Pediatric Rhabdomyosarcomas: Three-Dimensional Radiological Assessments after Induction Chemotherapy Predict Survival Better than One-Dimensional and Two-Dimensional Measurements
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
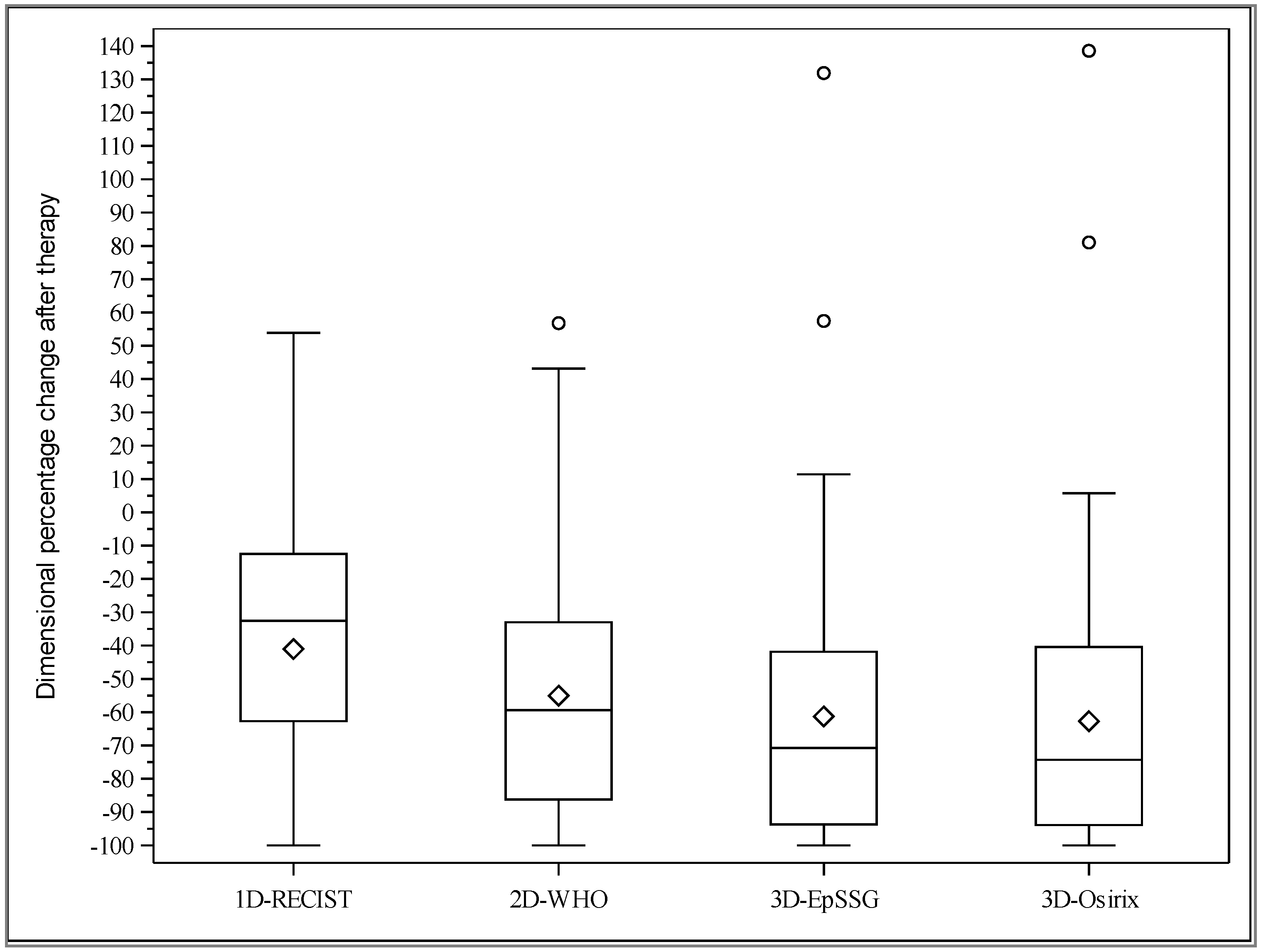
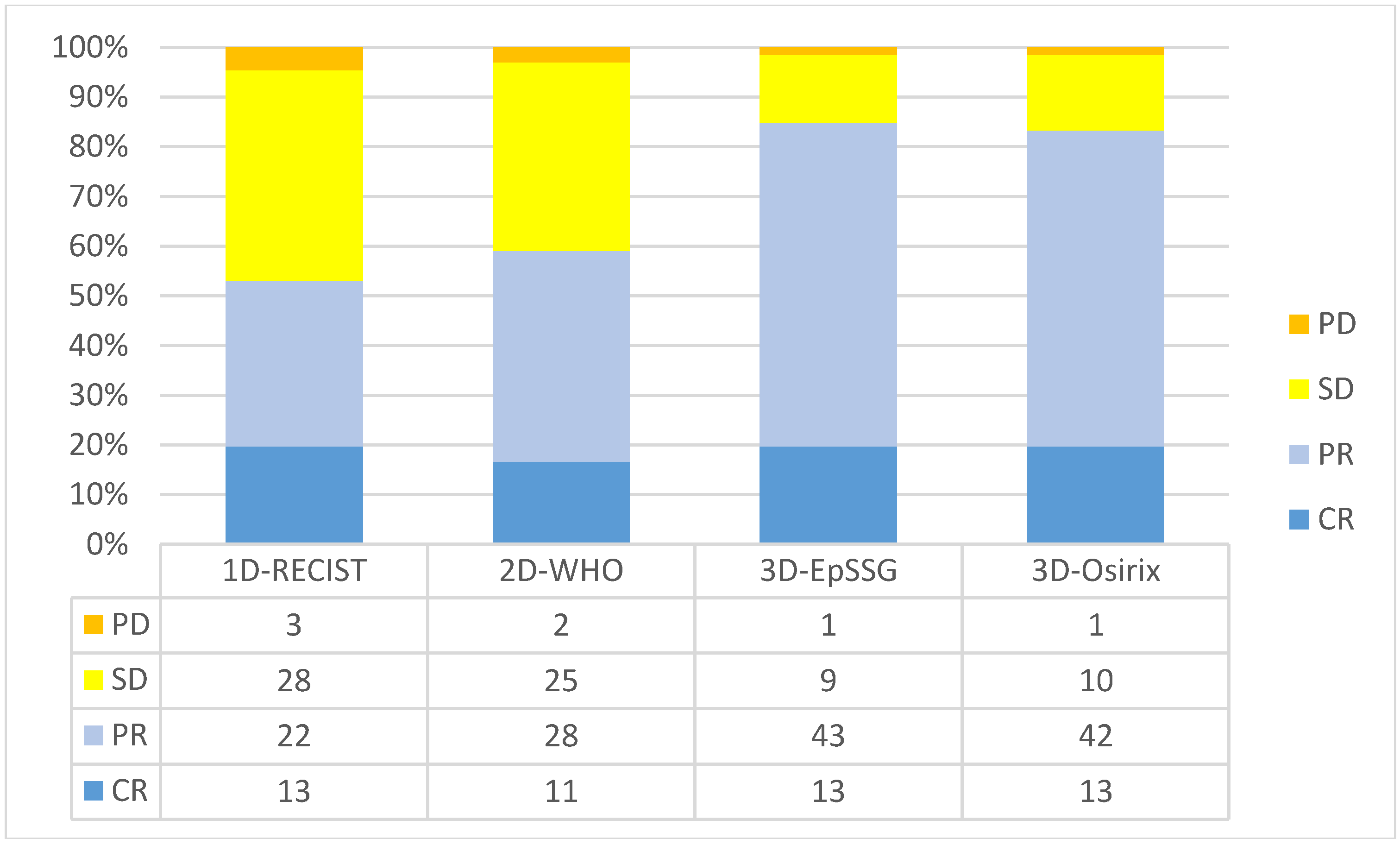
2.2. Agreement between Observers and Methods for Tumor Response Assessment
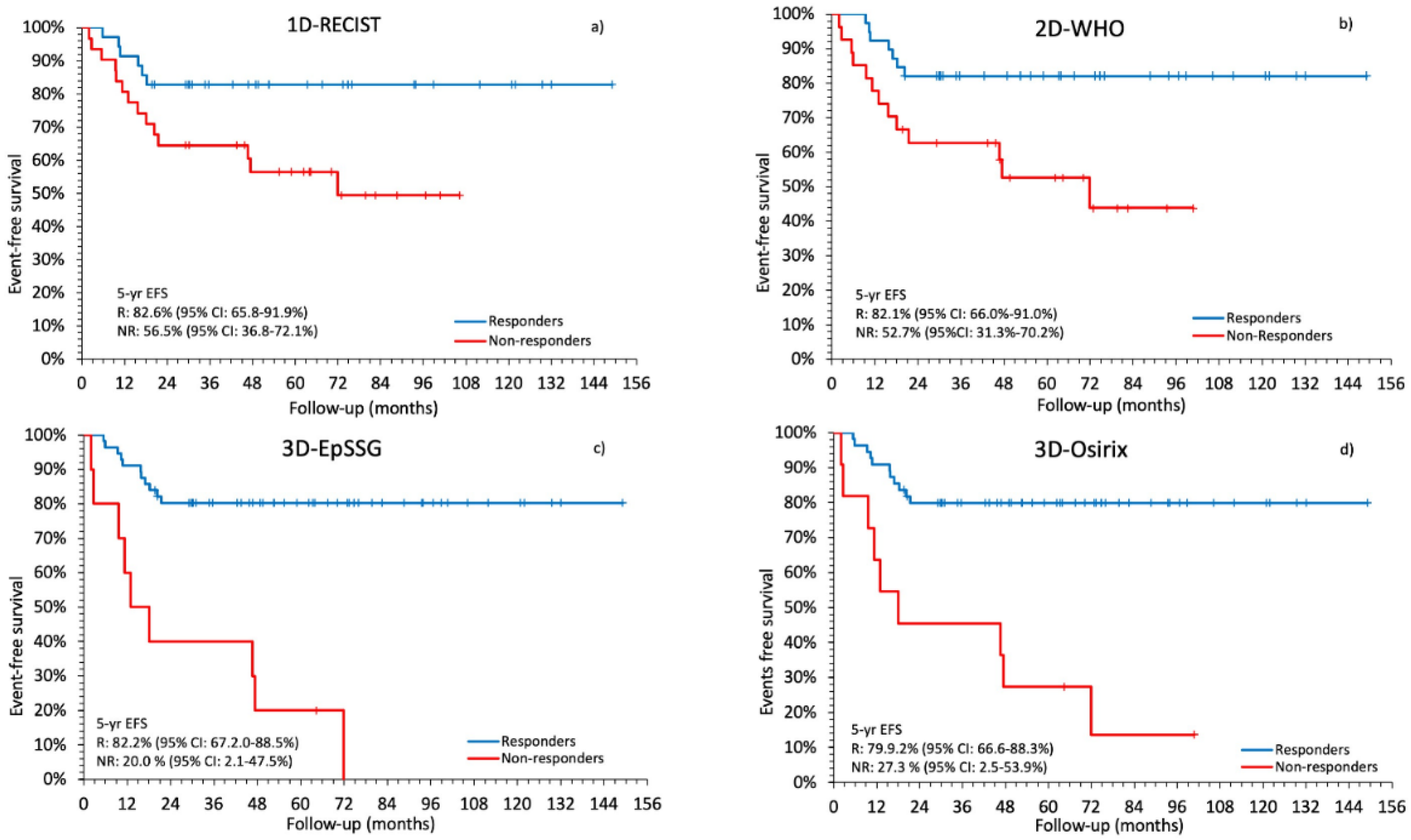
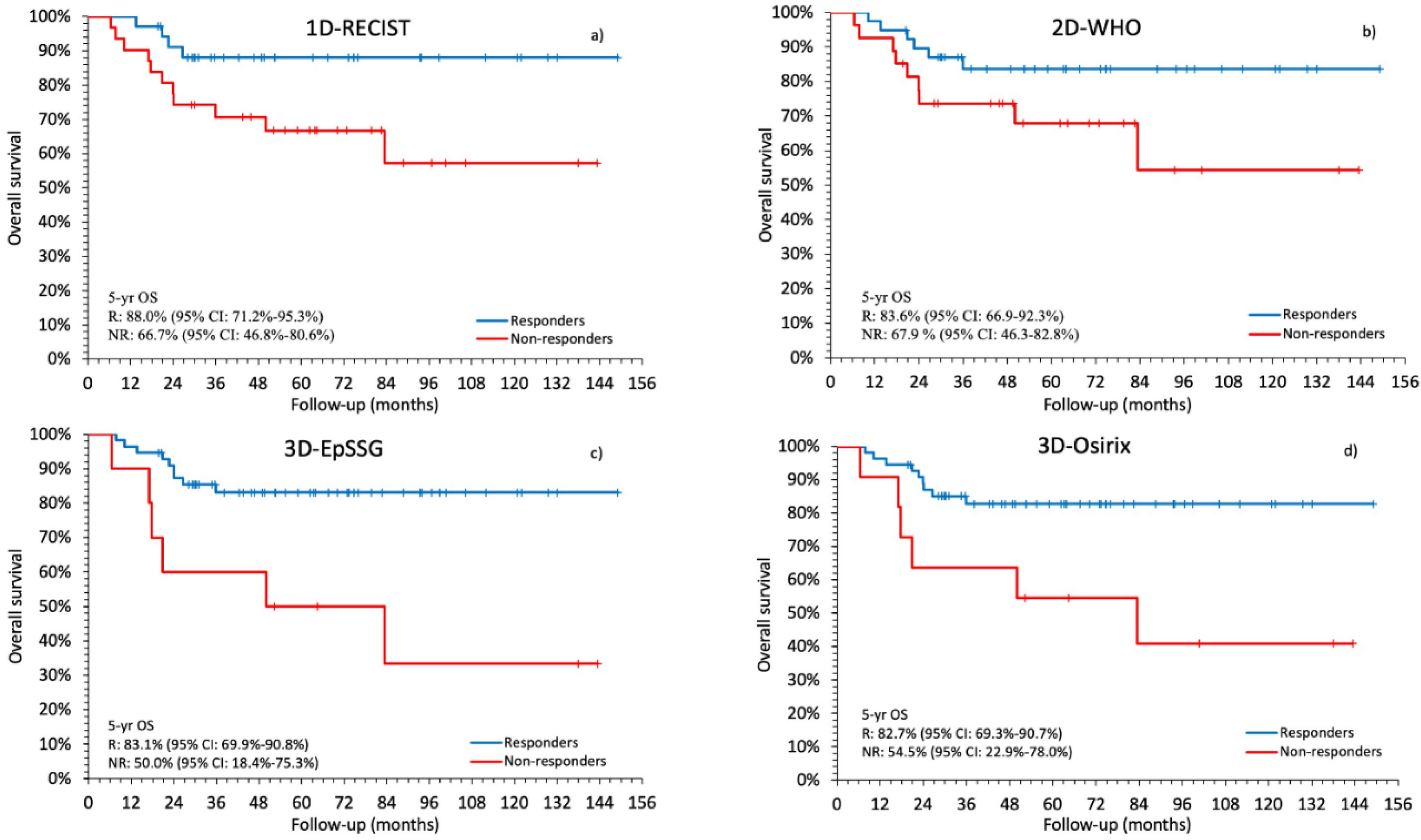
2.3. Comparison between Radiological Response and Survival
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Tumor Size Measurements
