Pigment Epithelium-Derived Factor and Sex Hormone-Responsive Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Sex Hormones
3. Background on Pigment Epithelium-Derived Factor (PEDF)
4. PEDF and Cancer
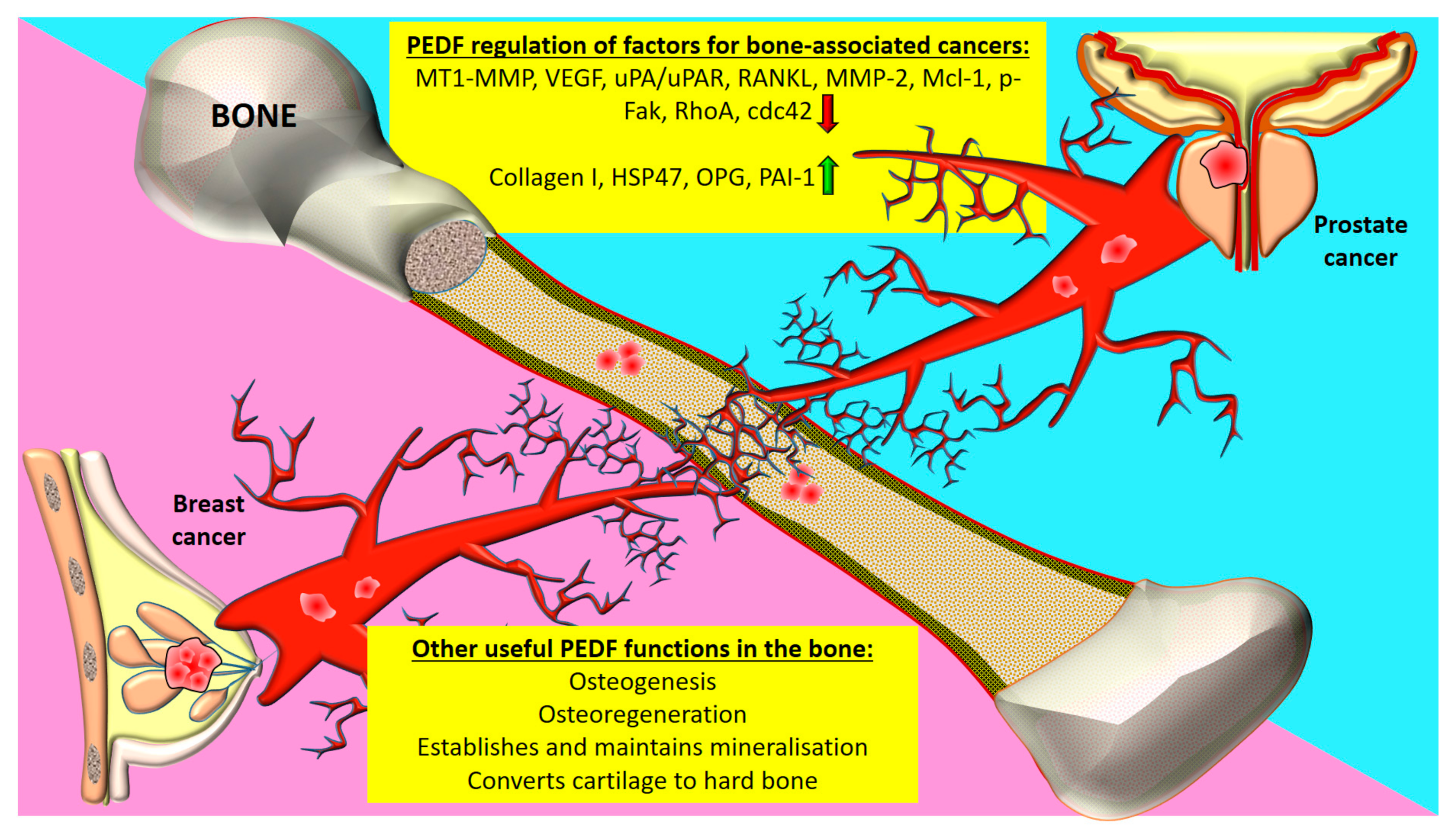
4.1. Prostate Cancer
4.2. Breast Cancer
4.3. Female Reproductive Tract Cancers
4.3.1. Ovarian Cancer
4.3.2. Endometrial Cancer
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| AR | Androgen Receptor |
| ATP5B | ATP Synthase F1 Subunit Beta |
| Cdc42 | Cell Division Control Protein 42 Homolog |
| CRPC | Castration-Resistant PC |
| CXCR1 | C-X-C Motif Receptor 1 |
| DHT | Dihydrotestosterone |
| EC | Endometrial Cancer |
| ECC | EC Cell |
| EMT | Epithelial-Mesenchymal Transition |
| ER | Oestrogen Receptor |
| ERK | Extracellular Signal-Regulated Kinase |
| FAK | Focal Adhesion Kinase |
| FasL | Fas Ligand |
| HIF | Hypoxia-Inducible Factor |
| HSP47 | Heat Shock Protein-47 |
| Il | Interleukin |
| JNK | c-Jun N-Terminal Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| Mcl-1 | Myeloid Cell Leukemia 1 |
| MMP | Matrix Metalloproteinase |
| mTOR | Mammalian Target of Rapamycin |
| MVD | Microvessel Density |
| NF-κB | Nuclear Factor-Kappa B |
| Nrf2 | NF-E2-Related Factor-2 |
| OC | Ovarian Cancer |
| PAI | Plasminogen Inhibitor Activator |
| PC | Prostate Cancer |
| PEDF | Pigment Epithelium-Derived Factor |
| PI3K | Phosphoinositide 3-Kinase |
| PKC | Protein Kinase C |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor-Gamma |
| PR | Progesterone Receptor |
| RET | Receptor Tyrosine Kinase Rearranged During Transfection |
| RhoA | Ras Homolog Family Member A |
| T | Testosterone |
| TME | Tumour Microenvironment |
| TNBC | Triple Negative Breast Cancer |
| TNF | Tumour Necrosis Factor |
| uPA | Urokinase Plasminogen Activator |
| uPAR | uPA Receptor |
| VEGF | Vascular Endothelial Growth Factor |
References
- Hilton, H.N.; Clarke, C.L.; Graham, J.D. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol. Cell. Endocrinol. 2018, 466, 2–14. [Google Scholar] [CrossRef]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R.; Zirkin, B.R. Androgen action in prostate function and disease. Am. J. Clin. Exp. Urol. 2018, 6, 62. [Google Scholar]
- Brook, N.; Brook, E.; Dharmarajan, A.; Dass, C.R.; Chan, A. Breast cancer bone metastases: Pathogenesis and therapeutic targets. Int. J. Biochem. Cell Biol. 2018, 96, 63–78. [Google Scholar] [CrossRef]
