The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of a Nationwide Observational Study (PROSa)
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- How is the HRQoL of sarcoma patients in Germany? How high is the percentage of patients with clinically important limitations and symptoms in the individual domains of HRQoL?
- (2)
- Which factors are associated with selected HRQoL domains? Are there differences between sarcoma subtypes with respect to histology and location?
2. Results
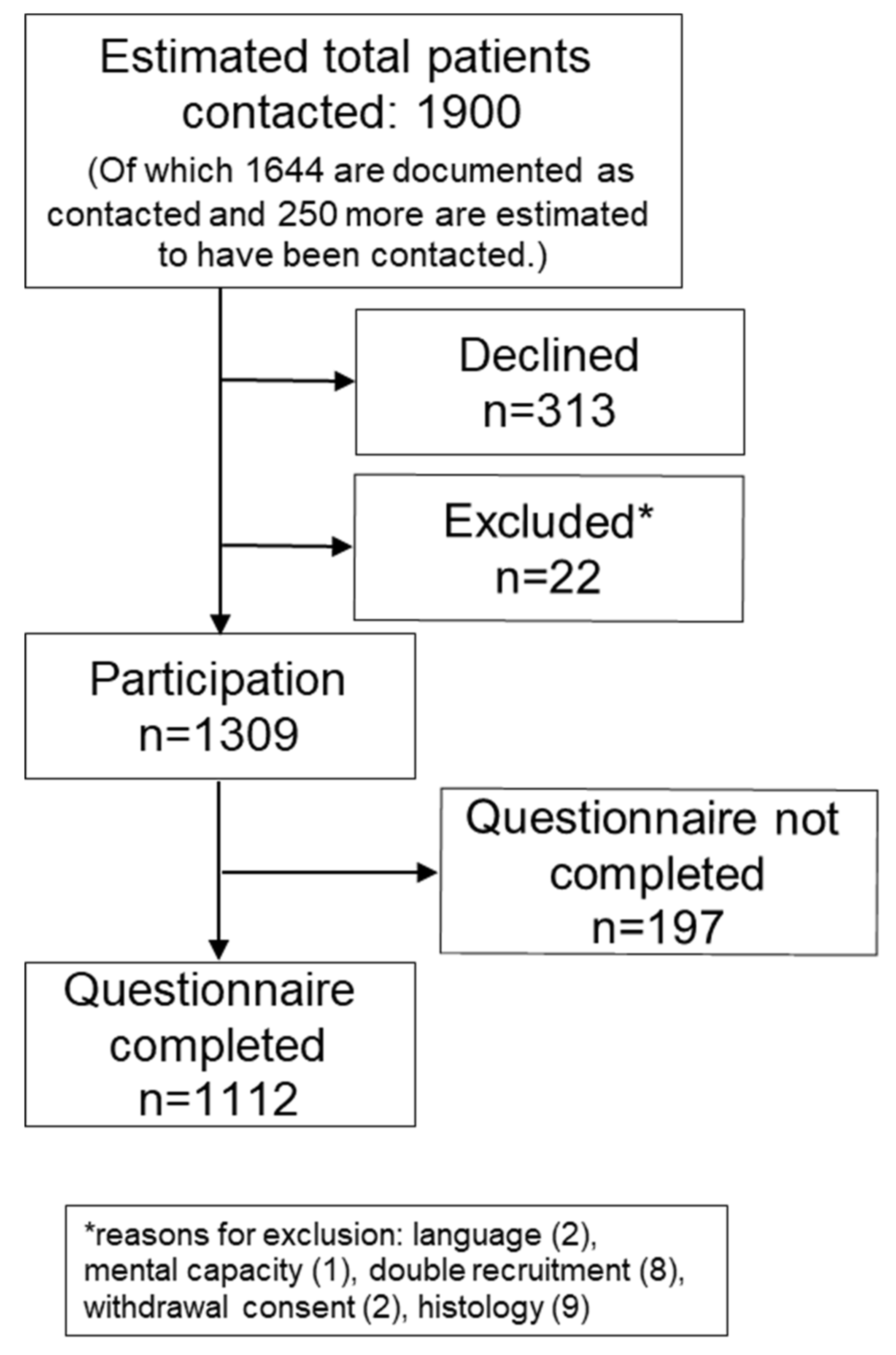
2.1. Participation and Sample Description
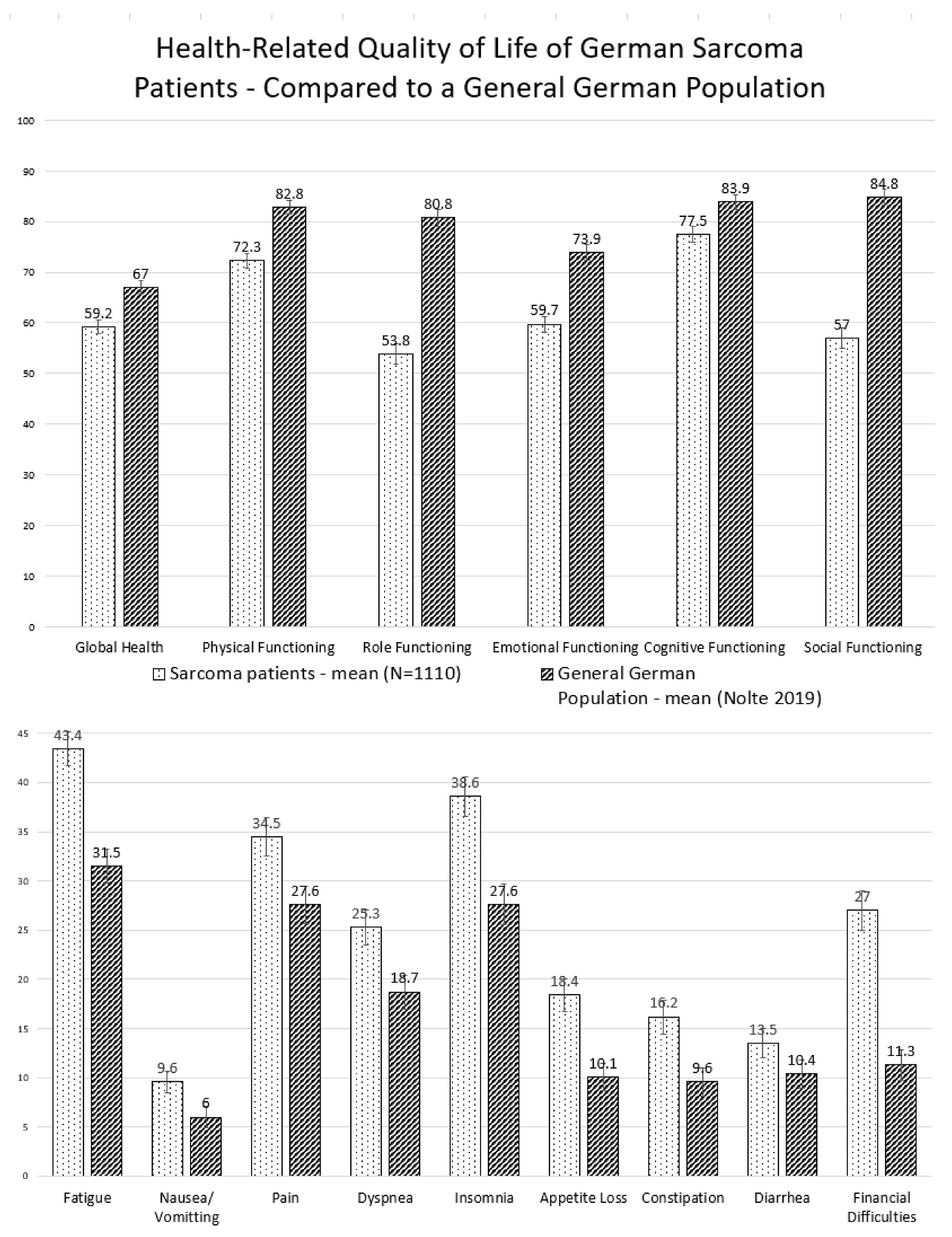
2.2. Health-Related Quality of Life
2.3. Clinically Important Restrictions and Symptoms
2.4. Stratified Analyses
2.5. Associated Factors in Multivariable Regression
2.5.1. Socio-Demographics
2.5.2. Tumor Sites
2.5.3. Sarcoma Types
2.5.4. Tumor-Related Factors
2.5.5. Disease and Treatment Status
2.5.6. Treatments Received
3. Discussion
3.1. Results in Context
3.2. Strengths and Limitations
4. Patients and Methods
4.1. Variables
4.2. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ressing, M.; Wardelmann, E.; Hohenberger, P.; Jakob, J.; Kasper, B.; Emrich, K.; Eberle, A.; Blettner, M.; Zeissig, S.R. Strengthening health data on a rare and heterogeneous disease: Sarcoma incidence and histological subtypes in Germany. BMC Public Health 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Stiller, C.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.; Casali, P. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.M. World Health Organization. International Agency for Research on Cancer (Hrsg.): WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC Press: Lyon, France, 2013. [Google Scholar]
- Casali, P.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; Broto, J.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.S.; Chow, W.; Reed, D.R.; Lucas, D.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Budd, G.T.; Curry, W.T.; et al. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 155–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, T.; Blay, J.-Y.; Hirota, S.; Kitagawa, Y.; Kang, Y.-K. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016, 19, 3–14. [Google Scholar] [CrossRef]
- Neuwirth, M.G.; Song, Y.; Sinnamon, A.J.; Fraker, D.L.; Zager, J.S.; Karakousis, G.C. Isolated Limb Perfusion and Infusion for Extremity Soft Tissue Sarcoma: A Contemporary Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2017, 24, 3803–3810. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Vujaskovic, Z. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients with Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483. [Google Scholar] [CrossRef]
- Soomers, V.L.M.N.; Husson, O.; Young, R.; Desar, I.; Van Der Graaf, W. The sarcoma diagnostic interval: A systematic review on length, contributing factors and patient outcomes. ESMO Open 2020, 5, e000592. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Kim, H.-S.; Han, I. Unplanned Excision of Extremity Soft Tissue Sarcoma in Korea: A Nationwide Study Based on a Claims Registry. Duan Z (Hrsg.). PLoS ONE 2015, 10, e0134354. [Google Scholar]
- Traub, F.; Griffin, A.M.; Wunder, J.S.; Ferguson, P.C. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma: Outcome of Unplanned Excisions in STS. Cancer 2018, 124, 3868–3875. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.krebsgesellschaft.de/zertkomm-protokolle.html?file=files/dkg/deutsche-krebsgesellschaft/content/pdf/Zertifizierung/Protokolle_Zertkomm/SAR_Protokoll_190225.pdf&cid=61328 (accessed on 30 November 2020).
- Dangoor, A.; Seddon, B.; Gerrand, C.; Grimer, R.J.; Whelan, J.S.; Judson, I. UK guidelines for the management of soft tissue sarcomas. Clin. Sarcoma Res. 2016, 6, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.sarkome.de/ (accessed on 30 November 2020).
- Available online: https://euracan.ern-net.eu (accessed on 30 November 2020).