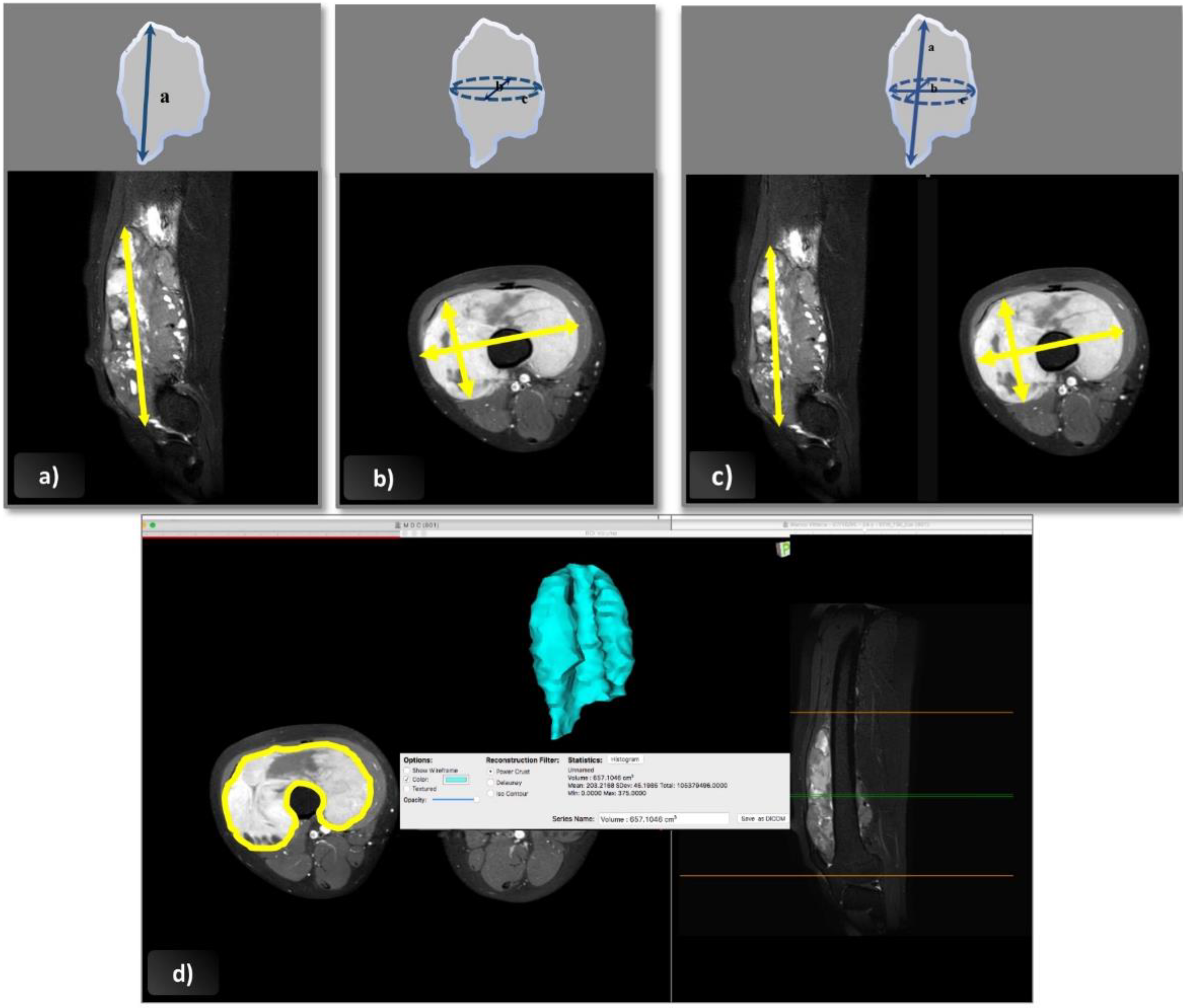
- Following the RECIST 1.1 guidelines, the longest tumor diameter was measured in any plane (axial, coronal, or sagittal) [25]. In the post-treatment assessment, the maximal diameter was measured in the same plane as at diagnosis, but not necessarily on the same slice level or in the same direction.
- Cross-sectional area according to the WHO criteria was obtained from the product of the longest overall tumor diameter in the axial plane and the longest perpendicular diameter [14].
- For the 3D-EpSSG assessment, a tumor’s three maximal diameters were assessed according to the guidelines in the EpSSG RMS 2005 protocol. The two maximal perpendicular diameters (a and b) were assessed in the axial plane on the section with the largest tumor surface area; the cranio-caudal dimension (c) was measured on sagittal or coronal images. Tumor volume was obtained with the following formula: a × b × c × π/6, which approximates the geometry of the tumor to an ellipsoid [2].
- Software-assisted volume assessment was based on 2D cross-sectional measurements followed by volume rendering using Osirix software, version 5.6, 64 bit (Geneva, Switzerland). This software can calculate the volume of a solid lesion starting from a series of 2D regions of interest drawn by the operator. The tumor’s boundaries were outlined manually with the tool’s pencil, selecting one region of interest for each slice containing visible tumor on the axial plane, and avoiding the inclusion of any perilesional edema with the help of coronal and sagittal images. Then, the 3D tumor volume was reconstructed using the “multiplanar reformation compute volume” application, and the corresponding volume in cubic centimeters was recorded.
