Outcome of Patients with NSCLC and Brain Metastases Treated with Immune Checkpoint Inhibitors in a ‘Real-Life’ Setting
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
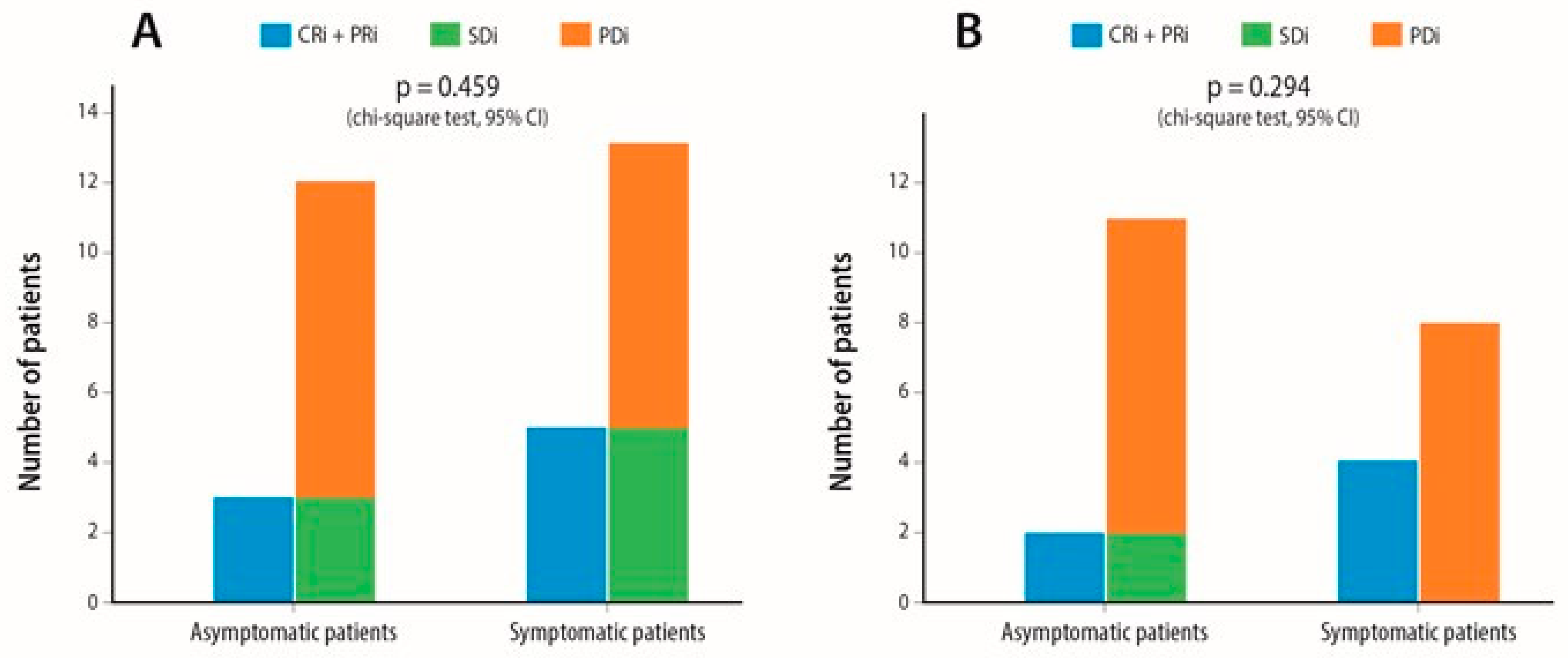
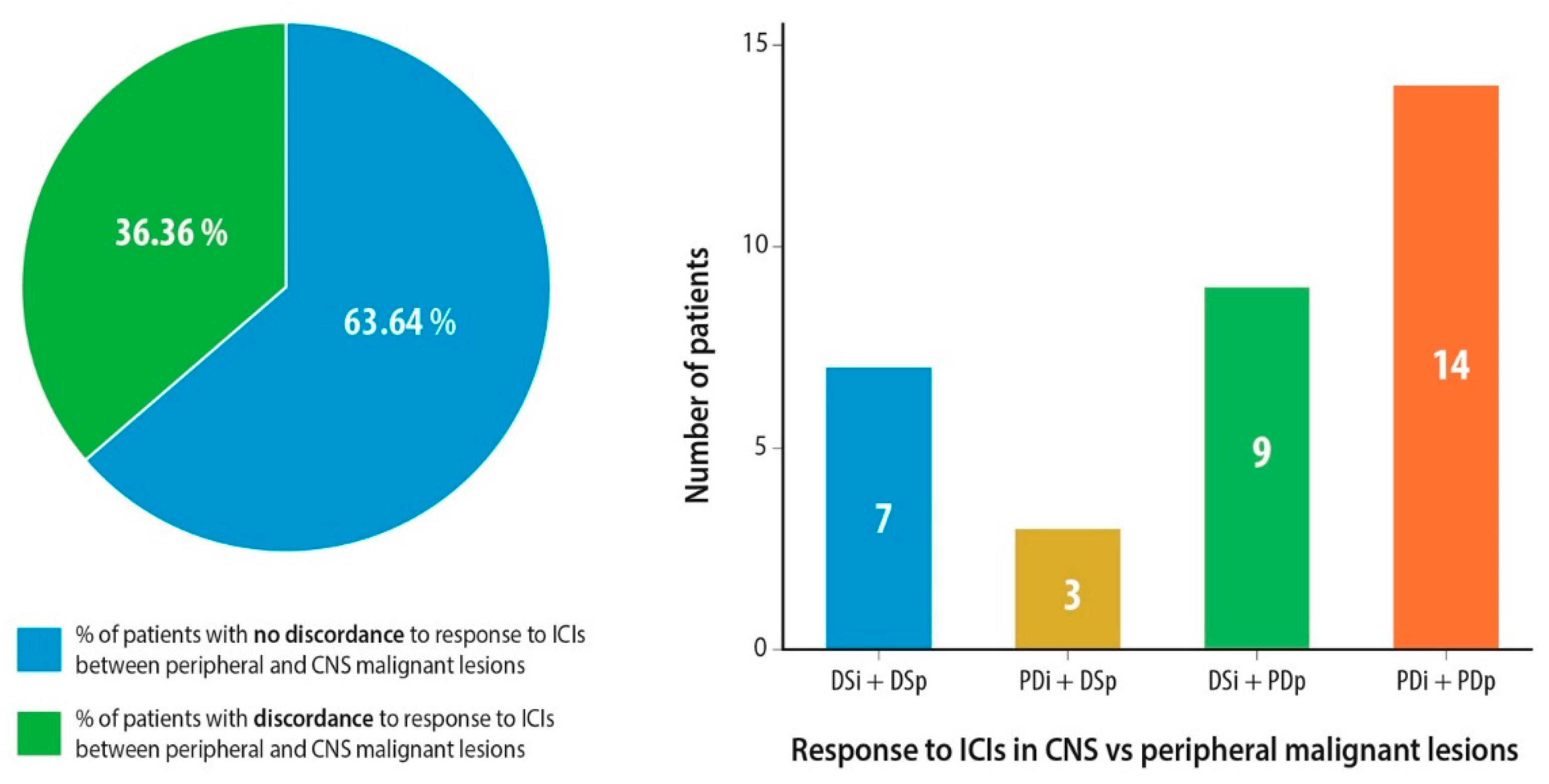
2.2. Response Assessment
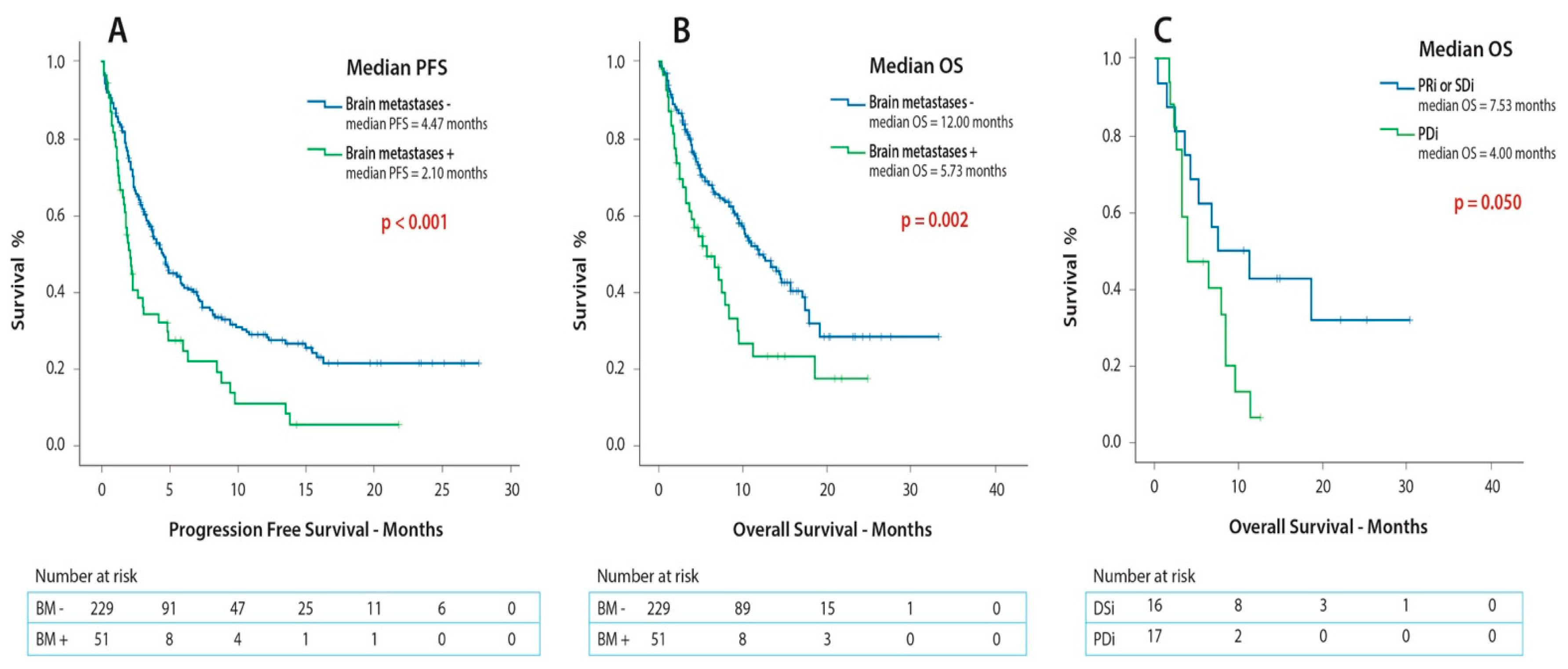
2.3. Survival Outcomes
3. Discussion
4. Methods
4.1. Patient Population/Study Design
4.2. Outcome Assessment
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, C.J.; Mehta, M.P. Current management of brain metastases, with a focus on systemic options. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.N.; Samson, P.P.; Robinson, C.G.; Bradley, J.; Devarakonda, S.; Du, L.; Govindan, R.; Gao, F.; Puri, V.; Morgensztern, D. Non-small-cell Lung Cancer With Brain Metastasis at Presentation. Clin. Lung Cancer 2018, 19, e373–e379. [Google Scholar] [CrossRef]
- Kalkanis, S.N.; Kondziolka, D.; Gaspar, L.E.; Burri, S.H.; Asher, A.L.; Cobbs, C.S.; Ammirati, M.; Robinson, P.D.; Andrews, D.W.; Loeffler, J.S.; et al. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010, 96, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahgal, A.; Aoyama, H.; Kocher, M.; Neupane, B.; Collette, S.; Tago, M.; Shaw, P.; Beyene, J.; Chang, E.L. Phase 3 Trials of Stereotactic Radiosurgery with or Without Whole-Brain Radiation Therapy for 1 to 4 Brain Metastases: Individual Patient Data Meta-Analysis. Int. J. Radiat. Oncol. 2015, 91, 710–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulvenna, P.; Nankivell, M.; Barton, R.; Faivre-Finn, C.; Wilson, P.; McColl, E.; Moore, B.; Brisbane, I.; Ardron, D.; Holt, T.; et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet Lond. Engl. 2016, 388, 2004–2014. [Google Scholar] [CrossRef] [Green Version]
