaplastic large cell lymphoma (ALCL). Cancers 2018, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Oishi, N.; Brody, G.S.; Ketterling, R.P.; Viswanatha, D.S.; He, R.; Dasari, S.; Mai, M.; Benson, H.K.; Sattler, C.A.; Boddicker, R.L.; et al. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood 2018, 132, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Kadin, M.E.; Deva, A.; Xu, H.; Morgan, J.; Khare, P.; MacLeod, R.A.; Van Natta, B.W.; Adams, W.P., Jr.; Brody, G.S.; Epstein, A.L. Biomarkers provide clues to early events in the pathogenesis of breast implant-associated anaplastic large cell lymphoma. Aesthetic Surg. J. 2016, 36, 773–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewold, S.; Olsson, H.; Gustafson, P.; Rydholm, A.; Lidgren, L. Overall cancer incidence not increased after prosthetic knee replacement: 14,551 patients followed for 66,622 person-years. Int. J. Cancer 1996, 68, 30–33. [Google Scholar] [CrossRef]
- Lidgren, L. Chronic inflammation, joint replacement and malignant lymphoma. J. Bone Joint Surg. Br. 2008, 90, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, B.C.; Hiro, M.E.; Payne, W.G. Implant-associated anaplastic large cell lymphoma: Beyond breast prostheses. Ann. Plast. Surg. 2014, 73, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Palraj, B.; Paturi, A.; Stone, R.G.; Alvarez, H.; Sebenik, M.; Perez, M.T.; Bush, L.M. Soft tissue anaplastic large T-cell lymphoma associated with a metallic orthopedic implant: Case report and review of the current literature. J. Foot Ankle Surg. 2010, 49, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Choe, J.Y.; Jeon, Y.K. Mucosal CD30-positive T-cell lymphoproliferative disorder arising in the oral cavity following dental implants: Report of the first case. Int. J. Surg. Pathol. 2015, 23, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Manikkam Umakanthan, J.; McBride, C.L.; Greiner, T.; Yuan, J.; Sanmann, J.; Bierman, P.J.; Lunning, M.A.; Bociek, R.G. Bariatric implant-associated anaplastic large-cell lymphoma. J. Oncol. Pract. 2017, 13, 838–839. [Google Scholar] [CrossRef]
- Shauly, O.; Gould, D.J.; Siddiqi, I.; Patel, K.M.; Carey, J. The first reported case of gluteal implant-associated anaplastic large cell lymphoma (ALCL). Aesthetic Surg. J. 2019, 39, NP253–NP258. [Google Scholar] [CrossRef]
- Engberg, A.K.; Bunick, C.G.; Subtil, A.; Ko, C.J.; Girardi, M. Development of a plaque infiltrated with large CD30+ T cells over a silicone-containing device in a patient with history of Sezary syndrome. J. Clin. Oncol. 2013, 31, e87–e89. [Google Scholar] [CrossRef] [Green Version]
- Hallab, N.J.; Samelko, L.; Hammond, D. The inflammatory effects of breast implant particulate shedding: Comparison with orthopedic implants. Aesthetic Surg. J. 2019, 39, S36–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haussmann, P. Long-term results after silicone prosthesis replacement of the proximal pole of the scaphoid bone in advanced scaphoid nonunion. J. Hand Surg. Br. 2002, 27, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.T. Silicone synovitis. The current role of silicone elastomer implants in joint reconstruction. J. Hand Surg. Br. 1993, 18, 679–686. [Google Scholar] [CrossRef]
- Hirakawa, K.; Bauer, T.W.; Culver, J.E.; Wilde, A.H. Isolation and quantitation of debris particles around failed silicone orthopedic implants. J. Hand Surg. Am. 1996, 21, 819–827. [Google Scholar] [CrossRef]