4.3. Tumor Response Assessment and Classification
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hawkins, D.S.; Spunt, S.L.; Skapek, S.X. Children’s Oncology Group’s 2013 Blueprint for Research: Soft Tissue Sarcomas. Pediatr. Blood Cancer 2013, 60, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, G.; Jenney, M.; Bergeron, C.; Melcón, S.G.; Ferrari, A.; Oberlin, O.; Carli, M.; Stevens, M.; Kelsey, A.; De Paoli, A.; et al. Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1061–1071. [Google Scholar] [CrossRef]
- Koscielniak, E.; Harms, D.; Henze, G.; Jürgens, H.; Gadner, H.; Herbst, M.; Klingebiel, T.; Schmidt, B.F.; Morgan, M.; Knietig, R.; et al. Results of Treatment for Soft Tissue Sarcoma in Childhood and Adolescence: A Final Report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J. Clin. Oncol. 1999, 17, 3706–3719. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.C.G.; Rey, A.; Bouvet, N.; Ellershaw, C.; Flamant, F.; Habrand, J.L.; Marsden, H.B.; Martelli, H.; De Toledo, J.S.; Spicer, R.D.; et al. Treatment of Nonmetastatic Rhabdomyosarcoma in Childhood and Adolescence: Third Study of the International Society of Paediatric Oncology—SIOP Malignant Mesenchymal Tumor 89. J. Clin. Oncol. 2005, 23, 2618–2628. [Google Scholar] [CrossRef]
- Haveman, L.M.; Ranft, A.; Berg, H.V.; Smets, A.; Kruseova, J.; Ladenstein, R.; Brichard, B.; Paulussen, M.; Kuehne, T.; Juergens, H.; et al. The relation of radiological tumor volume response to histological response and outcome in patients with localized Ewing Sarcoma. Cancer Med. 2019, 8, 1086–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacci, G.; Longhi, A.; Versari, M.; Mercuri, M.; Briccoli, A.; Picci, P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Cancer 2006, 106, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Kim, J.-S.; Sung, K.W.; Jeon, T.Y.; Choi, J.Y.; Moon, S.H.; Son, M.H.; Lee, S.H.; Yoo, K.H.; Koo, H.H. The degree of tumor volume reduction during the early phase of induction chemotherapy is an independent prognostic factor in patients with high-risk neuroblastoma. Cancer 2012, 119, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Hutchings, M.; Rigacci, L.; Specht, L.; Merli, F.; Hansen, M.; Patti, C.; Loft, A.; Di Raimondo, F.; D’Amore, F.; et al. Early Interim 2-[18F]Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography Is Prognostically Superior to International Prognostic Score in Advanced-Stage Hodgkin’s Lymphoma: A Report From a Joint Italian-Danish Study. J. Clin. Oncol. 2007, 25, 3746–3752. [Google Scholar] [CrossRef] [Green Version]
- Vaarwerk, B.; Van Der Lee, J.H.; Breunis, W.B.; Orbach, D.; Chisholm, J.C.; Cozic, N.; Jenney, M.; Van Rijn, R.R.; McHugh, K.; Gallego, S.; et al. Prognostic relevance of early radiologic response to induction chemotherapy in pediatric rhabdomyosarcoma: A report from the International Society of Pediatric Oncology Malignant Mesenchymal Tumor 95 study. Cancer 2017, 124, 1016–1024. [Google Scholar] [CrossRef]
- Burke, M.; Anderson, J.R.; Kao, S.C.; Rodeberg, D.; Qualman, S.J.; Wolden, S.; Meyer, W.H.; Breitfeld, P.P. Assessment of Response to Induction Therapy and Its Influence on 5-Year Failure-Free Survival in Group III Rhabdomyosarcoma: The Intergroup Rhabdomyosarcoma Study-IV Experience—A Report From the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J. Clin. Oncol. 2007, 25, 4909–4913. [Google Scholar] [CrossRef]