- Tsakonas, G.; De Petris, L.; Ekman, S. Management of brain metastasized non-small cell lung cancer (NSCLC)—From local treatment to new systemic therapies. Cancer Treat. Rev. 2017, 54, 122–131. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet Lond. Engl. 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef]
- Moro-Sibilot, D.; Smit, E.; de Castro Carpeño, J.; Lesniewski-Kmak, K.; Aerts, J.G.; Villatoro, R.; Kraaij, K.; Nacerddine, K.; Dyachkova, Y.; Smith, K.T.; et al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: Analysis from the European FRAME study. Lung Cancer Amst. Neth. 2015, 90, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet Lond. Engl. 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet Lond. Engl. 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Kudo, Y.; Haymaker, C.; Zhang, J.; Reuben, A.; Duose, D.Y.; Fujimoto, J.; Roy-Chowdhuri, S.; Solis Soto, L.M.; Dejima, H.; Parra, E.R.; et al. Suppressed immune microenvironment and repertoire in brain metastases from patients with resected non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Tsakonas, G.; Lewensohn, R.; Botling, J.; Ortiz-Villalon, C.; Micke, P.; Friesland, S.; Nord, H.; Lindskog, M.; Sandelin, M.; Hydbring, P.; et al. An immune gene expression signature distinguishes central nervous system metastases from primary tumours in non-small-cell lung cancer. Eur. J. Cancer 2020, 132, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Svokos, K.A.; Salhia, B.; Toms, S.A. Molecular biology of brain metastasis. Int. J. Mol. Sci. 2014, 15, 9519–9530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, I.; Muranski, P.; Enose-Akahata, Y.; Ha, S.