- De Almeida Chuffa, L.G.; Lupi-Júnior, L.A.; Costa, A.B.; de Arruda Amorim, J.P.; Seiva, F.R.F. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids 2017, 118, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Potter, B.V. The structural biology of oestrogen metabolism. J. Steroid Biochem. Mol. Biol. 2013, 137, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Secky, L.; Svoboda, M.; Klameth, L.; Bajna, E.; Hamilton, G.; Zeillinger, R.; Jäger, W.; Thalhammer, T. The sulfatase pathway for estrogen formation: Targets for the treatment and diagnosis of hormone-associated tumors. J. Drug Deliv. 2013, 2013, 957605. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Lin, C.-Y. Oestrogen receptors in breast cancer: Basic mechanisms and clinical implications. Ecancermedicalscience 2013, 7, 370. [Google Scholar]
- Amanatullah, D.F.; Tamaresis, J.S.; Chu, P.; Bachmann, M.H.; Hoang, N.M.; Collyar, D.; Mayer, A.T.; West, R.B.; Maloney, W.J.; Contag, C.H. Local estrogen axis in the human bone microenvironment regulates estrogen receptor-positive breast cancer cells. Breast Cancer Res. 2017, 19, 1–16. [Google Scholar]
- Schiffer, L.; Arlt, W.; Storbeck, K.-H. Intracrine androgen biosynthesis, metabolism and action revisited. Mol. Cell. Endocrinol. 2018, 465, 4–26. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, physiology, and clinical implications of elevated blood levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar]
- Mostaghel, E.A.; Nelson, P.S. Intracrine androgen metabolism in prostate cancer progression: Mechanisms of castration resistance and therapeutic implications. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 243–258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaipainen, A.; Zhang, A.; Gil da Costa, R.M.; Lucas, J.; Marck, B.; Matsumoto, A.M.; Morrissey, C.; True, L.D.; Mostaghel, E.A.; Nelson, P.S. Testosterone accumulation in prostate cancer cells is enhanced by facilitated diffusion. Prostate 2019, 79, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Stanbrough, M.; Bubley, G.J.; Ross, K.; Golub, T.R.; Rubin, M.A.; Penning, T.M.; Febbo, P.G.; Balk, S.P. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006, 66, 2815–2825. [Google Scholar] [CrossRef] [PubMed]
- Tombran-Tink, J.; Pawar, H.; Swaroop, A.; Rodriguez, I.; Chader, G.J. Localization of the gene for pigment epithelium-derived factor (PEDF) to chromosome 17p13. 1 and expression in cultured human retinoblastoma cells. Genomics 1994, 19, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Brook, N.; Brook, E.; Dharmarajan, A.; Chan, A.; Dass, C.R. The role of pigment epithelium-derived factor in protecting against cellular stress. Free Radic. Res. 2019, 53, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Brook, N.; Brook, E.; Dharmarajan, A.; Chan, A.; Dass, C.R. Pigment epithelium-derived factor regulation of neuronal and stem cell fate. Exp. Cell Res. 2020, 389, 111891. [Google Scholar] [CrossRef]
