Tumour Stroma Ratio Assessment Using Digital Image Analysis Predicts Survival in Triple Negative and Luminal Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Tumour Stroma Ratios (TSR)
Association of TSR with Clinico-Pathological Features
2.2. Survival Analyses
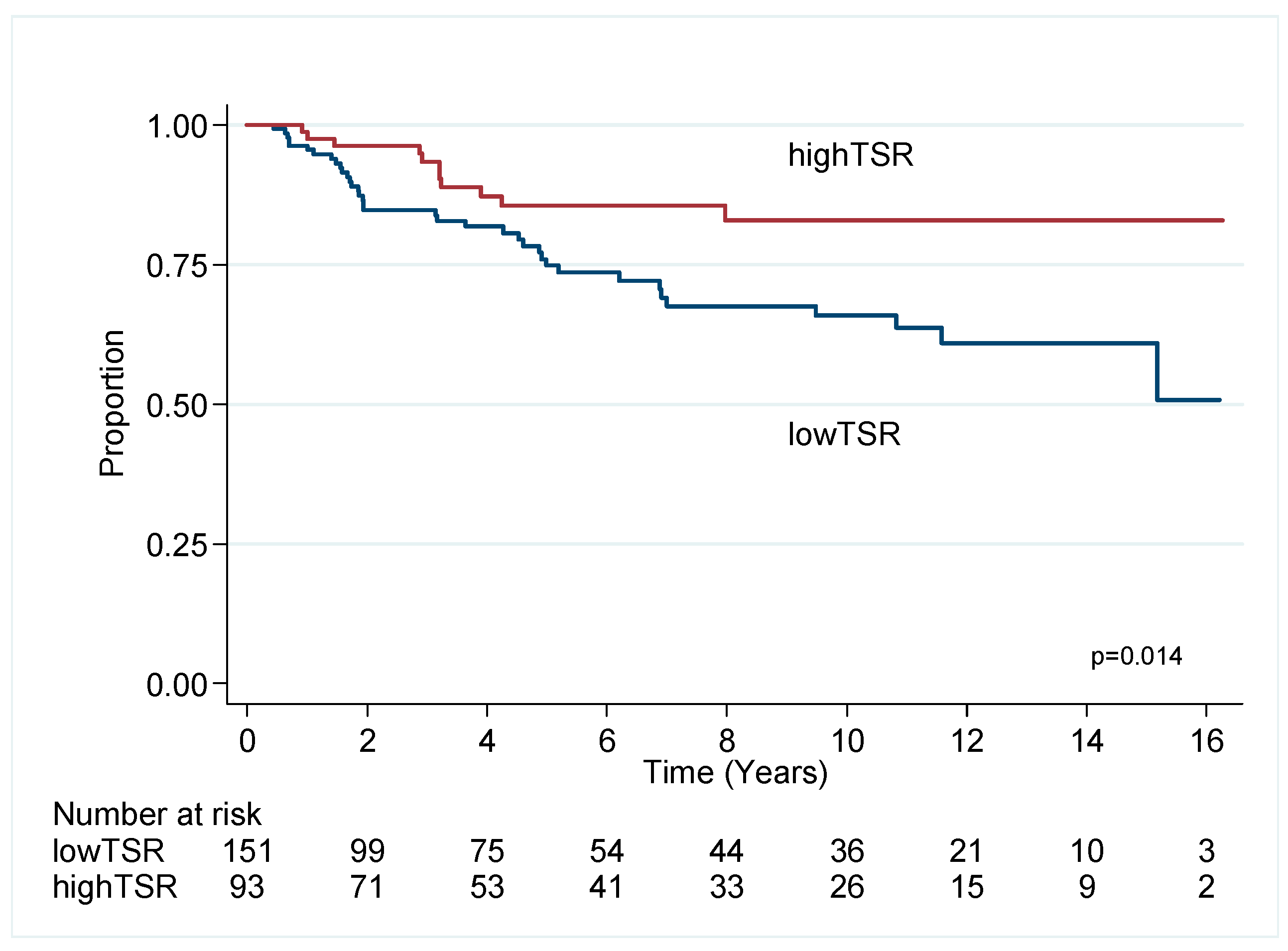
2.2.1. Triple Negative Breast Cancer
2.2.2. Combined TSR and TILs Impact on Outcome in TNBC
2.2.3. Chemotherapy and TSR in TNBC
2.3. Luminal Breast Cancer
Endocrine Therapy and TSR
3. Discussion
4. Materials and Methods
4.1. Clinical Cohorts
4.1.1. Luminal Cohort
4.1.2. TNBC Cohort
4.2. TMA Construction
4.3. Digital Scanning
Image Analysis
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Farmer, P.; Bonnefoi, H.; Anderle, P.; Cameron, D.; Wirapati, P.; Becette, V.; André, S.; Piccart, M.; Campone, M.; Brain, E.; et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat. Med. 2009, 15, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Plava, J.; Cihova, M.; Burikova, M.; Matuskova, M.; Kucerova, L.; Miklikova, S. Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol. Cancer 2019, 18, 67. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Brechbuhl, H.M.; Finlay-Schultz, J.; Yamamoto, T.M.; Gillen, A.E.; Cittelly, D.M.; Tan, A.C.; Sams, S.B.; Pillai, M.M.; Elias, A.D.; Robinson, W.A.; et al. Fibroblast Subtypes Regulate Responsiveness of Luminal Breast Cancer to Estrogen. Clin. Cancer Res. 2017, 23, 1710–1721. [Google Scholar] [CrossRef]
- Wu, J.; Liang, C.; Chen, M.; Su, W. Association between tumor-stroma ratio and prognosis in solid tumor patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 68954–68965. [Google Scholar] [CrossRef]
- Kramer, C.J.H.; Vangangelt, K.M.H.; van Pelt, G.W.; Dekker, T.J.A.; Tollenaar, R.; Mesker, W.E. The prognostic value of tumour-stroma ratio in primary breast cancer with special attention to triple-negative tumours: A review. Breast Cancer Res. Treat. 2019, 173, 55–64. [Google Scholar] [CrossRef]
- de Kruijf, E.M.; van Nes, J.G.; van de Velde, C.J.; Putter, H.; Smit, V.T.; Liefers, G.J.; Kuppen, P.J.; Tollenaar, R.A.; Mesker, W.E. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res. Treat. 2011, 125, 687–696. [Google Scholar] [CrossRef]