-K.; Smith, B.; Monaco, M.; Ryschkewitsch, C.; Major, E.O.; Ohayon, J.; Schindler, M.K.; et al. Pembrolizumab Treatment for Progressive Multifocal Leukoencephalopathy. N. Engl. J. Med. 2019, 380, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 52–60. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, S.B.; Schalper, K.A.; Gettinger, S.N.; Mahajan, A.; Herbst, R.S.; Chiang, A.C.; Lilenbaum, R.; Wilson, F.H.; Omay, S.B.; Yu, J.B.; et al. Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 655–663. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Henon, C.; Auclin, E.; Mezquita, L.; Ferrara, R.; Audigier-Valette, C.; Mazieres, J.; Lefebvre, C.; Rabeau, A.; Le Moulec, S.; et al. Outcome of Patients with Non-Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 1244–1254. [Google Scholar] [CrossRef]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Hyperprogressive Disease in Patients with Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Total Patients with Brain Metastases (n = 51) | Total Patient Population (n = 280) | |
|---|---|---|---|
| Age (years) | Median | 69 | 69.8 |
| Range | 40–84 | 35–84 | |
| Gender (male) | 54.9% | 55.7% | |
| Performance status 0–1 | 74.5% | 83.5% | |
| Smoking status (former or active) | 86.3% | 92.8% | |
| Histology (non-squamous) | 84.3% | 67% | |
| PD-L1 expression extracranially | <1% | 7.8% | 10% |
| 1%≤PD-L1<50% | 25.5% | 28.9% | |
| PD-L1≥50% | 58.5% | 34.6% | |
| No data | 19.6% | 26% | |
| Line of treatment of ICI administration (first line) | 19.6% | 21% | |
| Types of ICI Therapy Administered | Atezolizumab | 3.9% | |