- Meropol, N.J.; Egleston, B.L.; Buzaglo, J.S.; Benson, A.B.; Cegala, D.J.; Diefenbach, M.A.; Fleisher, L.; Miller, S.M.; Sulmasy, D.P.; Weinfurt, K.P.; et al. Cancer patient preferences for quality and length of life. Cancer 2008, 113, 3459–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higginson, I.J.; Gomes, B.; Calanzani, N.; Gao, W.; Bausewein, C.; A Daveson, B.; Deliens, L.; Ferreira, P.L.; Toscani, F.; Gysels, M.; et al. Priorities for treatment, care and information if faced with serious illness: A comparative population-based survey in seven European countries. Palliat. Med. 2014, 28, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winnette, R.; Hess, L.M.; Nicol, S.J.; Tai, D.F. Copley-Merriman C: The Patient Experience with Soft Tissue Sarcoma: A Systematic Review of the Literature. Patient Patient-Cent. Outcomes Res. 2016, 10, 153–162. [Google Scholar] [CrossRef]
- McDonough, J.; Eliott, J.; Neuhaus, S.J.; Reid, J.; Butow, P. Health-related quality of life, psychosocial functioning, and unmet health needs in patients with sarcoma: A systematic review. Psycho Oncol. 2019, 28, 653–664. [Google Scholar] [CrossRef]
- Coens, C.; Van Der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Judson, I.; Sanfilippo, R.; Lugowska, I. Health-related quality-of-life results from PALETTE: A randomized, double-blind, phase 3 trial of pazopanib versus placebo in patients with soft tissue sarcoma whose disease has progressed during or after prior chemotherapy-a European Organization for res: HRQoL of Pazopanib in Pretreated STS. Cancer 2015, 121, 2933–2941. [Google Scholar]
- Poveda, A.; López-Pousa, A.; Martín, J.; Del Muro, J.G.; Bernabé, R.; Casado, A.; Balañá, C.; Sanmartín, O.; Menéndez, M.D.; Escudero, P.; et al. Phase II Clinical Trial with Pegylated Liposomal Doxorubicin (CAELYX®/Doxil®) and Quality of Life Evaluation (EORTC QLQ-C30) in Adult Patients With Advanced Soft Tissue Sarcomas: A study of the Spanish Group for Research in Sarcomas (GEIS). Sarcoma 2005, 9, 127–132. [Google Scholar] [CrossRef]
- Schreiber, D.; Bell, R.S.; Wunder, J.S.; O’Sullivan, B.; Turcotte, R.; Masri, B.A.; Davis, A.M. Evaluating Function and Health Related Quality of Life in Patients Treated for Extremity Soft Tissue Sarcoma. Qual. Life Res. 2006, 15, 1439–1446. [Google Scholar] [CrossRef]
- Kwong, T.N.K.; Furtado, S.; Gerrand, C. What do we know about survivorship after treatment for extremity sarcoma? A systematic review. Eur. J. Surg. Oncol. 2014, 40, 1109–1124. [Google Scholar] [CrossRef]
- Saebye, C.; Fugloe, H.M.; Nymark, T.; Safwat, A.; Petersen, M.M.; Baad-Hansen, T.; Krarup-Hansen, A.; Keller, J. Factors associated with reduced functional outcome and quality of life in patients having limb-sparing surgery for soft tissue sarcomas–A national multicenter study of 128 patients. Acta Oncol. 2017, 56, 239–244. [Google Scholar] [CrossRef]
- Gough, N.J.; Smith, C.; Ross, J.R.; Riley, J.; Judson, I. Symptom Burden, Survival and Palliative Care in Advanced Soft Tissue Sarcoma. Sarcoma 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, N.; Koffman, J.; Ross, J.R.; Riley, J.; Judson, I. Symptom Burden in Advanced Soft-Tissue Sarcoma. J. Pain Symptom Manag. 2017, 53, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichardt, P.; Leahy, M.; Del Muro, X.G.; Ferrari, S.; Martin, J.; Gelderblom, H.; Wang, J.; Krishna, A.; Eriksson, J.; Staddon, A.; et al. Quality of Life and Utility in Patients with Metastatic Soft Tissue and Bone Sarcoma: The Sarcoma Treatment and Burden of Illness in North America and Europe (SABINE) Study. Sarcoma 2012, 2012, 740279. [Google Scholar] [CrossRef]
- Chan, A.; Lim, E.; Ng, T.; Shih, V.; Quek, R.; Cheung, Y.T. Symptom burden and medication use in adult sarcoma patients. Support. Care Cancer 2015, 23, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, I.; Hollander, D.D.; Desar, I.M.; Soomers, V.L.; Van De Sande, M.A.; De Haan, J.J.; Verhoef, C.; Vriens, I.J.; Bonenkamp, J.J.; Van Der Graaf, W.T.; et al. Unraveling the Heterogeneity of Sarcoma Survivors’ Health-Related Quality of Life Regarding Primary Sarcoma Location: Results from the SURVSARC Study. Cancers 2020, 12, 3083. [Google Scholar] [CrossRef]