- Dekker, T.J.; van de Velde, C.J.; van Pelt, G.W.; Kroep, J.R.; Julien, J.P.; Smit, V.T.; Tollenaar, R.A.; Mesker, W.E. Prognostic significance of the tumor-stroma ratio: Validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res. Treat. 2013, 139, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Roeke, T.; Sobral-Leite, M.; Dekker, T.J.A.; Wesseling, J.; Smit, V.; Tollenaar, R.; Schmidt, M.K.; Mesker, W.E. The prognostic value of the tumour-stroma ratio in primary operable invasive cancer of the breast: A validation study. Breast Cancer Res. Treat. 2017, 166, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Downey, C.L.; Simpkins, S.A.; White, J.; Holliday, D.L.; Jones, J.L.; Jordan, L.B.; Kulka, J.; Pollock, S.; Rajan, S.S.; Thygesen, H.H.; et al. The prognostic significance of tumour-stroma ratio in oestrogen receptor-positive breast cancer. Br. J. Cancer 2014, 110, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. Embo J. 2020, 39, e104063. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.S.; Hui, M.N.; Elsworth, B.L.; Wu, S.Z.; Roden, D.; Chan, C.L.; Skhinas, J.N.; Collot, R.; Yang, J.; Harvey, K.; et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 2018, 9, 2897. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Sansone, P.; Berishaj, M.; Rajasekhar, V.K.; Ceccarelli, C.; Chang, Q.; Strillacci, A.; Savini, C.; Shapiro, L.; Bowman, R.L.; Mastroleo, C.; et al. Evolution of Cancer Stem-like Cells in Endocrine-Resistant Metastatic Breast Cancers Is Mediated by Stromal Microvesicles. Cancer Res. 2017, 77, 1927–1941. [Google Scholar] [CrossRef]
- Ruocco, M.R.; Avagliano, A.; Granato, G.; Imparato, V.; Masone, S.; Masullo, M.; Nasso, R.; Montagnani, S.; Arcucci, A. Involvement of Breast Cancer-Associated Fibroblasts in Tumor Development, Therapy Resistance and Evaluation of Potential Therapeutic Strategies. Curr. Med. Chem. 2018, 25, 3414–3434. [Google Scholar] [CrossRef]
- Sebastian, A.; Hum, N.R.; Martin, K.A.; Gilmore, S.F.; Peran, I.; Byers, S.W.; Wheeler, E.K.; Coleman, M.A.; Loots, G.G. Single-Cell Transcriptomic Analysis of Tumor-Derived Fibroblasts and Normal Tissue-Resident Fibroblasts Reveals Fibroblast Heterogeneity in Breast Cancer. Cancers 2020, 12, 1307. [Google Scholar] [CrossRef]
- Smit, M.; van Pelt, G.; Roodvoets, A.; Meershoek-Klein Kranenbarg, E.; Putter, H.; Tollenaar, R.; van Krieken, J.H.; Mesker, W. Uniform Noting for International Application of the Tumor-Stroma Ratio as an Easy Diagnostic Tool: Protocol for a Multicenter Prospective Cohort Study. JMIR Res. Protoc. 2019, 8, e13464. [Google Scholar] [CrossRef]