- Wei, Y.; Elahy, M.; Friedhuber, A.M.; Wong, J.Y.; Hughes, J.D.; Doschak, M.R.; Dass, C.R. Triple-threat activity of PEDF in bone tumors: Tumor inhibition, tissue preservation and cardioprotection against doxorubicin. Bone 2019, 124, 103–117. [Google Scholar] [CrossRef]
- Cheung, L.W.; Au, S.C.; Cheung, A.N.; Ngan, H.Y.; Tombran-Tink, J.; Auersperg, N.; Wong, A.S. Pigment epithelium-derived factor is estrogen sensitive and inhibits the growth of human ovarian cancer and ovarian surface epithelial cells. Endocrinology 2006, 147, 4179–4191. [Google Scholar] [CrossRef]
- Chuderland, D.; Ben-Ami, I.; Kaplan-Kraicer, R.; Grossman, H.; Komsky, A.; Satchi-Fainaro, R.; Eldar-Boock, A.; Ron-El, R.; Shalgi, R. Hormonal regulation of pigment epithelium-derived factor (PEDF) in granulosa cells. Mol. Hum. Reprod. 2013, 19, 72–81. [Google Scholar] [CrossRef]
- Chuderland, D.; Ben-Ami, I.; Friedler, S.; Hasky, N.; Ninio-Many, L.; Goldberg, K.; Bar-Joseph, H.; Grossman, H.; Shalgi, R. Hormonal regulation of pigment epithelium-derived factor (PEDF) expression in the endometrium. Mol. Cell. Endocrinol. 2014, 390, 85–92. [Google Scholar] [CrossRef]
- Doll, J.A.; Stellmach, V.M.; Bouck, N.P.; Bergh, A.R.; Lee, C.; Abramson, L.P.; Cornwell, M.L.; Pins, M.R.; Borensztajn, J.; Crawford, S.E. Pigment epithelium–derived factor regulates the vasculature and mass of the prostate and pancreas. Nat. Med. 2003, 9, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Huang, M.; Lewis-Wambi, J. Loss of pigment epithelium-derived factor: A novel mechanism for the development of endocrine resistance in breast cancer. Breast Cancer Res. 2012, 14, R146. [Google Scholar] [CrossRef] [PubMed]
- Daubriac, J.; Pandya, U.M.; Huang, K.-T.; Pavlides, S.C.; Gama, P.; Blank, S.V.; Shukla, P.; Crawford, S.E.; Gold, L.I. Hormonal and growth regulation of epithelial and stromal cells from the normal and malignant endometrium by pigment epithelium-derived factor. Endocrinology 2017, 158, 2754–2773. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhu, L.; Huang, Z.; Luo, C.; Zhou, T.; Li, L.; Wang, G.; Yang, Z.; Qi, W.; Yang, X. Stem-like tumor cells involved in heterogeneous vasculogenesis in breast cancer. Endocr. Relat. Cancer 2020, 27, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, M.; Xu, P.; Yu, Y.; Ye, G.; Zhang, L.; Wu, A. Expression of pigment epithelium-derived factor is associated with a good prognosis and is correlated with epithelial-mesenchymal transition-related genes in infiltrating ductal breast carcinoma. Oncol. Lett. 2016, 11, 116–124. [Google Scholar] [CrossRef]
