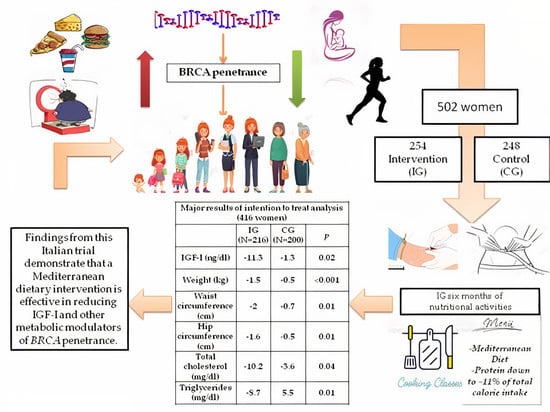
A Mediterranean Dietary Intervention in Female Carriers of BRCA Mutations: Results from an Italian Prospective Randomized Controlled Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
3. Discussion
4. Patients and Methods
4.1. Study and Subjects
4.2. Data Collection and Measurements
- to complete a questionnaire on their medical history and major cancer risk factors (reproductive and behavioral factors);
- to attend a clinic for anthropometric measurements. Height and body weight were collected without shoes and heavy clothes. Waist circumference was measured with a professional measuring tape at natural waist or, if not identifiable, at the midpoint between the iliac crest and the lower rib according to standard techniques. Hip circumference was measured at the level of the greater throcanter. Blood pressure was measured using an electronic sphygmomanometer;
- to give a 20-mL blood sample for metabolic/hormonal assays;
- to provide information on their health and to allow the study officials to contact their usual physicians, to consult clinical notes and to examine biopsy material, as necessary;
- to fill in a 24-h food frequency diary of each previous day’s food intake (65 food items) and the validated 14-point Mediterranean Diet Adherence Screener [27]. The 24-h food frequency diary contained a list of 65 food items. The diary did not include information on portion size or weight, nor on recipes. Women had to indicate only whether, the previous day, they had or not eaten the specified food at breakfast, lunch, dinner and breaks. MEDAS [27] consists of 14 questions on food consumption frequency and two on eating habits.
4.3. Dietary Intervention
- reduce the overall consumption of animal food in order to lower protein intake to ~11% of total calorie intake. Animal milk, associated with higher plasma levels of IGF-I [29], was markedly limited. The use of plant-based unsweetened milk and creams was encouraged. Among animal food, fish, especially cold-water fish (e.g., salmon and mackerel), rich in omega-3 poly-unsaturated fatty acids, was privileged. The lower safe limit of protein intake for lean adults is about 0.6 g of protein per kg body weight; to ensure a 25% margin of safety, the current recommended lower limit of protein intake has been set at 0.75 g/kg body weight [30,31]. Our goal was ~0.9–1.1 g/kg body weight. IG women received the menus supplied during cookery classes, recipes and handouts with illustrations of the food portions, especially of food with high protein content;
- markedly reduce the consumption of refined foods, such as sugar, sugary drinks, refined food, potatoes and sweets, that promote pro-inflammatory cytokines. The consumption of whole-grain food was encouraged in order to attenuate the postprandial glucose response and to acutely improve insulin homeostasis [32]. Desserts were prepared without adding sugars, using dried fruits. Nuts and legume flours were proposed to prepare sweets and for savory cookery recipes;
- help participants distinguish between the different types of fat in foods (saturated, trans-, mono- and poly-unsaturated). Processed and red meat (rich in saturated fats), but also some food of vegetable origin, such as margarine (which contains trans-fatty acids), promote inflammation and needed to be substantially reduced. Cold-pressed extra-virgin olive oil was the main source of fat;
- encourage an improvement of dietary habits, as defined by macro- and micronutrient needs.
- Nutritional requirements, menus and recipes were standardized among study centers.