- Moorman, A.M.; Vink, R.; Heijmans, H.J.; van der Palen, J.; Kouwenhoven, E.A. The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur. J. Surg. Oncol. 2012, 38, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Vangangelt, K.M.H.; Green, A.R.; Heemskerk, I.M.F.; Cohen, D.; van Pelt, G.W.; Sobral-Leite, M.; Schmidt, M.K.; Putter, H.; Rakha, E.A.; Tollenaar, R.; et al. The prognostic value of the tumor-stroma ratio is most discriminative in patients with grade III or triple-negative breast cancer. Int. J. Cancer 2020, 146, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Downey, C.L.; Thygesen, H.H.; Sharma, N.; Shaaban, A.M. Prognostic significance of tumour stroma ratio in inflammatory breast cancer. Springerplus 2015, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Geessink, O.G.F.; Baidoshvili, A.; Klaase, J.M.; Ehteshami Bejnordi, B.; Litjens, G.J.S.; van Pelt, G.W.; Mesker, W.E.; Nagtegaal, I.D.; Ciompi, F.; van der Laak, J. Computer aided quantification of intratumoral stroma yields an independent prognosticator in rectal cancer. Cell. Oncol. (Dordr.) 2019, 42, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Park, J.H.; Jonas, S.F.; Bataillon, G.; Criscitiello, C.; Salgado, R.; Loi, S.; Viale, G.; Lee, H.J.; Dieci, M.V.; Kim, S.B.; et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann. Oncol. 2019, 30, 1941–1949. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Mesker, W.E.; Junggeburt, J.M.; Szuhai, K.; de Heer, P.; Morreau, H.; Tanke, H.J.; Tollenaar, R.A. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell. Oncol. 2007, 29, 387–398. [Google Scholar] [CrossRef]
- Gujam, F.J.; Edwards, J.; Mohammed, Z.M.; Going, J.J.; McMillan, D.C. The relationship between the tumour stroma percentage, clinicopathological characteristics and outcome in patients with operable ductal breast cancer. Br. J. Cancer 2014, 111, 157–165. [Google Scholar] [CrossRef]
- Vangangelt, K.M.H.; Tollenaar, L.S.A.; van Pelt, G.W.; de Kruijf, E.M.; Dekker, T.J.A.; Kuppen, P.J.K.; Tollenaar, R.; Mesker, W.E. The prognostic value of tumor-stroma ratio in tumor-positive axillary lymph nodes of breast cancer patients. Int. J. Cancer 2018, 143, 3194–3200. [Google Scholar] [CrossRef]
- Saltz, J.; Gupta, R.; Hou, L.; Kurc, T.; Singh, P.; Nguyen, V.; Samaras, D.; Shroyer, K.R.; Zhao, T.; Batiste, R.; et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep. 2018, 23, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Dennison, J.B.; Shahmoradgoli, M.; Liu, W.; Ju, Z.; Meric-Bernstam, F.; Perou, C.M.; Sahin, A.A.; Welm, A.; Oesterreich, S.; Sikora, M.J.; et al. High Intratumoral Stromal Content Defines Reactive Breast Cancer as a Low-risk Breast Cancer Subtype. Clin. Cancer Res. 2016, 22, 5068–5078. [Google Scholar] [CrossRef] [PubMed]