- Sachsenmaier, S.; Ipach, I.; Kluba, T. Quality of Life, Physical and Mental Status and Contentment of Patients with Localized Soft Tissue or Bone Sarcoma: A Questionnaire Analysis. Orthop. Rev. 2015, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Van Der Geest, I.; Knoop, H.; Veth, R.P.; Schreuder, H.W.; Bleijenberg, G. High fatigue scores before and after surgical treatment of bone and soft tissue tumors. Exp. Ther. Med. 2013, 5, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Trautmann, F.; Singer, S.; Schmitt, J. Patients with soft tissue sarcoma comprise a higher probability of comorbidities than cancer-free individuals. A secondary data analysis. Eur. J. Cancer Care 2017, 26, e12605. [Google Scholar] [CrossRef]
- Schwarz, R.; Hinz, A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur. J. Cancer 2001, 37, 1345–1351. [Google Scholar] [CrossRef]
- Hechtner, M.; Eichler, M.; Wehler, B.; Buhl, R.; Sebastian, M.; Stratmann, J.; Schmidberger, H.; Gohrbandt, B.; Peuser, J.; Kortsik, C.; et al. Quality of Life in NSCLC Survivors—A Multicenter Cross-Sectional Study. J. Thorac. Oncol. 2019, 14, 420–435. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewski, K.A.; Püsküllüoğlu, M.; Biesiada, K.; Bochenek, J.; Ochenduszko, S.; Tomaszewska, I.M.; Krzemieniecki, K. Main influencing factors and health-related quality of life issues in patients with oesophago-gastric cancer–As measured by EORTC tools. Współczesna Onkol. 2013, 3, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husson, O.; Hollander, D.D.; Van Der Graaf, W.T.A. The complexity of assessing health-related quality of life among sarcoma patients. Qual. Life Res. 2020, 29, 2613–2614. [Google Scholar] [CrossRef] [PubMed]
- Gough, N.; Koffman, J.; Ross, J.R.; Riley, J.; Judson, I. Does palliative chemotherapy really palliate and are we measuring it correctly? A mixed methods longitudinal study of health related quality of life in advanced soft tissue sarcoma. PLoS ONE 2019, 14, e0210731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichler, M.; Schmitt, J.; Schuler, M.K. Die Dauer von Ethikvoten in Deutschland—Am Beispiel einer nicht-interventionellen Beobachtungsstudie mit 44 teilnehmenden Zentren (PROSa). Z. Evidenz Fortbild. Qual. Gesundh. 2019, 146, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Lampert, T.; Kroll, L.E.; Müters, S.; Stolzenberg, H. Messung des sozioökonomischen Status in der Studie Gesundheit in Deutschland aktuell (GEDA). Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Nolte, S.; Liegl, G.; Petersen, M.; Aaronson, N.K.; Costantini, A.; Fayers, P.; Groenvold, M.; Holzner, B.; Johnson, C.; Kemmler, G.; et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur. J. Cancer 2019, 107, 153–163. [Google Scholar] [CrossRef]
- Cocks, K.; King, M.T.; Velikova, G.; St-James, M.M.; Fayers, P.M.; Brown, J.M. Evidence-Based Guidelines for Determination of Sample Size and Interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J. Clin. Oncol. 2011, 29, 89–96. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Giesinger, J.; Loth, F.L.; Aaronson, N.K.; Arraras, J.I.; Caocci, G.; Efficace, F.; Groenvold, M.; Van Leeuwen, M.; Petersen, M.A.; Ramage, J.; et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J. Clin. Epidemiol. 2020, 118, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Textor, J.; Hardt, J.; Knüppel, S. DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology 2011, 22, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Value | N | % | Missing (Out of 1113) |
|---|---|---|---|---|
| Sex * | female | 541 | 48.7 | 2 |
| Age */** at study entry (mean: 52.6; SD: 16.3) | 18–<40 | 185 | 16.6 | 1 |
| ≥40–<55 | 264 | 23.7 | ||
| ≥55–<65 | 297 | 26.7 | ||
| ≥65–<75 | 233 | 21.0 | ||
| ≥75 | 133 | 12.0 | ||
| Time since diagnosis * (median: 2.3; IQR: 0.7–5.8) | 0–<0.5 years | 213 | 19.2 | 3 |
| ≥0.5–<1 year | 126 | 11.4 | ||
| ≥1–<2 years | 165 | 14.9 | ||
| ≥2–<5 years | 293 | 26.4 | ||
| ≥5 years | 313 | 28.2 | ||
| Socio-economic status (Winkler-Index) */**/*** | low (3.0–7.9) | 113 | 10.6 | 44 |
| medium (8.0–13.8) | 526 | 49.2 | ||