4.4. Laboratory Methods
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Project Administration
Acknowledgments
Conflicts of Interest
References
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, Å.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrino, J.; Berrino, F.; Francisci, S.; Peissel, B.; Azzollini, J.; Pensotti, V.; Radice, P.; Pasanisi, P.; Manoukian, S. Estimate of the penetrance of BRCA mutation and the COS software for the assessment of BRCA mutation probability. Fam.Cancer 2015, 14, 117–1128. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, F.; Evans, D.G. Familial breast cancer. Clin. Genet. 2012, 82, 105–114. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; Van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of breast, ovarian, and contralateral breast cancer for brca1 and brca2 mutation carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkondjock, A.; Ghadirian, P. Epidemiology of breast cancer among BRCA mutation carriers: An overview. Cancer Lett. 2004, 205, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pettapiece-Phillips, R.; Narod, S.A.; Kotsopoulos, J. The role of body size and physical activity on the risk of breast cancer in BRCA mutation carriers. Cancer Causes Control. 2015, 26, 333–344. [Google Scholar] [CrossRef]
- Bordeleau, L.; Lipscombe, L.; Lubinski, J.; Ghadirian, P.; Foulkes, W.D.; Neuhausen, S.; Ainsworth, P.; Pollak, M.; Sun, P.; Narod, S.A.; et al. Diabetes and breast cancer among women with BRCA1 and BRCA2 mutations. Cancer 2011, 117, 1812–1818. [Google Scholar] [CrossRef]
- Dumais, V.; Lumingu, J.; Bedard, M.; Paquet, L.; Verma, S.; Fontaine-Bisson, B. Prevalence of insulin resistance, metabolic syndrome, and type 2 diabetes in Canadian women at high risk for breast cancer. Breast J. 2017, 23, 482–483. [Google Scholar] [CrossRef]
- Pasanisi, P.; Bruno, E.; Venturelli, E.; Manoukian, S.; Barile, M.; Peissel, B.; De Giacomi, C.; Bonanni, B.; Berrino, J.; Berrino, F. Serum levels of IGF-I and BRCA penetrance: A case control study in breast cancer families. Fam. Cancer. 2011, 10, 521–538. [Google Scholar] [CrossRef]
- Bruno, E.; Manoukian, S.; Venturelli, E.; Oliverio, A.; Rovera, F.; Iula, G.; Morelli, D.; Peissel, B.; Azzolini, J.; Roveda, E.; et al. Adherence to mediterranean diet and metabolic syndrome in BRCA mutation carriers. Integr. Cancer Ther. 2018, 17, 153–160. [Google Scholar] [CrossRef]
- Daniele, A.; Paradiso, A.V.; Divella, R.; Digennaro, M.; Patruno, M.; Tommasi, S.; Pilato, B.; Tufaro, A.; Barone, M.; Minoia, C.; et al. The role of circulating adiponectin and SNP276G>T at ADIPOQ Gene in BRCA-mutant women. Cancer Genom. Proteom. 2020, 17, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Pasanisi, P.; Bruno, E.; Manoukian, S.; Berrino, F. A randomized controlled trial of diet and physical activity in BRCA mutation carriers. Fam.Cancer 2014, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Pasanisi, P.; Bruno, E.; Venturelli EMorelli, D.; Oliverio, A.; Baldassari, I.; Rovera, F.; Iula, G.; Taborelli, M.; Peissel, B.; Azzollini, J.; et al. A dietary intervention to lower serum levels of IGF-I in BRCA mutation carriers. Cancers (Basel) 2018, 10, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, E.; Oliverio, A.; Paradiso, A.; Daniele, A.; Tommasi, S.; Terribile, D.A.; Filippone, A.; Digennaro, M.; Pilato, B.; Danza, K.; et al. Lifestyle characteristics in BRCA-mutant women: Results from an Italian trial cohort. Clin. Breast Cancer 2020. [Google Scholar] [CrossRef]