- Vangangelt, K.M.H.; van Pelt, G.W.; Engels, C.C.; Putter, H.; Liefers, G.J.; Smit, V.; Tollenaar, R.; Kuppen, P.J.K.; Mesker, W.E. Prognostic value of tumor-stroma ratio combined with the immune status of tumors in invasive breast carcinoma. Breast Cancer Res. Treat. 2018, 168, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Braman, N.; Prasanna, P.; Whitney, J.; Singh, S.; Beig, N.; Etesami, M.; Bates, D.D.B.; Gallagher, K.; Bloch, B.N.; Vulchi, M.; et al. Association of Peritumoral Radiomics with Tumor Biology and Pathologic Response to Preoperative Targeted Therapy for HER2 (ERBB2)-Positive Breast Cancer. JAMA Netw. Open 2019, 2, e192561. [Google Scholar] [CrossRef] [PubMed]
- Dilorenzo, G.; Telegrafo, M.; La Forgia, D.; Stabile Ianora, A.A.; Moschetta, M. Breast MRI background parenchymal enhancement as an imaging bridge to molecular cancer sub-type. Eur. J. Radiol. 2019, 113, 148–152. [Google Scholar] [CrossRef] [PubMed]
- La Forgia, D.; Fanizzi, A.; Campobasso, F.; Bellotti, R.; Didonna, V.; Lorusso, V.; Moschetta, M.; Massafra, R.; Tamborra, P.; Tangaro, S.; et al. Radiomic Analysis in Contrast-Enhanced Spectral Mammography for Predicting Breast Cancer Histological Outcome. Diagnostics 2020, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lövrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Single-cell analysis reveals diverse stromal subsets associated with immune evasion in triple-negative breast cancer. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sherratt, M.J.; McConnell, J.C.; Streuli, C.H. Raised mammographic density: Causative mechanisms and biological consequences. Breast Cancer Res. 2016, 18, 45. [Google Scholar] [CrossRef]
- Bredfeldt, J.S.; Liu, Y.; Conklin, M.W.; Keely, P.J.; Mackie, T.R.; Eliceiri, K.W. Automated quantification of aligned collagen for human breast carcinoma prognosis. J. Pathol. Inform. 2014, 5, 28. [Google Scholar] [CrossRef]
- Li, H.; Bera, K.; Gilmore, H.; Davidson, N.E.; Goldstein, L.J.; Madabhushi, A. Abstract P5-06-16: Histomorphometric measure of disorder of collagen fiber orientation is associated with risk of recurrence in ER+ breast cancers in ECOG-ACRIN E2197 and TCGA-BRCA. Cancer Res. 2020, 80, P5-06-16. [Google Scholar] [CrossRef]
- Beretov, J.; Wasinger, V.C.; Graham, P.H.; Millar, E.K.; Kearsley, J.H.; Li, Y. Proteomics for breast cancer urine biomarkers. Adv. Clin. Chem. 2014, 63, 123–167. [Google Scholar] [CrossRef] [PubMed]
- Millar, E.; Browne, L.; Slapetova, I.; Shang, F.; Ren, Y.; Bradshaw, R.; Ann Brauer, H.; O’Toole, S.; Beretov, J.; Whan, R.; et al. TILs Immunophenotype in Breast Cancer Predicts Local Failure and Overall Survival: Analysis in a Large Radiotherapy Trial with Long-Term Follow-Up. Cancers 2020, 12, 2365. [Google Scholar] [CrossRef] [PubMed]