- Pearle, A.D.; Crow, M.K.; Rakshit, D.S.; Wohlgemuth, J.; Nestor, B.J. Distinct inflammatory gene pathways induced by particles. Clin. Orthop. Relat. Res. 2007, 458, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Fleury, E.F.; Rego, M.M.; Ramalho, L.C.; Ayres, V.J.; Seleti, R.O.; Ferreira, C.A.; Roveda, D., Jr. Silicone-induced granuloma of breast implant capsule (SIGBIC): Similarities and differences with anaplastic large cell lymphoma (ALCL) and their differential diagnosis. Breast Cancer 2017, 9, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Bizjak, M.; Selmi, C.; Praprotnik, S.; Bruck, O.; Perricone, C.; Ehrenfeld, M.; Shoenfeld, Y. Silicone implants and lymphoma: The role of inflammation. J. Autoimmun. 2015, 65, 64–73. [Google Scholar] [CrossRef]
- Webb, L.H.; Aime, V.L.; Do, A.; Mossman, K.; Mahabir, R.C. Textured breast implants: A closer look at the surface debris under the microscope. Plast. Surg. 2017, 25, 179–183. [Google Scholar] [CrossRef]
- Loch-Wilkinson, A.; Beath, K.; Knight, R.J.W.; Wessels, W.L.F.; Magnusson, M.; Papadopoulos, T.; Connell, T.; Lofts, J.; Locke, M.; Hopper, I.; et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: High surface area textured implants are associated with increased risk. Plast. Reconstr. Surg. 2017, 140, 645–654. [Google Scholar] [CrossRef]
- Flassbeck, D.; Pfleiderer, B.; Klemens, P.; Heumann, K.G.; Eltze, E.; Hirner, A.V. Determination of siloxanes, silicon, and platinum in tissues of women with silicone gel-filled implants. Anal. Bioanal. Chem. 2003, 375, 356–362. [Google Scholar] [CrossRef]
- Potter, M. Silicone gels induce plasmacytomas in BALB/c mice. NIH Catal. 1994, 19, 22–23. [Google Scholar]
- Food and Drug Administration. FDA Backgrounder on Platinum in Silicone Breast Implants. Available online: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM064040 (accessed on 9 July 2018).
- Brook, M.A. Platinum in silicone breast implants. Biomaterials 2006, 27, 3274–3286. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.R.; Fried, M.; Schnur, P.L.; Tofield, J.J. Capsules, infection, and intraluminal antibiotics. Plast. Reconstr. Surg. 1981, 68, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rieger, U.M.; Mesina, J.; Kalbermatten, D.F.; Haug, M.; Frey, H.P.; Pico, R.; Frei, R.; Pierer, G.; Luscher, N.J.; Trampuz, A. Bacterial biofilms and capsular contracture in patients with breast implants. Br. J. Surg. 2013, 100, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Pajkos, A.; Deva, A.K.; Vickery, K.; Cope, C.; Chang, L.; Cossart, Y.E. Detection of subclinical infection in significant breast implant capsules. Plast. Reconstr. Surg. 2003, 111, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P., Jr. Capsular contracture: What is it? What causes it? How can it be prevented and managed? Clin. Plast. Surg. 2009, 36, 119–126, vii. [Google Scholar] [CrossRef] [PubMed]
- Spear, S.L.; Baker, J.L., Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plast. Reconstr. Surg. 1995, 96, 1119–1123; discussion 1124. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of human breast tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [Green Version]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef]
- Bartsich, S.; Ascherman, J.A.; Whittier, S.; Yao, C.A.; Rohde, C. The breast: A clean-contaminated surgical site. Aesthetic Surg. J. 2011, 31, 802–806. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How biofilms evade host defenses. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deva, A.K.; Adams, W.P., Jr.; Vickery, K. The role of bacterial biofilms in device-associated infection. Plast. Reconstr. Surg. 2013, 132, 1319–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, W.P., Jr.; Rios, J.L.; Smith, S.J. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: Six-year prospective clinical study. Plast. Reconstr. Surg. 2006, 117, 30–36. [Google Scholar] [CrossRef] [PubMed]