- Bissonauth, V.; Shatenstein, B.; Fafard, E.; Maugard, C.; Robidoux, A.; Narod, S.; Ghadirian, P. Weight history, smoking, physical activity and breast cancer risk among French-Canadian women non-carriers of more frequent BRCA1/2 mutations. J. Cancer Epidemiol. 2009, 2009, 748367. [Google Scholar] [CrossRef] [Green Version]
- Pasanisi, P.; Berrino, J.; Fusconi, E.; Curtosi, P.; Berrino, F. European case-only study (COS) on familial breast cancer. J. Nutr. 2005, 135, S3040–S30411. [Google Scholar]
- World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition, Physical activity and the Prevention of Cancer: A Global Perspective; AICR: Washington DC, USA, 2007. [Google Scholar]
- Berrino, F.; Bellati, C.; Secreto, G.; Amerini, E.; Pala, V.; Panico, S.; Allegro, G.; Kaaks, R. Reducing bioavailable sex hormones through a comprehensive change in diet: The diet and androgens (DIANA) randomized trial. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 25–33. [Google Scholar]
- Fontana, L.; Villareal, D.T.; Das, S.K.; Smith, S.R.; Meydani, S.N.; Pittas, A.G.; Klein, S.; Bhapkar, M.; Rochon, J.; Ravussin, E.; et al. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: A randomized clinical trial. Aging Cell 2016, 15, 22–27. [Google Scholar] [CrossRef]
- Kaaks, R.; Bellati, C.; Venturelli, E.; Rinaldi, S.; Secreto, G.; Biessy, C.; Pala, V.; Sieri, S.; Berrino, F. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: The diet and androgens (DIANA) randomised trial. Eur. J. Clin. Nutr. 2003, 57, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008, 7, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Kiechle, M.; Dukatz, R.; Yahiaoui-Doktor, M.; Berling, A.; Basrai, M.; Staiger, V.; Niederberger, U.; Marter, N.; Lammert, J.; Grill, S.; et al. Feasibility of structured endurance training and Mediterranean diet in BRCA1 and BRCA2 mutation carriers—An interventional randomized controlled multicenter trial (LIBRE-1). BMC Cancer. 2017, 17, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, B.; Basrai, M.; Vetter WLehnert, K.; Engel, C.; Siniatchkin, M.; Halle, M.; Kiechle, M.; Bischoff, S.C. Fatty acid profiles in erythrocyte membranes following the Mediterranean diet—data from a multicenter lifestyle intervention study in women with hereditary breast cancer (LIBRE). Clin. Nutr. 2020, 39, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Endogenous Hormones and Breast Cancer Collaborative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar] [CrossRef] [Green Version]
- Muti, P.; Quattrin, T.; Grant, B.J.; Krogh, V.; Micheli, A.; Schünemann, H.J.; Ram, M.; Freudenheim, J.L.; Sieri, S.; Trevisan, M. Fasting glucose is a risk factor for breast cancer: A prospective study. Cancer Epidemiol Biomark Prev. 2002, 11, 1361–1368. [Google Scholar]