| high (13.9–21) | 430 | 40.2 | ||
| Early retirement/ old age pension * | yes | 369 | 33.2 | 0 |
| Sarcoma Types | soft tissue sarcoma | 782 | 70.5 | 4 |
| bone sarcoma | 197 | 17.8 | ||
| GIST | 130 | 11.7 | ||
| Sarcoma Types * | undifferentiated/unclassified | 165 | 14.9 | 4 **** |
| fibro-/myofibroblastic/fibrohistiocytic | 130 | 11.7 | ||
| liposarcoma | 211 | 19 | ||
| leiomyosarcoma | 132 | 11.9 | ||
| osteosarcoma | 71 | 6.4 | ||
| chondrosarcoma | 64 | 5.8 | ||
| synovial sarcoma | 48 | 4.3 | ||
| Ewing sarcoma | 45 | 4.1 | ||
| GIST | 130 | 11.7 | ||
| all other **** | 113 | 11.2 | ||
| Tumor site * | abdomen/retroperitoneum | 300 | 27.0 | |
| thorax | 90 | 8.1 | ||
| pelvis/urogenital | 162 | 14.6 | ||
| lower limbs | 402 | 36.1 | ||
| upper limbs | 85 | 7.6 | ||
| head & neck | 35 | 3.1 | ||
| back/spine | 30 | 2.7 | ||
| other/unknown | 9 | 0.8 | ||
| Grading * | low grade (G1) | 138 | 13.2 | 64 **** |
| high grade (G2, G3) | 603 | 57.5 | ||
| not applicable ***** | 308 | 29.3 | ||
| T stage */**** | T1 | 172 | 21.2 | 310 **** |
| T2-T4 | 518 | 63.7 | ||
| Tx**** | 13 | 1.6 | ||
| Tumor without classification **** | 110 | 13.5 | ||
| Aggressiveness of tumor * | locally aggressive + rarely metastatic | 87 | 7.8 | 4 |
| malignant | 1022 | 92.2 | ||
| Metastasis at study inclusion * | yes | 369 | 37.8 | 138 |
| Tumor recurrence * | no | 801 | 71.4 | 20 **** |
| yes | 280 | 25.6 | ||
| suspicion **** | 12 | 1.1 | ||
| Treatment intention at study inclusion | palliative | 261 | 24.0 | 26 |
| Comorbidities */** | 0 | 548 | 49.2 | |
| 1 | 363 | 32.6 | ||
| 2 | 152 | 13.7 | ||
| 3 | 39 | 3.5 | ||
| ≥4 | 11 | 1.0 | ||
| Disease status * | complete remission | 492 | 50.1 | 130 |
| partial remission + stable disease | 330 | 33.5 | ||
| progress | 161 | 16.4 | ||
| Treatment status * | in treatment | 369 | 33.4 | 7 |
| Received treatment—surgery | yes | 975 | 88.3 | 9 |
| Received treatment—chemotherapy | yes | 527 | 47.9 | 13 |
| Received treatment—radiotherapy | yes | 431 | 39.8 | 29 |
| Received treatment—TKI | TKI − all | 170 | 15.9 | 45 |
| TKI + surgery | 144 | 11.4 | ||
| Combined treatments * | surgery alone | 356 | 32.2 | 6 **** |
| OP + CT | 222 | 20.1 | ||
| OP + RT | 174 | 15.7 | ||
| OP + CT + RT | 222 | 20.1 | ||
| CT alone | 56 | 5.1 | ||
| RT alone **** | 9 | 0.8 | ||
| CT + RT **** | 26 | 2.3 | ||
| no therapy (yet) **** | 27 | 2.4 | ||
| other therapies **** | 15 | 1.4 |
| Variable | All Patients (N = 1003–1100) | Curative (N = 818–825) | Palliative (N = 258–261) | Soft Tissue Sarcoma (N = 775–780) | Bone Sarcoma (N = 195–197) | GIST (N = 129–130) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Physical Functioning | 59.5 | 56.5 | 55.6 | 52.1 | 70.8 | 64.8 | 58.8 | 55.2 | 67.0 | 60.2 | 50.8 | 41.9 |
| 62.4 | 59.0 | 76.2 | 62.3 | 73.5 | 59.6 | |||||||
| Role Functioning | 50.7 | 47.7 | 47.3 | 43.8 | 61.4 | 55.2 | 49.1 | 45.5 | 61.9 | 54.8 | 42.6 | 34.0 |
| 67.4 | 52.7 | 68.7 | 51.6 | |||||||||
| 53.7 | 50.7 | |||||||||||
| Emotional Functioning | 62.7 | 59.8 | 61.2 | 57.8 | 67.7 | 61.6 | 63.2 | 59.7 | 61.4 | 54.2 | 61.5 | 52.6 |
| 65.6 | 64.6 | 73.3 | 66.6 | 68.3 | 70.0 | |||||||
| Cognitive Functioning | 39.0 | 36.1 | 37.3 | 34.0 | 44.8 | 38.7 | 39.4 | 35.9 | 38.6 | 31.8 | 36.9 | 28.6 |
| 42.0 | 40.7 | 51.1 | 42.9 | 45.8 | 45.8 | |||||||
| Social Functioning | 45.7 | 42.8 | 42.3 | 38.9 | 57.9 | 51.6 | 45.3 | 41.8 | 52.3 | 45.1 | 37.7 | 29.4 |
| 48.7 | 45.7 | 63.9 | 48.9 | 59.4 | 46.7 | |||||||
| Fatigue | 50.9 | 48.0 | 46.4 | 43.0 | 65.1 | 59.0 | 51.2 | 47.6 | 53.3 | 46.1 | 45.0 | 36.2 |
| 70.9 | 54.7 | 60.4 | 54.0 | |||||||||
| 53.9 | 49.9 | |||||||||||
| Nausea/Vomiting | 27.5 | 24.9 | 23.4 | 20.6 | 40.6 | 34.6 | 25.4 | 22.4 | 29.9 | 23.7 | 35.4 | 27.2 |
| 44.3 | ||||||||||||
| 30.2 | 28.6 | 36.9 | ||||||||||
| 26.5 | 46.8 | |||||||||||
| Pain | 55.5 | 52.6 | 53.9 | 50.4 | 60.5 | 54.3 | 54.0 | 50.4 | 65.0 | 57.9 | 50.8 | 41.9 |
| 59.6 | ||||||||||||
| 58.5 | 66.5 | 57.5 | 71.6 | |||||||||
| 57.3 | ||||||||||||
| Dyspnea | 49.2 | 46.2 | 44.6 | 41.2 | 63.7 | 57.3 | 51.4 | 47.8 | 41.1 | 34.2 | 48.5 | 39.6 |