- McGuire, P.; Reisman, N.R.; Murphy, D.K. Risk factor analysis for capsular contracture, malposition, and late seroma in subjects receiving Natrelle 410 form-stable silicone breast implants. Plast. Reconstr. Surg. 2017, 139, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalanis, G.C.; Liu, E.W.; Cheng, H.T. Efficacy and safety of povidone-iodine irrigation in reducing the risk of capsular contracture in aesthetic breast augmentation: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2015, 136, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P., Jr.; Calobrace, M.B. Discussion: The questionable role of antibiotic irrigation in breast augmentation. Plast. Reconstr. Surg. 2019, 144, 253–257. [Google Scholar] [CrossRef]
- Jacombs, A.; Tahir, S.; Hu, H.; Deva, A.K.; Almatroudi, A.; Wessels, W.L.; Bradshaw, D.A.; Vickery, K. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast. Reconstr. Surg. 2014, 133, 471e–480e. [Google Scholar] [CrossRef]
- Loch-Wilkinson, A.; Beath, K.J.; Magnusson, M.R.; Cooter, R.; Shaw, K.; French, J.; Vickery, K.; Prince, H.M.; Deva, A.K. Breast implant-associated anaplastic large cell lymphoma in Australia: A longitudinal study of implant and other related risk factors. Aesthetic Surg. J. 2019. [Google Scholar] [CrossRef]
- Hu, H.; Jacombs, A.; Vickery, K.; Merten, S.L.; Pennington, D.G.; Deva, A.K. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: Implications for breast implant-associated lymphoma. Plast. Reconstr. Surg. 2015, 135, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Fadl, A.A.; Tammali, R.; Reddy, A.B.; Chopra, A.K.; Srivastava, S.K. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J. Biol. Chem. 2006, 281, 33019–33029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triantafilou, M.; Triantafilou, K. The dynamics of LPS recognition: Complex orchestration of multiple receptors. J. Endotoxin Res. 2005, 11, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.T.; Ignarro, L.J. Lipopolysaccharide-induced expression of interferon-beta mediates the timing of inducible nitric-oxide synthase induction in RAW 264.7 macrophages. J. Biol. Chem. 2001, 276, 47950–47957. [Google Scholar] [CrossRef] [Green Version]
- Pollara, G.; Handley, M.E.; Kwan, A.; Chain, B.M.; Katz, D.R. Autocrine type I interferon amplifies dendritic cell responses to lipopolysaccharide via the nuclear factor-kappaB/p38 pathways. Scand. J. Immunol. 2006, 63, 151–154. [Google Scholar] [CrossRef]
- Jewell, M.L.; Adams, W.P., Jr. Betadine and Breast Implants. Aesthetic Surg. J. 2018, 38, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Adams, W.P., Jr.; Culbertson, E.J.; Deva, A.K.; Magnusson, R.M.; Layt, C.; Jewell, M.L.; Mallucci, P.; Heden, P. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: Experience in 42,000 implants. Plast. Reconstr. Surg. 2017, 140, 427–431. [Google Scholar] [CrossRef]
- Cummins, J.; Tangney, M. Bacteria and tumours: Causative agents or opportunistic inhabitants? Infect. Agents Cancer 2013, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.; Carter, J.; Harari, S.; Pei, Z. The interrelationships of the gut microbiome and inflammation in colorectal carcinogenesis. Clin. Lab. Med. 2014, 34, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Mima, K.; Nakagawa, S.; Sawayama, H.; Ishimoto, T.; Imai, K.; Iwatsuki, M.; Hashimoto, D.; Baba, Y.; Yamashita, Y.I.; Yoshida, N.; et al. The microbiome and hepatobiliary-pancreatic cancers. Cancer Lett. 2017, 402, 9–15. [Google Scholar] [CrossRef]