| Nivolumab | 51.0% | ||
| Pembrolizumab | 45.1% | ||
| Mean number of ICI cycles | 6.2 (range: 1–42) | ||
| Primary CNS metastases at diagnosis | 60.8% | ||
| Neurological symptoms associated with CNS metastases | 43.1% | ||
| Radiological evaluation method of CNS metastases (MRI) | 60.8% | ||
| Size of largest CNS metastasis (mm) | Median | 17 | |
| Range | 4–50 | ||
| > 3 CNS metastases | 33.3% | ||
| High disease burden * | 31.4% | ||
| Steroid administration > 10 mg for ≥10 days | 54.1% | ||
| Active brain metastases | 84.2% | ||
| Previous local CNS treatment modality | WBRT | 33.3% | |
| Surgical excision | 5.9% | ||
| SRS | 29.4% | ||
| Surgical excision + SRS | 2.0% | ||
| WBRT + SRS | 2.0% | ||
| No local treatment | 27.5% | ||
| Timeline of local CNS treatment modality administration | ≥3 months before ICI administration | 33.3% | |
| <3 months before or after ICI administration | 39.2% | ||
| No local treatment | 27.4% | ||
| Range | 0.4–22.9 | ||
| Patient Outcomes | Total (n = 33) | Total (n = 51) | 95% Confidence Intervals | |
|---|---|---|---|---|
| Intracranial response rates to ICIs | CRi | 3.0% | ||
| PRi | 21.2% | |||
| SDi | 24.2% | |||
| PDi | 51.5% | |||
| Intracranial response rates to ICIs in patients with active brain metastases | CRi | 4.0% | ||
| PRi | 20.0% | |||
| SDi | 8.0% | |||
| PDi | 68.0% | |||
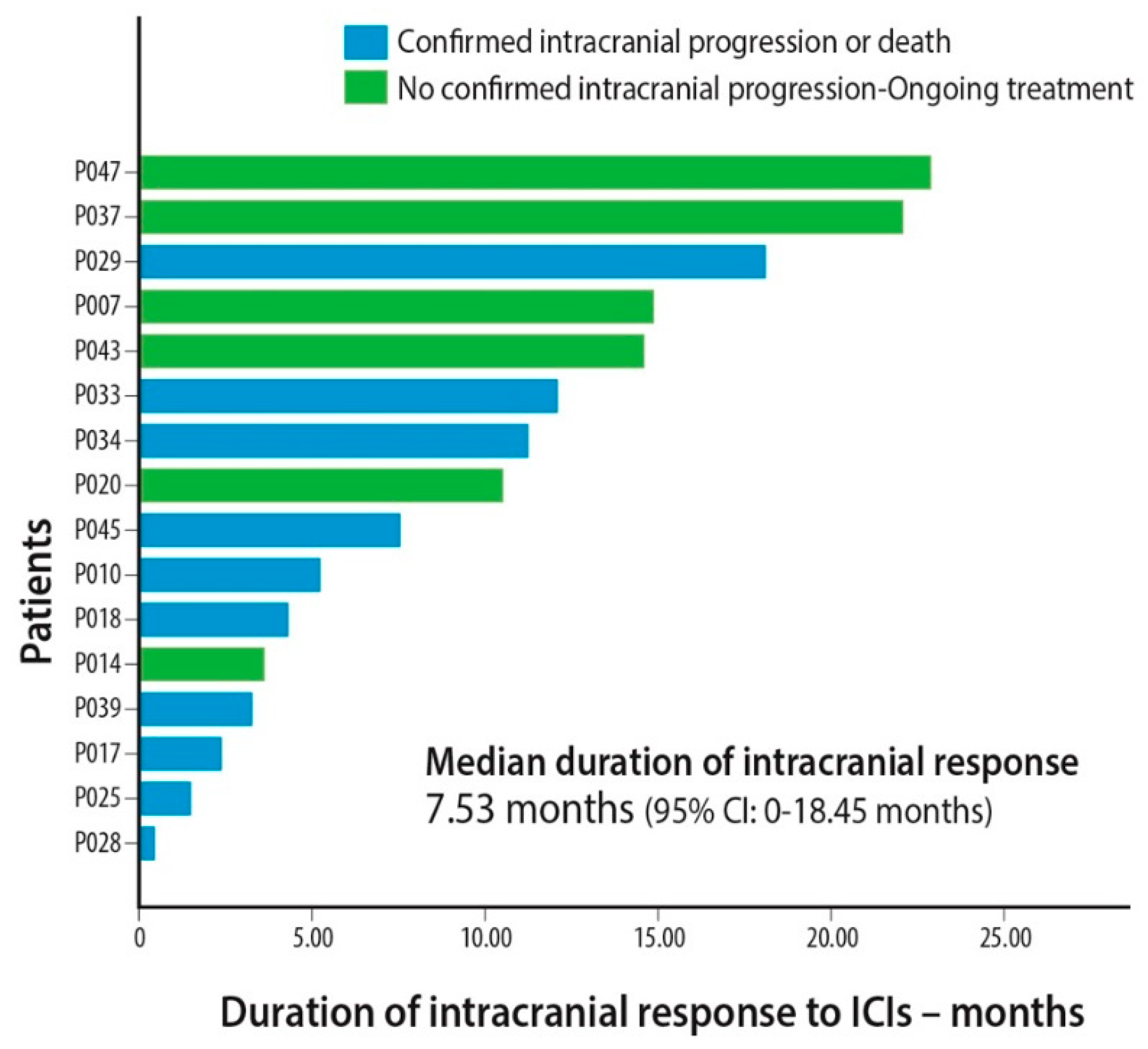
| Duration of intracranial response (months) (patients that achieved PRi or SDi) | Median | 7.5 | 0.0–18.5 | |
| Range | 0.5–22.9 | |||
| Disease progression | 88.2% | |||
| Death | 78.4% | |||
| Time to intracranial progression (months) | Median | 2.5 | 1.5–3.6 | |