| 52.2 | 69.6 | 55.0 | 48.3 | 57.4 | ||||||||
| 48.1 | ||||||||||||
| Insomnia | 35.3 | 32.4 | 35.4 | 32.1 | 36.8 | 30.9 | 34.7 | 31.4 | 33.5 | 27.0 | 41.1 | 32.5 |
| 38.2 | 38.8 | 43.0 | 38.2 | 40.6 | 50.1 | |||||||
| Appetite Loss | 15.8 | 13.7 | 12.7 | 10.5 | 24.5 | 19.4 | 15.0 | 12.6 | 16.8 | 11.8 | 16.9 | 10.9 |
| 22.7 | 24.5 | |||||||||||
| 18.0 | 30.2 | |||||||||||
| 15.2 | 17.7 | |||||||||||
| Constipation | 13.6 | 11.6 | 11.6 | 9.5 | 18.4 | 13.9 | 12.5 | 10.2 | 16.8 | 11.8 | 14.6 | 9.0 |
| 22.7 | 21.9 | |||||||||||
| 15.7 | 14.0 | 23.6 | 15.0 | |||||||||
| Diarrhea | 26.0 | 23.5 | 21.8 | 19.0 | 40.0 | 34.0 | 23.6 | 20.6 | 23.9 | 18.1 | 44.6 | 35.9 |
| 28.7 | 53.6 | |||||||||||
| 46.2 | 26.7 | 30.4 | ||||||||||
| 24.8 | ||||||||||||
| Financial Difficulties | 45.1 | 42.2 | 43.8 | 40.4 | 49.6 | 43.4 | 45.2 | 41.6 | 48.7 | 41.5 | 39.2 | 30.8 |
| 48.2 | ||||||||||||
| 48.1 | 55.9 | 56.0 | ||||||||||
| 47.3 | 48.7 | |||||||||||
| Variable | Value | General Health (R2 = 0.16) | Physical Functioning (R2 = 0.19) | Social Functioning (R2 = 0.16) | Emotional Functioning (R2 = 0.08) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI (l;u) | p | B | 95% CI (l;u) | p | B | 95% CI (l;u) | p | B | 95% CI (l;u) | p | ||
| Sex | male vs. female | −2.91 * | −5.56; −0.27 | 0.03 | −4.77 * | −7.53; −2.00 | <0.01 | −5.23 ** | −9.07; −1.39 | 0.01 | −6.62 ## | −9.83; −3.41 | <0.01 |
| Age | increase per year | −0.19 ** | −0.33; −0.06 | 0.01 | −0.31 *** | −0.45; −0.17 | <0.01 | −0.15 | −0.34; 0.05 | 0.14 | −0.08 | −0.24; 0.08 | 0.34 |
| SES ¶ | increase per point | 0.66 *** | 0.31; 1.02 | <0.01 | 0.92 *** | 0.55; 1.29 | <0.01 | 0.27 | −0.24; 0.78 | 0.30 | 0.39 | −0.04; 0.82 | 0.08 |
| Old age pension/early retirement | no vs. yes | 4.59 ** | 0.74; 8.43 | 0.02 | 5.45 ** | 1.42; 9.47 | 0.01 | 8.31 ** | 2.73; 13.88 | <0.01 | 9.86 ### | 5.18; 14.54 | <0.01 |
| Sarcoma Type | liposarcoma | reference | |||||||||||
| undifferentiated/unclassified | −4.24 | −9.18; 0.71 | 0.09 | −9.77 ** | −14.95; −4.60 | <0.01 | −6.69 | −13.85; 0.48 | 0.07 | −6.56 ## | −12.56; −0.56 | 0.03 | |
| fibro-/myofibroblastic | −4.76 | −9.98; −0.46 | 0.07 | −3.04 | −8.51; 2.42 | 0.28 | −0.75 | −9.32; 6.83 | 0.85 | −3.42 | −9.75; 2.92 | 0.29 | |
| GIST | 0.46 | −6.41; 7.34 | 0.90 | −0.62 | −7.83; 6.59 | 0.87 | 0.74 | −9.24; 10.72 | 0.88 | −0.07 | −8.42; 8.28 | 0.98 | |
| leiomyosarcoma | 0.07 | −5.17; 5.31 | 0.98 | −1.20 | −6.60; 4.27 | 0.67 | 2.21 | −5.37; 9.78 | 0.57 | −4.01 | −10.34: 2.33 | 0.22 | |
| osteosarcoma | −7.43 ** | −14.50; −0.36 | 0.04 | −15.01 *** | −22.46; −7.68 | <0.01 | −11.96 *** | −22.21; −1.71 | 0.02 | −9.75 ## | −18.33; −1.18 | 0.03 | |
| synovial sarcoma | −8.74 ** | −16.09; −1.39 | 0.02 | −7.67 | −15.36; 0.23 | 0.051 | −7.11 | −17.78; 3.56 | 0.19 | −3.40 | −12.32; 5.52 | 0.46 | |
| Ewing sarcoma | −13.24 *** | −21.50; −4.99 | <0.01 | −11.69 ** | −20.33; −3.05 | 0.01 | −12.85 *** | −24.83; −0.87 | 0.04 | −6.39 | −16.41; 3.63 | 0.21 | |
| chondrosarcoma | −10.56 *** | −17.19; −3.94 | <0.01 | −15.82 *** | −22.76; −8.89 | <0.01 | −18.8 **** | −28.38; −9.15 | <0.01 | −6.10 | −14.14; 1.95 | 0.14 | |
| all other | −0.56 | −6.23; 4.95 | 0.82 | −5.66 | −11.54; 0.22 | 0.06 | −5.06 | −13.19; 3.08 | 0.22 | −4.37 | −11.17; 2.43 | 0.21 | |
| Tumor Site | lower limbs | reference | |||||||||||
| abdomen/retroperitoneum | 4.10 | −0.28; 8.47 | 0.07 | 5.83 ** | 1.26; 10.40 | 0.01 | 7.17 ** | 0.83; 13.51 | 0.03 | 0.13 | −5.18; 5.43 | 0.96 | |
| thorax | 3.76 | −1.45; 8.97 | 0.16 | 8.38 ** | 2.90; 13.86 | <0.01 | 8.94 ** | 1.37; 16.51 | 0.02 | −2.38 | −8.71; 3.96 | 0.46 | |
| pelvis/urogenital | 0.25 | −4.00; 4.51 | 0.91 | 1.59 | −2.86; 6.04 | 0.48 | −0.43 | −6.60; 5.74 | 0.89 | 0.31 | −4.86; 5.48 | 0.91 | |
| upper limbs | 9.16 ** | 3.91; 14.39 | <0.01 | 12.54 ** | 7.07; 18.02 | <0.01 | 12.04 *** | 4.74; 19.64 | <0.01 | 1.99 | −4.37; 8.35 | 0.54 | |
| head & neck | 7.29 | −0.38; 14.96 | 0.06 | 10.78* | 2.64; 18.81 | 0.01 | 16.30 **** | 5.15; 27.44 | <0.01 | 11.39 ### | 2.07; 20.70 | 0.02 | |