- Rosenberg, A.R.; Anderson, J.R.; Lyden, E.; Rodeberg, D.A.; Wolden, S.L.; Kao, S.C.; Parham, D.M.; Arndt, C.; Hawkins, D.S. Early response as assessed by anatomic imaging does not predict failure-free survival among patients with Group III rhabdomyosarcoma: A report from the Children’s Oncology Group. Eur. J. Cancer 2014, 50, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.; Miceli, R.; Meazza, C.; Casanova, M.; Favini, F.; Morosi, C.; Trecate, G.; Marchianò, A.; Luksch, R.; Cefalo, G.; et al. Comparison of the Prognostic Value of Assessing Tumor Diameter Versus Tumor Volume at Diagnosis or in Response to Initial Chemotherapy in Rhabdomyosarcoma. J. Clin. Oncol. 2010, 28, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Dantonello, T.M.; Stark, M.; Timmermann, B.; Fuchs, J.; Selle, B.; Linderkamp, C.; Handgretinger, R.; Hagen, R.; Feuchtgruber, S.; Kube, S.; et al. Tumour volume reduction after neoadjuvant chemotherapy impacts outcome in localised embryonal rhabdomyosarcoma. Pediatr. Blood Cancer 2014, 62, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Julka, P.K.; Doval, D.C.; Gupta, S.; Rath, G.K. Response assessment in solid tumours: A comparison of WHO, SWOG and RECIST guidelines. Br. J. Radiol. 2008, 81, 444–449. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Eisenhauer, E.; Christian, M.; Terenziani, M.; Vena, D.; Muldal, A.; Therasse, P. Measuring Response in Solid Tumors: Unidimensional Versus Bidimensional Measurement. J. Natl. Cancer Inst. 1999, 91, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.; Jacobsson, H.; Hatschek, T.; Torkzad, M.R.; Bodén, K.; Eriksson-Alm, Y.; Berg, E.; Fujii, H.; Kubo, A.; Blomqvist, L. Radiologic Measurements of Tumor Response to Treatment: Practical Approaches and Limitations. Radiographics 2008, 28, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Tirkes, T.; Hollar, M.A.; Tann, M.; Kohli, M.D.; Akisik, F.; Sandrasegaran, K. Response Criteria in Oncologic Imaging: Review of Traditional and New Criteria. Radiographics 2013, 33, 1323–1341. [Google Scholar] [CrossRef]
- Aghighi, M.; Boe, J.; Rosenberg, J.; Von Eyben, R.; Gawande, R.S.; Petit, P.; Sethi, T.K.; Sharib, J.; Marina, N.M.; Dubois, S.G.; et al. Three-dimensional Radiologic Assessment of Chemotherapy Response in Ewing Sarcoma Can Be Used to Predict Clinical Outcome. Radiology 2016, 280, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Schoot, R.A.; McHugh, K.; Van Rijn, R.R.; Kremer, L.C.M.; Chisholm, J.C.; Caron, H.N.; Merks, J.H.M. Response Assessment in Pediatric Rhabdomyosarcoma: Can Response Evaluation Criteria in Solid Tumors Replace Three-dimensional Volume Assessments? Radiology 2013, 269, 870–878. [Google Scholar] [CrossRef]
- Galanis, E.; Buckner, J.C.; Maurer, M.J.; Sykora, R.; Castillo, R.; Ballman, K.V.; Bradley, J. Erickson for the North Central Cancer Treatment Group Validation of neuroradiologic response assessment in gliomas: Measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods1. Neuro-Oncology 2006, 8, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheng, J.-L.; Han, Y.-H.; Li, Y.-L.; Dai, J.-P.; Shi, D. Measurement of tumor size in adult glioblastoma: Classical cross-sectional criteria on 2D MRI or volumetric criteria on high resolution 3D MRI? Eur. J. Radiol. 2012, 81, 2370–2374. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.D.; Kesari, S.; Xu, R.; Batchelor, T.T.; O’Neill, A.M.; Hochberg, F.H.; Levy, B.; Bradshaw, J.; Wen, P.Y. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas1. Neuro-oncology 2006, 8, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.G.; Patel, S.; Harmath, C.; Bridges, S.; Synnott, J.; Sievers, A.; Yoon, Y.-H.; Lee, E.J.; Yang, M.C.; Lewis, R.F.; et al. Comparison of Diameter and Perimeter Methods for Tumor Volume Calculation. J. Clin. Oncol. 2001, 19, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; De Vries, E.G.E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsatti, G.; Beltrame, V.; Crimì, F.; Frigo, A.C.; Bisogno, G.; Stramare, R. Radiologic Response Assessment in Pediatric Soft Tissue Sarcoma: Computed-Assisted Volume Evaluation. J. Pediatr. 2017, 182, 327–334.e2. [Google Scholar] [CrossRef]
- Schiavon, G.; Ruggiero, A.; Schöffski, P.; Van Der Holt, B.; Bekers, D.J.; Eechoute, K.; Vandecaveye, V.; Krestin, G.P.; Verweij, J.; Sleijfer, S.; et al. Tumor Volume as an Alternative Response Measurement for Imatinib Treated GIST Patients. PLoS ONE 2012, 7, e48372. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Schwartz, L.H.; Moskowitz, C.S.; Ginsberg, M.S.; Rizvi, N.A.; Kris, M.G. Lung Cancer: Computerized Quantification of Tumor Response—Initial Results. Radiology 2006, 241, 892–898. [Google Scholar] [CrossRef]