- Millar, E.K.; Graham, P.H.; McNeil, C.M.; Browne, L.; O’Toole, S.A.; Boulghourjian, A.; Kearsley, J.H.; Papadatos, G.; Delaney, G.; Fox, C.; et al. Prediction of outcome of early ER+ breast cancer is improved using a biomarker panel, which includes Ki-67 and p53. Br. J. Cancer 2011, 105, 272–280. [Google Scholar] [CrossRef]
- Millar, E.K.; Graham, P.H.; O’Toole, S.A.; McNeil, C.M.; Browne, L.; Morey, A.L.; Eggleton, S.; Beretov, J.; Theocharous, C.; Capp, A.; et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J. Clin. Oncol. 2009, 27, 4701–4708. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting recommendations for tumor marker prognostic studies. J. Clin. Oncol. 2005, 23, 9067–9072. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]




| Overall Survival | Univariate | Multivariable (n = 238, Events n = 66) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | n | HR | 95%CI | p | HR | 95%CI | p | |
| TSR | ≤2 vs. >2 | 151 vs. 93 | 1.54 | 0.93–2.55 | 0.093 | 1.90 | 1.10–3.29 | 0.021 |
| TILs | ≤30 vs. >30 | 133 vs. 111 | 1.66 | 1.02–2.70 | 0.040 | |||
| Age | ≤55 vs. >55 | 105 vs. 138 | 0.48 | 0.29–0.80 | 0.004 | 0.41 | 0.24–0.70 | 0.001 |
| Size | ≤20 vs. >20 | 113 vs. 129 | 0.54 | 0.33–0.89 | 0.016 | 0.51 | 0.30–0.87 | 0.014 |
| Grade | 1,2 vs. 3 | 12 vs. 232 | 1.46 | 0.63–3.38 | 0.379 | |||
| LN | neg vs. pos | 156 vs. 85 | 0.43 | 0.27–0.70 | 0.001 | 0.46 | 0.28–0.76 | 0.003 |
| Chemo | yes vs. no | 174 vs. 58 | 0.49 | 0.30–0.81 | 0.006 | |||
| Breast Cancer Specific Survival | Univariate | Multivariable (n = 238, Events n = 46) | ||||||
| Variables | n | HR | 95%CI | p | HR | 95%CI | p | |
| TSR | ≤2 vs. >2 | 151 vs. 93 | 2.34 | 1.19–4.59 | 0.014 | 2.64 | 1.31–5.35 | 0.007 |
| TILs | ≤30 vs. >30 | 133 vs. 111 | 1.89 | 1.03–3.44 | 0.038 | |||
| Age | ≤55 vs. >55 | 105 vs. 138 | 0.50 | 0.27–0.91 | 0.022 | 0.43 | 0.23–0.80 | 0.008 |
| Size | ≤20 vs. >20 | 113 vs. 129 | 0.45 | 0.24–0.85 | 0.013 | 0.46 | 0.24–0.89 | 0.020 |
| Grade | 1,2 vs. 3 | 12 vs. 232 | 1.48 | 0.53–4.14 | 0.453 | |||
| LN | neg vs. pos | 156 vs. 85 | 0.31 | 0.17–0.55 | <0.001 | 0.32 | 0.18–0.59 | <0.001 |
| Chemo | yes vs. no | 174 vs. 58 | 0.69 | 0.37–1.30 | 0.253 | |||
| Overall Survival | Univariate | Multivariable (n = 402, Events n = 149) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | n | HR | 95%CI | p | HR | 95%CI | p | |
| TSR | ≤0.74 vs. >0.74 | 202 vs. 201 | 0.65 | 0.47–0.90 | 0.010 | 0.56 | 0.40–0.77 | 0.001 |