| back/spine | −1.17 | −9.67; 7.33 | 0.79 | 3.71 | −5.18; 12.60 | 0.41 | 0.27 | −12.08; 12.61 | 0.97 | 1.57 | −8.76; 11.89 | 0.77 | |
| all other | 15.34 | −4.14; 34.81 | 0.12 | 6.86 | −13.53; 27.25 | 0.51 | 5.60 | −22.70; 33.89 | 0.70 | 3.04 | −20.63; 26.71 | 0.80 | |
| Grading | G1 | reference | |||||||||||
| G2/G3 | −0.45 | −4.87; 3.98 | 0.84 | −3.44 | −8.07; 1.18 | 0.15 | −6.84 ** | −13.27; −0.43 | 0.04 | −1.58 | −6.94; 3.79 | 0.57 | |
| other (unknown/na) | 0.56 | −4.91; 6.03 | 0.84 | −1.10 | −6.84; 4.64 | 0.71 | −5.45 | −13.40; 2.50 | 0.18 | −3.82 | −10.47; 2.83 | 0.26 | |
| T-Stadium | T1 | reference | |||||||||||
| T2-T4 | 1.06 | −3.07; 5.18 | 0.62 | −2.54 | −6.84; 1.77 | 0.25 | −4.86 | −10.85; 1.13 | 0.11 | −2.54 | −7.55; 2.47 | 0.32 | |
| other (unknown/na) | 2.56 | −1.95; 7.06 | 0.27 | 0.68 | −4.02; 5.37 | 0.78 | −2.93 | −9.46; 3.60 | 0.40 | −0.75 | −6.21; 4.72 | 0.79 | |
| Aggressiveness Tumor | malignant vs. locally aggressive + rarely metastatic | 2.96 | −4.06; 9.99 | 0.41 | −2.16 | −9.51; 5.18 | 0.56 | −4.34 | −14.54; 5.86 | 0.40 | −3.24 | −11.78; 5.29 | 0.46 |
| Metastasis till study inclusion | no | reference | |||||||||||
| yes | −1.86 | −5.47; 1.75 | 0.31 | −3.07 | −6.85; 0.70 | 0.11 | −3.83 | −9.06; 1.41 | 0.15 | −1.70 | −6.08; 2.68 | 0.45 | |
| unknown | −1.82 | −6.18; 2.54 | 0.41 | −2.14 | −6.69; 2.41 | 0.36 | 0.86 | −5.47; 7.20 | 0.79 | −2.17 | −7.47; 3.13 | 0.42 | |
| Comorbidities | increase per comorbidity | −2.78 *** | −4.43; −1.12 | <0.01 | −3.43** | −5.16; −1.69 | <0.01 | −3.41 *** | −5.81; −1.00 | 0.01 | −1.90 | −3.91; 0.07 | 0.07 |
| Disease status | complete remission | reference | |||||||||||
| part. remission + stable disease | −3.17 | −6.77; 0.43 | 0.08 | −0.14 | −3.91; 3.63 | 0.94 | −5.51 ** | −10.74; −0.28 | 0.04 | −4.11 | −8.48; 0.27 | 0.07 | |
| progress | −7.98 ** | −12.87; −3.09 | <0.01 | −2.24 | −7.34; 2.86 | 0.39 | −6.95 | −14.03; 0.13 | 0.054 | −5.32 | −11.25; 0.61 | 0.08 | |
| unknown | −0.39 | −5.46; 4.69 | 0.88 | 0.68 | −4.63; 5.99 | 0.80 | −8.54 ** | −15.91; −1.17 | 0.02 | −4.25 | −10.42; 1.91 | 0.18 | |
| Treatment status | no vs. yes | −6.32 ** | −9.90; −2.75 | <0.01 | −5.03** | −8.76; −1.30 | 0.01 | −5.90 ** | −11.08; −0.71 | 0.03 | −0.67 | −5.01; 3.66 | 0.76 |
| Combined Treatments | surgery alone | reference | |||||||||||
| surgery + CT | −0.39 | −4.65; 3.88 | 0.86 | −4.63** | −9.10; −0.16 | 0.04 | −4.64 | −10.84; 1.56 | 0.14 | 2.72 | −2.46; 7.91 | 0.30 | |
| surgery + RT | 0.02 | −4.32; 4.37 | 0.99 | −2.35 | −6.89; 2.20 | 0.31 | −4.71 | −11.02; 1.61 | 0.14 | −0.40 | −5.68; 4.88 | 0.88 | |
| surgery + CT + RT | −1.17 | −5.61; 3.27 | 0.61 | −5.25** | −9.89; −0.61 | 0.03 | −4.95 | −11.39; 1.49 | 0.13 | −2.72 | −8.10; 2.67 | 0.32 | |
| CT alone | −0.56 | −6.57; 7.68 | 0.88 | −6.94 | −14.43; 0.55 | 0.07 | −5.78 | −16.13; 4.57 | 0.27 | 7.40 | −1.32; 16.12 | 0.10 | |
| all other | −1.81 | −7.66; 4.04 | 0.54 | −3.76 | −9.86; 2.34 | 0.23 | −4.31 | −12.78; 4.16 | 0.32 | −2.31 | −9.40; 4.77 | 0.52 | |
| Time since diagnosis | 0–<6months | reference | |||||||||||
| 6–<12months | 0.92 | −4.26; 6.10 | 0.73 | −2.89 | −8.32; 2.54 | 0.30 | −3.18 | −10.70; 4.35 | 0.41 | −0.08 | −6.37; 6.22 | 0.98 | |
| 12–<24months | 6.68 ** | 1.64; 11.72 | 0.01 | −1.67 | −6.93; 3.60 | 0.54 | 3.91 | −3.41; 11.22 | 0.30 | 2.52 | −3.62; 8.66 | 0.42 | |
| 24–<60months | 8.67 ** | 3.89; 13.44 | <0.01 | 0.91 | −4.07; 5.90 | 0.72 | 6.25 | −0.68; 13.18 | 0.08 | 2.69 | −3.11; 8.49 | 0.36 | |
| 60 months or more | 7.73 ** | 2.60; 12.87 | <0.01 | −1.88 | −7.24; 3.49 | 0.49 | 9.54 ** | 2.09; 16.99 | 0.01 | 7.15 ## | 0.91; 13.39 | 0.03 | |
| Tumor recurrence | no | reference | |||||||||||
| yes | −4.96 * | −8.43; −1.49 | 0.01 | −3.67 * | −7.30; −0.04 | 0.04 | −8.35 ** | −13.38; −3.32 | <0.01 | −5.52 ## | −9.73; −1.31 | 0.01 | |
| unknown | 0.73 | −7.75; 9.21 | 0.87 | −2.80 | −11.67; 6.09 | 0.54 | 2.27 | −10.05; 14.59 | 0.72 | −1.26 | −11.75; 9.23 | 0.81 | |
| Variable | Value | Role Functioning (R2 = 0.16) | Pain (R2 = 0.10) | Fatigue (R2 = 0.16) | Dyspnea (R2 = 0.14) | ||||||||
| B | 95% CI (l;u) | p | B | 95% CI (l;u) | p | B | 95% CI (l;u) | p | B | 95% CI (l;u) | p | ||