| Range | 0.2–22.9 | |||
| PFS (months) | Median | 1.9 | 1.5–2.3 | |
| Range | 0.1–22.1 | |||
| OS (months) | Median | 5.7 | 2.6–8.9 | |
| Range | 0.4–30.3 | |||
| Follow-up (months, median) | 5.7 | 2.2–9.3 | ||
| COX REGRESSION | PFS | OS | ||
|---|---|---|---|---|
| Univariate Analysis | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age > 70 years old | 0.858 (0.648–1.136) | 0.285 | 0.794 (0.574–1.099) | 0.164 |
| Performance status = 2 | 1.517 (1.064–2.163) | 0.021 | 2.132 (1.445–3.144) | <0.001 |
| Non-squamous histology | 0.999 (0.747–1.337) | 0.997 | 1.065 (0.765–1.484) | 0.708 |
| ICI administration as 2nd or subsequent line of therapy | 2.284 (1.519–3.436) | < 0.001 | 2.840 (1.665–4.846) | <0.001 |
| Brain metastases | 1.838 (1.314–2.569) | < 0.001 | 1.810 (1.230–2.662) | 0.003 |
| High disease burden * | 1.746 (1.256–2.427) | 0.001 | 1.961 (1.363–2.821) | <0.001 |
| PD-L1 < 50% | 2.083 (1.464–2.958) | < 0.001 | 1.915 (1.269–2.898) | 0.002 |
| Multivariate Analysis | ||||
| Performance status = 2 | 1.377 (0.882–2.149) | 0.159 | 2.106 (1.269–3.497) | 0.004 |
| ICI administration as 2nd or subsequent line of therapy | 1.672 (1.022–2.736) | 0.041 | 2.898 (1.638–5.125) | <0.001 |
| Brain metastases | 2.265 (1.529–3.356) | < 0.001 | 1.577 (0.965–2.597) | 0.069 |
| High disease burden * | 1.447 (0.909–2.303) | 0.119 | 2.219 (1.380–3.579) | 0.001 |
| PD-L1 < 50% | 1.709 (1.184–2.645) | 0.005 | 1.390 (0.861–2.242) | 0.178 |
| Variable | OVERALL SURVIVAL | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (CI 95%) | p-Value | HR (CI 95%) | p-Value | |
| Age > 70 years old | 1.723 (0.913–3.252) | 0.093 | ||
| Performance status = 2 | 1.460 (0.739–2.883) | 0.276 | ||