- Deike, K.; Wiestler, B.; Graf, M.; Reimer, C.; Floca, R.; Bäumer, P.; Vollmuth, P.; Heiland, S.; Schlemmer, H.-P.; Wick, W.; et al. Prognostic value of combined visualization of MR diffusion and perfusion maps in glioblastoma. J. Neuro-Oncol. 2015, 126, 463–472. [Google Scholar] [CrossRef]
- Abudu, A.; Davies, A.M.; Pynsent, P.B.; Mangham, D.C.; Tillman, R.M.; Carter, S.R.; Grimer, R.J. Tumour volume as a predictor of necrosis after chemotherapy in Ewing’s sarcoma. J. Bone Jt. Surg. Br. Vol. 1999, 81, 317–322. [Google Scholar] [CrossRef]
- Chhabra, A.; Ashikyan, O.; Slepicka, C.; Dettori, N.; Hwang, H.; Callan, A.; Sharma, R.R.; Xi, Y. Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: Correlation with histologic grading. Eur. Radiol. 2018, 29, 4485–4494. [Google Scholar] [CrossRef] [PubMed]
- Crist, W.M.; Anderson, J.R.; Meza, J.L.; Fryer, C.; Raney, R.B.; Ruymann, F.B.; Breneman, J.; Qualman, S.J.; Wiener, E.; Wharam, M.; et al. Intergroup Rhabdomyosarcoma Study-IV: Results for Patients With Nonmetastatic Disease. J. Clin. Oncol. 2001, 19, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Meza, J.; Anderson, J.; Pappo, A.S.; Meyer, W.H. Analysis of Prognostic Factors in Patients With Nonmetastatic Rhabdomyosarcoma Treated on Intergroup Rhabdomyosarcoma Studies III and IV: The Children’s Oncology Group. J. Clin. Oncol. 2006, 24, 3844–3851. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N | % |
|---|---|---|
| Sex | ||
| Male | 34 | 51.5 |
| Female | 32 | 48.5 |
| Age (y) | ||
| Median (range) | 6.1 (0.01–21) | |
| Mean ± sd | 7.8 ± 6.1 | |
| ≤10 | 47 | 71.2 |
| >10 | 19 | 28.8 |
| Tumor Site | ||
| Orbit | 6 | 9.1 |
| Head and neck parameningeal | 28 | 42.4 |
| Head and neck non parameningeal | 3 | 4.6 |
| Genitourinary, bladder or prostate | 8 | 12.1 |
| Genitourinary, non-bladder or prostate | 1 | 1.5 |
| Extremities | 7 | 10.6 |
| Other | 13 | 19.7 |
| Histology | ||
| Embryonal | 49 | 74.2 |
| Alveolar | 16 | 24.3 |
| Not otherwise specified | 1 | 1.5 |
| Tumor Size at Diagnosis | ||
| Diameter ≤ 5 cm | 42 | |
| Diameter >5 cm | 24 | |
| Median maximal diameter (iq range), cm | 5.85 (1.58–20.1) | |
| Median cross sectional area (iq range), cm2 | 17.01 (1.24–126) | |
| Median 3d-epssg volume (iq range), cm3 | 41.09 (2.58–950.1) | 63.6 |
| Median 3d-osirix volume (iq range), cm3 | 39.42 (2.41–927.0) | 36.4 |
| Tumor Size after Treatment | ||
| Median maximal diameter (iq range), cm | 3.17 (0–22.0) | |
| Median cross sectional area (iq range), cm2 | 5.03(0–128.3) | |
| Median 3d-epssg volume (iq range), cm3 | 7.18 (0–1441.5) | |
| Median 3d-osirix volume (iq range), cm3 | 7.09 (0–1500.4) | |
| T Status | ||
| T1 (without local invasion) | 19 | 28.8 |
| T2 (with local invasion) | 47 | 71.2 |
| N Status | ||
| N0 (without regional nodal metastases) | 53 | 80.3 |
| N1 (regional nodal metastases) | 12 | 18.2 |
| Unknown | 1 | 1.5 |
| Risk Group | ||
| Standard | 13 | 19.7 |
| High | 49 | 74.2 |
| Very High | 4 | 6.1 |
| Chemotherapy Regimen | ||
| 9 iva | 25 | 37.9 |
| 9 iva + 6 vnl/cpm | 11 | 16.7 |
| 4 ivado + 5 iva | 8 | 12.1 |
| 4 ivado + 5 iva + 6 vnl/cpm | 13 | 19.7 |
| 4 iva + 5 topo/carbo | 7 | 10.6 |
| 4 iva * | 2 | 3 |
| Delayed Surgery | ||
| Yes | 29 | 43.9 |
| No | 37 | 56.1 |
| Radiotherapy | ||
| Yes | 61 | 92.4 |
| No | 5 | 7.6 |
| Methods | κ | 95% CI | N of Cases of Disagreement |
|---|---|---|---|
| Inter-observer agreement | |||
| 1D-RECIST | 0.83 | 0.72–0.94 | 9 |
| 2D-WHO | 0.71 | 0.55–0.86 | 11 |
| 3D-EpSSG | 0.86 | 0.75–0.97 | 5 |
| 3D-Osirix | 0.95 | 0.89–1.00 | 2 |
| Inter-method agreement | |||
| 1D-RECIST/2D-WHO | 0.68 | 0.53–0.83 | 16 |
| 1D-RECIST/3D-EpSSG | 0.57 | 0.43–0.71 | 24 |
| 1D-RECIST/3D-Osirix | 0.59 | 0.45–0.73 | 23 |
| 2D-WHO/3D-EpSSG | 0.60 | 0.45–0.75 | 20 |