| Sex | male vs. female | −6.11 ** | −10.01; −2.22 | <0.01 | 4.16 * | 0.36; 7.97 | 0.03 | 8.45 ** | 5.13; 11.76 | <0.01 | 5.50 ** | 1.95; 9.05 | <0.01 |
| Age | increase per year | −0.20 ** | −0.40; −0.004 | 0.045 | 0.12 | −0.07; 0.31 | 0.22 | 0.26 *** | 0.10; 0.43 | <0.01 | 0.33 **** | 0.15; 0.51 | <0.01 |
| SES¶ | increase per point | 0.45 | −0.07; 0.97 | 0.09 | −1.25 **** | −1.76; −0.74 | <0.01 | −0.59 ** | −1.03; −0.15 | 0.01 | −0.23 | −0.71; 0.24 | 0.33 |
| Old age pension/early retirement | no vs. yes | 8.18 ** | 2.51; 13.85 | 0.01 | −2.96 | −8.49; 2.53 | 0.29 | −8.82 ** | −13.64; −4.00 | <0.01 | −6.78 ** | −11.98; −1.62 | 0.01 |
| Sarcoma Type | liposarcoma | reference | |||||||||||
| undifferentiated/unclassified | −9.74 ** | −17.02; −2.47 | 0.01 | 5.14 | −1.95; 12.38 | 0.16 | 4.54 | −1.64; 10.73 | 0.15 | 1.13 | −5.52; 7.77 | 0.74 | |
| fibro-/myofibroblastic | −5.62 | −13.31; 2.07 | 0.15 | 4.68 | −2.83; 12.19 | 0.22 | 3.25 | −3.29; 9.79 | 0.33 | −0.53 | −7.55; 6.49 | 0.88 | |
| GIST | 6.88 | −3.26; 17.02 | 0.18 | 5.71 | −4.18; 15.61 | 0.26 | 0.30 | −8.33; 8.83 | 0.95 | −9.76 *** | −18.98; −0.54 | 0.04 | |
| leiomyosarcoma | 1.95 | −5.75; 9.63 | 0.62 | −2.40 | −9.92; 5.12 | 0.53 | 0.08 | −6.46; 6.62 | 0.98 | −2.56 | −9.58; 4.46 | 0.48 | |
| osteosarcoma | −13.79 ** | −24.21; −3.38 | 0.01 | 8.94 | −1.22; 19.11 | 0.09 | 10.25 ** | 1.40; 19.10 | 0.02 | 5.10 | −4.37; 14.58 | 0.29 | |
| synovial sarcoma | −8.72 | −19.55; 2.12 | 0.12 | 4.69 | −5.89; 15.27 | 0.38 | 5.05 | −4.17; 14.26 | 0.28 | 7.45 | −2.40; 17.23 | 0.14 | |
| Ewing sarcoma | −13.50 ** | −25.67; −1.33 | 0.03 | 8.67 | −3.21; 20.55 | 0.15 | 11.94 ** | 1.60; 22.29 | 0.02 | 2.93 | −8.13; 13.99 | 0.60 | |
| chondrosarcoma | −19.55 *** | −29.31; −9.78 | <0.01 | 14.78 *** | 5.24; 24.31 | <0.01 | 9.09 ** | 0.79; 17.34 | 0.03 | 1.43 | −7.45; 10.32 | 0.75 | |
| all other | −4.85 | −13.11; 3.41 | 0.25 | 2.78 | −5.29; 10.84 | 0.50 | 5.26 | −1.76; 12.28 | 0.14 | −0.40 | −7.92; 7.11 | 0.92 | |
| Tumor Site | lower limbs | reference | |||||||||||
| abdomen/ retroperitoneum | 9.12 ** | 2.68; 15.57 | 0.01 | −8.81 ** | −15.1; −2.52 | 0.01 | −0.90 | −6.28; 4.58 | 0.75 | 4.11 | −1.76; 9.99 | 0.17 | |
| thorax | 9.53 ** | 1.87; 17.25 | 0.02 | −12.21 ** | −19.7; −4.71 | <0.01 | −1.33 | −7.89; 5.21 | 0.69 | 8.33 *** | 1.34; 15.31 | 0.02 | |
| pelvis/urogenital | 7.10 ** | 0.83; 13.37 | 0.03 | −2.99 | −9.11; 3.14 | 0.34 | 1.05 | −4.28; 6.38 | 0.70 | −2.12 | −7.84; 3.59 | 0.47 | |
| upper limbs | 10.47 ** | 2.75; 18.19 | 0.01 | −14.28 *** | −21.8; −6.74 | <0.01 | −7.41 ** | −13.98; −0.85 | 0.03 | −1.55 | −8.56; 5.46 | 0.67 | |
| head & neck | 13.45 ** | 2.14; 24.77 | 0.02 | −14.03 *** | −25.0; −2.98 | 0.01 | −6.25 | −15.89; 3.38 | 0.20 | −4.45 | −14.87; 5.98 | 0.40 | |
| back/spine | −2.55 | −15.09; 9.99 | 0.69 | 1.65 | −10.59; 13.9 | 0.79 | 4.13 | −6.53; 14.79 | 0.45 | 6.17 | −5.21; 17.56 | 0.29 | |
| all other | 9.13 | −19.62; 37.87 | 0.53 | −25.55 | −53.60; 2.51 | 0.07 | 0.27 | −24.17; 24.71 | 0.98 | −7.45 | −33.54; 18.63 | 0.58 | |
| Grading | G1 | reference | |||||||||||
| G2/G3 | −4.71 | −11.23; 1.81 | 0.16 | 2.82 | −3.55; 9.19 | 0.39 | 3.83 | −1.72; 9.37 | 0.18 | 0.26 | −5.70; 6.21 | 0.93 | |
| other (unknown/na) | −5.44 | −13.52; 2.54 | 0.19 | 1.88 | −6.00; 9.76 | 0.64 | 3.36 | −3.51; 10.22 | 0.34 | 3.41 | −3.95; 10.77 | 0.36 | |
| T-Stadium | T1 | reference | |||||||||||
| T2–T4 | −1.91 | −7.98; 4.16 | 0.54 | 0.28 | −5.65; 6.20 | 0.93 | −2.17 | −7.34; 3.00 | 0.41 | −2.47 | −8.02; 3.08 | 0.38 | |
| other (unknown/na) | 2.61 | −4.01; 9.23 | 0.44 | 1.00 | −5.37; 7.57 | 0.74 | −6.27 ** | −11.91; −0.63 | 0.03 | −6.72 ** | −12.79; −0.66 | 0.03 | |
| Aggressiveness Tumor | malignant vs. locally aggressive + rarely metastatic | 1.14 | −9.21; 11.50 | 0.83 | 8.44 | −1.67; 18.54 | 0.10 | −2.80 | −11.61; 6.01 | 0.53 | −3.71 | −13.17; 5.74 | 0.44 |
| Metastasis till study inclusion | no | reference | |||||||||||
| yes | −5.07 | −10.38; 0.25 | 0.06 | −0.11 | −5.30; 5.08 | 0.97 | 4.49 | −0.03; 9.02 | 0.052 | 7.81 ** | 2.96; 12.67 | <0.01 | |