| Non-adenocarcinoma histology | 2.150 (0.937–4.931) | 0.071 | ||
| Second or subsequent line of ICI administration | 1.628 (0.680–3.898) | 0.274 | ||
| High disease burden *1 | 2.390 (1.161–4.922) | 0.018 | 2.390 (1.161–4.922) | 0.018 |
| Non-primary CNS metastases *2 | 1.436 (0.760–2.711) | 0.265 | ||
| PD-L1 extracranially ≥ 50% | 1.021 (0.506–2.058) | 0.954 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skribek, M.; Rounis, K.; Makrakis, D.; Agelaki, S.; Mavroudis, D.; De Petris, L.; Ekman, S.; Tsakonas, G. Outcome of Patients with NSCLC and Brain Metastases Treated with Immune Checkpoint Inhibitors in a ‘Real-Life’ Setting. Cancers 2020, 12, 3707. https://doi.org/10.3390/cancers12123707
Skribek M, Rounis K, Makrakis D, Agelaki S, Mavroudis D, De Petris L, Ekman S, Tsakonas G. Outcome of Patients with NSCLC and Brain Metastases Treated with Immune Checkpoint Inhibitors in a ‘Real-Life’ Setting. Cancers. 2020; 12(12):3707. https://doi.org/10.3390/cancers12123707
Chicago/Turabian StyleSkribek, Marcus, Konstantinos Rounis, Dimitrios Makrakis, Sofia Agelaki, Dimitris Mavroudis, Luigi De Petris, Simon Ekman, and Georgios Tsakonas. 2020. "Outcome of Patients with NSCLC and Brain Metastases Treated with Immune Checkpoint Inhibitors in a ‘Real-Life’ Setting" Cancers 12, no. 12: 3707. https://doi.org/10.3390/cancers12123707
APA StyleSkribek, M., Rounis, K., Makrakis, D., Agelaki, S., Mavroudis, D., De Petris, L., Ekman, S., & Tsakonas, G. (2020). Outcome of Patients with NSCLC and Brain Metastases Treated with Immune Checkpoint Inhibitors in a ‘Real-Life’ Setting. Cancers, 12(12), 3707. https://doi.org/10.3390/cancers12123707