- Thomson, C.A.; Giuliano, A.; Rock, C.L.; Ritenbaugh, C.K.; Flatt, S.W.; Faerber, S.; Newman, V.; Caan, B.; Graver, E.; Hartz, V.; et al. Measuring dietary change in a diet intervention trial: Comparing food frequency questionnaire and dietary recalls. Am. J. Epidemiol. 2003, 157, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.; Fito, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-SalvadóLamuela-Raventós, J.; Ros, E.; Salaverría, I.; Fiol, M. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Norat, T.; Dossus, L.; Rinaldi, S.; Overvad, K.; Grønbæk, H.; Tjønneland, A.; Olsen, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Boeing, H.; et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur. J. Clin. Nutr. 2007, 61, 91–98. [Google Scholar] [CrossRef]
- Millward, D.J. An adaptive metabolic demand model for protein and amino acid requirements. Br. J. Nutr. 2003, 90, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Young, V.R.; Borgonha, S. Nitrogen and amino acid requirements: The Massachusetts Institute of Technology amino acid requirement pattern. J. Nutr. 2000, 130, S1841–S1849. [Google Scholar] [CrossRef] [Green Version]
- Musa-Veloso, K.; Poon, T.; Harkness, L.S.; O’Shea, M.; Chu, Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018, 108, 759–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bland, J.M.; Altman, D.G. Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials 2011, 12, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Mean ± SD | IG (254) | CG (248) |
|---|---|---|
| Age (yrs) | 47.6 ± 10.8 | 46.0 ± 11.1 |
| Menarche (yrs) | 12.4 ± 1.4 | 12.4 ± 1.6 |
| Age at first live birth (yrs) | 29.0 ± 5.9 | 28.9 ± 4.7 |
| Age at first diagnosis (yrs) (if affected) | 44.1 ± 8.4 | 43.2 ± 8.8 |
| Time from cancer diagnosis (yrs) (if affected) | 6.4 ± 7.4 | 6.4± 6.8 |
| Education (%) | ||
| First level | 16.9 | 16.9 |
| Second level | 44.1 | 43.6 |
| Third level | 39 | 39.5 |
| Pregnancy (%) | ||
| Yes | 71.3 | 70.8 |
| Number of children | ||
| ≤2 | 85.1 | 84.9 |
| ≥3 | 14.9 | 15.1 |
| Menopause (%) | 73.9 | 72.6 |
| Natural menopause (%) | 26.2 | 22.2 |
| Preventive salpingo-oophorectomy (%) | 25.6 | 22 |
| Oral contraceptive in the past (%) | 66.5 | 67.2 |
| Current smoking (%) | 12.1 | 13 |
| Physical Activity (%) | ||
| None | 36.3 | 36.2 |
| Moderate or Vigorous | 48 | 46.8 |
| Moderate and Vigorous | 15.7 | 17 |
| Mutated gene (%) | ||
| BRCA1 | 56.7 | 68.9 |
| BRCA2 | 43.3 | 31.1 |
| Cancer type if affected (%) | ||
| Breast | 81.4 | 81.1 |
| Breast and ovary | 5.8 | 2 |
| Ovary | 12.8 | 16.9 |
| Infiltrating duct BC (%) | 82.2 | 87.4 |
| ER-negative (%) | 44.6 | 41.2 |
| Axillary node metastasis (%) | 25.6 | 26.2 |
| Cancer hormonal treatment (%) | 46.8 | 50.4 |
| Current cancer hormonal treatment (%) | 26.9 | 25.7 |
| Variable | IG (216) | CG (200) | IG (216) | CG (200) | p ** | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean ± SD | Six Months Mean ± SD | p * | Baseline Mean ± SD | Six Months Mean ± SD | p * | Δ of Differences | Δ of Differences | ||
| Weight (Kg) | 62.1 ± 10.7 | 60.6 ± 10.8 | <0.001 | 65.6 ± 14.6 | 65.1 ± 14.5 | 0.01 | −1.5 | −0.5 | <0.001 |
| BMI (kg/m2) | 23.9 ± 4.4 | 23.3 ± 4.3 | <0.001 | 24.7 ± 5.1 | 24.5 ± 5.0 | 0.01 | −0.6 | −0.2 | <0.001 |
| Waist circ. (cm) | 77.1 ± 11.7 | 75.1 ± 10.2 | <0.001 | 79.0 ± 13.5 | 78.3 ± 13.0 | 0.02 | −2.0 | −0.7 | 0.01 |