| unknown | −2.19 | −8.60; 4.22 | 0.50 | 1.64 | −4.62; 7.89 | 0.61 | 2.92 | −2.55; 8.39 | 0.30 | 2.31 | −3.55; 8.17 | 0.44 | |
| Comorbidities | increase per comorbidity | −3.98 ** | −6.42; −1.54 | <0.01 | 2.69 ** | 0.30; 5.07 | 0.03 | 4.11 *** | 2.03; 6.19 | <0.01 | 4.44 **** | 2.23; 6.66 | <0.01 |
| Disease status | complete remission | reference | |||||||||||
| part. remission + stable disease | −3.95 | −9.26; 1.37 | 0.15 | 4.63 | −0.56; 9.82 | 0.08 | 5.13 ** | 0.61; 9.61 | 0.03 | 3.07 | −1.77; 7.92 | 0.21 | |
| progress | −5.65 | −12.84; 1.55 | 0.12 | 3.75 | −3.27; 10.77 | 0.30 | 2.96 | −3.16; 9.08 | 0.34 | 6.06 | −0.49; 12.61 | 0.07 | |
| unknown | −4.51 | −11.99; 2.97 | 0.23 | 3.17 | −4.13: 10.43 | 0.40 | 3.05 | −3.32; 9.41 | 0.35 | 4.71 | −2.09; 11.51 | 0.17 | |
| Treatment status | no vs. yes | −11.86 ** | −17.12; 6.60 | <0.01 | 4.34 | −0.80; 9.48 | 0.10 | 7.46 ** | 2.98; 11.93 | 0.001 | 6.63 ** | 1.85; 11.41 | 0.01 |
| Combined Treatments | surgery alone | reference | |||||||||||
| surgery + CT | −1.18 | −7.48; 5.13 | 0.71 | 1.09 | −5.06; 7.24 | 0.73 | 3.39 | −1.94; 8.75 | 0.21 | 4.53 | −1.20; 10.26 | 0.12 | |
| surgery + RT | −2.79 | −9.20; 3.61 | 0.39 | 5.74 | −0.52; 12.00 | 0.07 | 4.12 | −1.33; 9.57 | 0.14 | 4.32 | −1.53; 10.17 | 0.15 | |
| surgery + CT + RT | −2.64 | −9.18; 3.90 | 0.43 | 4.36 | −2.03; 10.75 | 0.18 | 7.93 ** | 2.36; 13.49 | 0.01 | 1.29 | −4.66; 7.24 | 0.67 | |
| CT alone | −0.71 | −11.22; 9.80 | 0.89 | 4.28 | −5.99; 14.56 | 0.41 | 10.28 ** | 1.34; 19.22 | 0.02 | 4.07 | −5.56; 13.70 | 0.41 | |
| all other | 2.18 | −6.43; 10.78 | 0.62 | 10.57 ** | 2.16; 18.98 | 0.01 | 5.50 | −1.82; 12.82 | 0.14 | −0.96 | −8.78; 6.87 | 0.81 | |
| Time since diagnosis | 0–<6 months | reference | |||||||||||
| 6–<12 months | −2.20 | −9.86; 5.45 | 0.57 | 5.27 | −2.20; 12.74 | 0.17 | 3.68 | −2.82; 10.18 | 0.27 | 1.31 | −5.66; 8.27 | 0.71 | |
| 12–<24 months | 1.30 | −6.12; 8.73 | 0.73 | 0.66 | −6.60; 7.91 | 0.86 | 4.16 | −2.16; 10.48 | 0.20 | 7.51 ** | 0.73; 14.31 | 0.03 | |
| 24–<60 months | 6.76 | −0.26; 13.79 | 0.06 | 2.56 | −4.31; 9.43 | 0.47 | 1.74 | −4.25; 7.72 | 0.57 | 3.21 | −3.20; 9.62 | 0.33 | |
| 60 months or more | 4.84 | −2.72; 12.40 | 0.21 | 0.13 | −7.26; 7.53 | 0.97 | 0.80 | −5.63; 7.24 | 0.81 | 4.80 | −2.10; 11.70 | 0.17 | |
| Tumor recurrence | no | reference | |||||||||||
| yes | −6.41 ** | −11.53; 1.30 | 0.01 | 6.31 ** | 1.32; 11.30 | 0.01 | 5.17 ** | 0.82; 9.52 | 0.02 | 0.87 | −3.78; 5.52 | 0.71 | |
| unknown | 1.12 | −11.40; 13.63 | 0.86 | −1.60 | −13.81; 10.6 | 0.80 | −2.35 | −12.99; 8.24 | 0.67 | 9.35 | −2.21; 20.91 | 0.11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eichler, M.; Hentschel, L.; Richter, S.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; Jakob, J.; Singer, S.; Grützmann, R.; et al. The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of a Nationwide Observational Study (PROSa). Cancers 2020, 12, 3590. https://doi.org/10.3390/cancers12123590
Eichler M, Hentschel L, Richter S, Hohenberger P, Kasper B, Andreou D, Pink D, Jakob J, Singer S, Grützmann R, et al. The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of a Nationwide Observational Study (PROSa). Cancers. 2020; 12(12):3590. https://doi.org/10.3390/cancers12123590
Chicago/Turabian StyleEichler, Martin, Leopold Hentschel, Stephan Richter, Peter Hohenberger, Bernd Kasper, Dimosthenis Andreou, Daniel Pink, Jens Jakob, Susanne Singer, Robert Grützmann, and et al. 2020. "The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of a Nationwide Observational Study (PROSa)" Cancers 12, no. 12: 3590. https://doi.org/10.3390/cancers12123590
APA StyleEichler, M., Hentschel, L., Richter, S., Hohenberger, P., Kasper, B., Andreou, D., Pink, D., Jakob, J., Singer, S., Grützmann, R., Fung, S., Wardelmann, E., Arndt, K., Heidt, V., Hofbauer, C., Fried, M., Gaidzik, V. I., Verpoort, K., Ahrens, M., ... the PROSa Study Group. (2020). The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of a Nationwide Observational Study (PROSa). Cancers, 12(12), 3590. https://doi.org/10.3390/cancers12123590