- Zhang, C.; Powell, S.E.; Betel, D.; Shah, M.A. The gastric microbiome and its influence on gastric carcinogenesis: Current knowledge and ongoing research. Hematol. Oncol. Clin. N. Am. 2017, 31, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Hansen, S.; Rodriguez, L.; Gelb, A.B.; Warnke, R.A.; Jellum, E.; Orentreich, N.; Vogelman, J.H.; Friedman, G.D. Helicobacter pylori infection and gastric lymphoma. New Engl. J. Med. 1994, 330, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Lamb, A.; Chen, L.F. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J. Cell. Biochem. 2013, 114, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melenotte, C.; Million, M.; Audoly, G.; Gorse, A.; Dutronc, H.; Roland, G.; Dekel, M.; Moreno, A.; Cammilleri, S.; Carrieri, M.P.; et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood 2016, 127, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Linnemann, T.; Gellrich, S.; Lukowsky, A.; Mielke, A.; Audring, H.; Sterry, W.; Walden, P. Polyclonal expansion of T cells with the TCR V beta type of the tumour cell in lesions of cutaneous T-cell lymphoma: Evidence for possible superantigen involvement. Br. J. Dermatol. 2004, 150, 1013–1017. [Google Scholar] [CrossRef]
- Llewelyn, M.; Sriskandan, S.; Terrazzini, N.; Cohen, J.; Altmann, D.M. The TCR Vbeta signature of bacterial superantigens spreads with stimulus strength. Int. Immunol. 2006, 18, 1433–1441. [Google Scholar] [CrossRef]
- Deva, A.K. Response to “breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): Why the search for an infectious etiology may be irrelevant”. Aesthetic Surg. J. 2017, 37, NP122–NP128. [Google Scholar] [CrossRef] [Green Version]
- Blombery, P.; Thompson, E.R.; Jones, K.; Arnau, G.M.; Lade, S.; Markham, J.F.; Li, J.; Deva, A.; Johnstone, R.W.; Khot, A.; et al. Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica 2016, 101, e387–e390. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.; Mempin, M.; Hu, H.; Chowdhury, D.; Foley, M.; Cooter, R.; Adams, W.P., Jr.; Vickery, K.; Deva, A.K. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast. Reconstr. Surg. 2018, 142, 837–849. [Google Scholar] [CrossRef]
- Di Napoli, A.; de Cecco, L.; Piccaluga, P.P.; Navari, M.; Cancila, V.; Cippitelli, C.; Pepe, G.; Lopez, G.; Monardo, F.; Bianchi, A.; et al. Transcriptional analysis distinguishes breast implant-associated anaplastic large cell lymphoma from other peripheral T-cell lymphomas. Mod. Pathol. 2019, 32, 216–230. [Google Scholar] [CrossRef]
- Kang, S.T.; Wang, H.C.; Yang, Y.T.; Kou, G.H.; Lo, C.F. The DNA virus white spot syndrome virus uses an internal ribosome entry site for translation of the highly expressed nonstructural protein ICP35. J. Virol. 2013, 87, 13263–13278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deva, A.K.; Turner, S.D.; Kadin, M.E.; Magnusson, M.R.; Prince, H.M.; Miranda, R.N.; Inghirami, G.G.; Adams, W.P., Jr. Etiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Current Directions in Research. Cancers 2020, 12, 3861. https://doi.org/10.3390/cancers12123861
Deva AK, Turner SD, Kadin ME, Magnusson MR, Prince HM, Miranda RN, Inghirami GG, Adams WP Jr. Etiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Current Directions in Research. Cancers. 2020; 12(12):3861. https://doi.org/10.3390/cancers12123861
Chicago/Turabian StyleDeva, Anand K., Suzanne D. Turner, Marshall E. Kadin, Mark R. Magnusson, H. Miles Prince, Roberto N. Miranda, Giorgio G. Inghirami, and William P. Adams, Jr. 2020. "Etiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Current Directions in Research" Cancers 12, no. 12: 3861. https://doi.org/10.3390/cancers12123861
APA StyleDeva, A. K., Turner, S. D., Kadin, M. E., Magnusson, M. R., Prince, H. M., Miranda, R. N., Inghirami, G. G., & Adams, W. P., Jr. (2020). Etiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Current Directions in Research. Cancers, 12(12), 3861. https://doi.org/10.3390/cancers12123861