| Hip circ. (cm) | 98.6 ± 9.2 | 97.0 ± 9.1 | <0.001 | 101.0 ± 10.4 | 100.4 ± 10.5 | 0.09 | −1.6 | −0.5 | 0.01 |
| Systolic press. (mmHg) | 125.9 ± 17.8 | 122.7 ± 14.3 | 0.002 | 124.9 ± 15.1 | 121.3 ± 14.1 | 0.003 | −3.2 | −3.6 | 0.53 |
| Diastolic press. (mmHg) | 82.0 ± 11.0 | 79.9 ±10.9 | 0.005 | 81.4 ± 10.3 | 79.5 ± 9.2 | 0.01 | −2.2 | −1.9 | 0.74 |
| Glycemia (mg/dL) | 101.2 ± 22.0 | 93.8 ± 18.3 | <0.001 | 101.4 ± 24.5 | 92.5 ± 19.5 | <0.001 | −7.4 | −8.8 | 0.51 |
| Total cholesterol (mg/dL) | 199.2 ± 38.3 | 189.0 ± 33.5 | <0.001 | 198.9 ± 37.8 | 195.3 ± 38.5 | 0.09 | −10.2 | −3.6 | 0.04 |
| HDL+ cholesterol (mg/dL) | 68.5 ± 16.0 | 66.6 ± 15.2 | 0.03 | 69.4 ± 18.0 | 69.7 ± 20.1 | 0.79 | −1.9 | +0.3 | 0.21 |
| LDL++ cholesterol (mg/dL) | 117.0 ± 35.6 | 111.4 ± 32.9 | <0.001 | 117.2 ± 35.0 | 112.1 ± 34.0 | 0.01 | −5.6 | −5.1 | 0.82 |
| Triglycerides (mg/dL) | 105.1 ± 71.0 | 96.4 ± 48.0 | 0.04 | 101.6 ± 57.7 | 107.1 ± 60.6 | 0.07 | −8.7 | +5.5 | 0.01 |
| IGF-I (ng/mL) | 178.9 ± 67.7 | 167.6 ± 72.0 | <0.001 | 173.1 ± 64.6 | 171.8 ± 62.4 | 0.67 | −11.3 | −1.3 | 0.02 |
| Insulin (µIU/mL) | 21.3 ± 18.7 | 13.5 ± 11.6 | <0.001 | 20.2± 16.9 | 14.7 ± 12.2 | <0.001 | −7.7 | −5.5 | 0.11 |
| Δ IGF-I | Δ Weight | Δ Animal Products | Δ Legumes | Δ Refined Products |
|---|---|---|---|---|
| 1st tertile (≥9.7 ng/mL) | 1 | 1 | 1 | 1 |
| 2nd tertile (9.6 to −22.2 ng/mL) | 1.01 (0.93–1.10) | 1.18 * (1.0–1.36) | 0.89 (0.64–1.24) | 1.12 (0.95–1.34) |
| 3rd tertile (> −22.3 ng/mL) | 1.04 (0.95–1.14) | 1.21 * (1.04–1.41) | 0.97 (0.70–1.35) | 1.05 (0.88–1.26) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, E.; Oliverio, A.; Paradiso, A.V.; Daniele, A.; Tommasi, S.; Tufaro, A.; Terribile, D.A.; Magno, S.; Filippone, A.; Venturelli, E.; et al. A Mediterranean Dietary Intervention in Female Carriers of BRCA Mutations: Results from an Italian Prospective Randomized Controlled Trial. Cancers 2020, 12, 3732. https://doi.org/10.3390/cancers12123732
Bruno E, Oliverio A, Paradiso AV, Daniele A, Tommasi S, Tufaro A, Terribile DA, Magno S, Filippone A, Venturelli E, et al. A Mediterranean Dietary Intervention in Female Carriers of BRCA Mutations: Results from an Italian Prospective Randomized Controlled Trial. Cancers. 2020; 12(12):3732. https://doi.org/10.3390/cancers12123732
Chicago/Turabian StyleBruno, Eleonora, Andreina Oliverio, Angelo Virgilio Paradiso, Antonella Daniele, Stefania Tommasi, Antonio Tufaro, Daniela Andreina Terribile, Stefano Magno, Alessio Filippone, Elisabetta Venturelli, and et al. 2020. "A Mediterranean Dietary Intervention in Female Carriers of BRCA Mutations: Results from an Italian Prospective Randomized Controlled Trial" Cancers 12, no. 12: 3732. https://doi.org/10.3390/cancers12123732
APA StyleBruno, E., Oliverio, A., Paradiso, A. V., Daniele, A., Tommasi, S., Tufaro, A., Terribile, D. A., Magno, S., Filippone, A., Venturelli, E., Morelli, D., Baldassari, I., Cravana, M. L., Manoukian, S., & Pasanisi, P. (2020). A Mediterranean Dietary Intervention in Female Carriers of BRCA Mutations: Results from an Italian Prospective Randomized Controlled Trial. Cancers, 12(12), 3732. https://doi.org/10.3390/cancers12123732