| 2D-WHO/3D-Osirix | 0.62 | 0.47–0.77 | 19 |
| 3D-EpSSG/3D-Osirix | 0.98 | 0.93–1.00 | 1 |
| Method | Hazard Ratio (95% CI) | p Value | Uno’s Concordance Statistic and Standard Error |
|---|---|---|---|
| Event-free survival | |||
| 1D-RECIST | 3.00 (1.15–7.81) | 0.0245 | 0.64 (0.094) |
| 2D-WHO | 3.29 (1.31–8.25) | 0.0113 | 0.66 (0.087) |
| 3D-EpSSG | 7.09 (2.91–17.25) | <0.0001 | 0.69 (0.058) |
| 3D-Osirix | 5.74 (2.37–13.91) | 0.0001 | 0.68 (0.061) |
| Overall survival | |||
| 1D-RECIST | 3.30 (1.05–10.36) | 0.0412 | 0.66 (0.106) |
| 2D-WHO | 2.45 (0.87–6.89) | 0.0897 | 0.64 (0.127) |
| 3D-EpSSG | 4.34 (1.54–12.26) | 0.0055 | 0.67 (0.074) |
| 3D-Osirix | 3.67 (1.30–18.38) | 0.0142 | 0.66 (0.072) |
| Method | Hazard Ratio * (95% CI) | p Value | Uno’s Concordance Statistic and Standard Error |
|---|---|---|---|
| Event-free survival | |||
| 1D-RECIST | 1.22 (1.05–1.42) | 0.0101 | 0.69 (0.126) |
| 2D-WHO | 1.17 (1.06–1.30) | 0.0031 | 0.70 (0.080) |
| 3D-EpSSG | 1.17 (1.07–1.29) | 0.0007 | 0.70 (0.092) |
| 3D-Osirix | 1.101 (1.03–1.18) | 0.0048 | 0.72 (0.065) |
| Overall survival | |||
| 1D-RECIST | 1.12 (0.97–1.28) | 0.1213 | 0.67 (0.15) |
| 2D-WHO | 1.11 (0.99–1.24) | 0.0684 | 0.66 (0.11) |
| 3D-EpSSG | 1.05 (0.962–1.15) | 0.2623 | 0.68 (0.13) |
| 3D-Osirix | 1.07 (0.98–1.15) | 0.1475 | 0.68 (0.11) |
| Method | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Event-free survival | ||
| 1D-RECIST | 3.57 (1.27–10.00) | 0.0158 |
| 2D-WHO | 5.05 (1.66–15.34) | 0.0042 |
| 3D-EpSSG | 14.40 (4.51–46.02) | <0.0001 |
| 3D-Osirix | 11.60 (3.61–37.29) | <0.0001 |
| Overall survival | ||
| 1D-RECIST | 3.74 (1.07–12.97) | 0.0375 |
| 2D-WHO | 2.77 (0.87–8.87) | 0.0849 |
| 3D-EpSSG | 7.90 (2.05–30.35) | 0.0026 |
| 3D-Osirix | 6.73 (1.74–26.00) | 0.0033 |
| Response CATEGORY | Measurement Method | |||
|---|---|---|---|---|
| 1D-Recist | 2D-WHO | 3D-EpSSG and 3D-Osirix | ||
| RESPONDERS | CR | 100% decrease | 100% decrease | 100% decrease |
| VGPR | NA | NA | ≥90% but <100% decrease | |
| PR | ≥30% but <100% decrease | ≥50% but <100% decrease | ≥66% but <90% decrease | |
| mPR | NA | NA | ≥33% but <66% decrease | |
| NON-RESPONDERS | SD | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD |
| PD | ≥20% increase | ≥25% increase | ≥40% increase | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsatti, G.; Morosi, C.; Giraudo, C.; Varotto, A.; Crimì, F.; Bonzini, M.; Minotti, M.; Frigo, A.C.; Zanetti, I.; Chiaravalli, S.; et al. Pediatric Rhabdomyosarcomas: Three-Dimensional Radiological Assessments after Induction Chemotherapy Predict Survival Better than One-Dimensional and Two-Dimensional Measurements. Cancers 2020, 12, 3808. https://doi.org/10.3390/cancers12123808
Orsatti G, Morosi C, Giraudo C, Varotto A, Crimì F, Bonzini M, Minotti M, Frigo AC, Zanetti I, Chiaravalli S, et al. Pediatric Rhabdomyosarcomas: Three-Dimensional Radiological Assessments after Induction Chemotherapy Predict Survival Better than One-Dimensional and Two-Dimensional Measurements. Cancers. 2020; 12(12):3808. https://doi.org/10.3390/cancers12123808
Chicago/Turabian StyleOrsatti, Giovanna, Carlo Morosi, Chiara Giraudo, Alessia Varotto, Filippo Crimì, Miriam Bonzini, Marta Minotti, Anna Chiara Frigo, Ilaria Zanetti, Stefano Chiaravalli, and et al. 2020. "Pediatric Rhabdomyosarcomas: Three-Dimensional Radiological Assessments after Induction Chemotherapy Predict Survival Better than One-Dimensional and Two-Dimensional Measurements" Cancers 12, no. 12: 3808. https://doi.org/10.3390/cancers12123808
APA StyleOrsatti, G., Morosi, C., Giraudo, C., Varotto, A., Crimì, F., Bonzini, M., Minotti, M., Frigo, A. C., Zanetti, I., Chiaravalli, S., Casanova, M., Ferrari, A., Bisogno, G., & Stramare, R. (2020). Pediatric Rhabdomyosarcomas: Three-Dimensional Radiological Assessments after Induction Chemotherapy Predict Survival Better than One-Dimensional and Two-Dimensional Measurements. Cancers, 12(12), 3808. https://doi.org/10.3390/cancers12123808