| TILs | ≤10 vs. >10 | 325 vs. 78 | 1.71 | 1.06–2.77 | 0.029 | 1.72 | 1.06–2.81 | 0.030 |
| Age | ≤55 vs. >55 | 139 vs. 264 | 0.34 | 0.22–0.52 | <0.001 | 0.31 | 0.20–0.48 | <0.001 |
| Size | ≤20 vs. >20 | 294 vs. 108 | 0.70 | 0.50–0.99 | 0.046 | 0.66 | 0.47–0.94 | 0.021 |
| Grade | 1,2 vs. 3 | 321 vs. 80 | 0.98 | 0.65–1.47 | 0.926 | |||
| LN | neg vs. pos | 282 vs. 121 | 0.71 | 0.51–0.99 | 0.043 | |||
| Chemo | yes vs. no | 63 vs. 340 | 0.69 | 0.42–1.15 | 0.153 | |||
| Horm | yes vs. no | 206 vs. 196 | 1.21 | 0.87–1.67 | 0.257 | |||
| Breast Cancer Specific Survival | Univariate | Multivariable (n = 400, Events n = 39) | ||||||
| Variables | n | HR | 95%CI | p | HR | 95%CI | p | |
| TSR | ≤0.74 vs. >0.74 | 202 vs. 201 | 0.39 | 0.20–0.78 | 0.007 | |||
| TILs | ≤10 vs. >10 | 325 vs. 78 | 0.93 | 0.43–2.03 | 0.860 | |||
| Age | ≤55 vs. >55 | 139 s 264 | 1.66 | 0.89–3.12 | 0.113 | |||
| Size | ≤20 vs. >20 | 294 vs. 108 | 0.29 | 0.16–0.55 | <0.001 | 0.45 | 0.23–0.87 | 0.021 |
| Grade | 1,2 vs. 3 | 321 vs. 80 | 0.30 | 0.16–0.57 | <0.001 | 0.38 | 0.20–0.72 | 0.003 |
| LN | neg vs. pos | 282 vs. 121 | 0.20 | 0.11–0.40 | <0.001 | 0.19 | 0.09–0.41 | <0.001 |
| Chemo | yes vs. no | 63 vs. 340 | 3.46 | 1.82–6.60 | <0.001 | |||
| Horm | yes vs. no | 206 vs. 196 | 1.11 | 0.59–2.09 | 0.742 | 0.45 | 0.23–0.88 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millar, E.K.; Browne, L.H.; Beretov, J.; Lee, K.; Lynch, J.; Swarbrick, A.; Graham, P.H. Tumour Stroma Ratio Assessment Using Digital Image Analysis Predicts Survival in Triple Negative and Luminal Breast Cancer. Cancers 2020, 12, 3749. https://doi.org/10.3390/cancers12123749
Millar EK, Browne LH, Beretov J, Lee K, Lynch J, Swarbrick A, Graham PH. Tumour Stroma Ratio Assessment Using Digital Image Analysis Predicts Survival in Triple Negative and Luminal Breast Cancer. Cancers. 2020; 12(12):3749. https://doi.org/10.3390/cancers12123749
Chicago/Turabian StyleMillar, Ewan KA, Lois H. Browne, Julia Beretov, Kirsty Lee, Jodi Lynch, Alexander Swarbrick, and Peter H. Graham. 2020. "Tumour Stroma Ratio Assessment Using Digital Image Analysis Predicts Survival in Triple Negative and Luminal Breast Cancer" Cancers 12, no. 12: 3749. https://doi.org/10.3390/cancers12123749
APA StyleMillar, E. K., Browne, L. H., Beretov, J., Lee, K., Lynch, J., Swarbrick, A., & Graham, P. H. (2020). Tumour Stroma Ratio Assessment Using Digital Image Analysis Predicts Survival in Triple Negative and Luminal Breast Cancer. Cancers, 12(12), 3749. https://doi.org/10.3390/cancers12123749