- Hong, H.; Zhou, T.; Fang, S.; Jia, M.; Xu, Z.; Dai, Z.; Li, C.; Li, S.; Li, L.; Zhang, T. Pigment epithelium-derived factor (PEDF) inhibits breast cancer metastasis by down-regulating fibronectin. Breast Cancer Res. Treat. 2014, 148, 61–72. [Google Scholar] [CrossRef]
- Filiz, G.; Dass, C.R. Reduction in tumour cell invasion by pigment epithelium-derived factor is mediated by membrane type-1 matrix metalloproteinase downregulation. Pharmazie 2012, 67, 1010–1014. [Google Scholar]
- Guan, M.; Jiang, H.; Xu, C.; Xu, R.; Chen, Z.; Lu, Y. Adenovirus-mediated PEDF expression inhibits prostate cancer cell growth and results in augmented expression of PAI-2. Cancer Biol. Ther. 2007, 6, 419–425. [Google Scholar] [CrossRef][Green Version]
- Matsui, T.; Ojima, A.; Higashimoto, Y.; Taira, J.; Fukami, K.; Yamagishi, S.I. Pigment epithelium-derived factor inhibits caveolin-induced interleukin-8 gene expression and proliferation of human prostate cancer cells. Oncol. Lett. 2015, 10, 2644–2648. [Google Scholar] [CrossRef][Green Version]
- Hirsch, J.; Johnson, C.L.; Nelius, T.; Kennedy, R.; de Riese, W.; Filleur, S. PEDF inhibits IL8 production in prostate cancer cells through PEDF receptor/phospholipase A2 and regulation of NFκB and PPARγ. Cytokine 2011, 55, 202–210. [Google Scholar] [CrossRef]
- Gong, Q.; Qiu, S.; Li, S.; Ma, Y.; Chen, M.; Yao, Y.; Che, D.; Feng, J.; Cai, W.; Ma, J. Proapoptotic PEDF functional peptides inhibit prostate tumor growth—A mechanistic study. Biochem. Pharmacol. 2014, 92, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Zolochevska, O.; Yu, G.; Gimble, J.M.; Figueiredo, M.L. Pigment epithelial-derived factor and melanoma differentiation associated gene-7 cytokine gene therapies delivered by adipose-derived stromal/mesenchymal stem cells are effective in reducing prostate cancer cell growth. Stem Cells Dev. 2012, 21, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, C.; Nelius, T.; Martinez-Marin, D.; Sennoune, S.R.; Filleur, S. Cabazitaxel regimens inhibit the growth of prostate cancer cells and enhances the anti-tumor properties of PEDF with various efficacy and toxicity. Prostate 2018, 78, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Nelius, T.; Martinez-Marin, D.; Hirsch, J.; Miller, B.; Rinard, K.; Lopez, J.; De Riese, W.; Filleur, S. Pigment epithelium-derived factor expression prolongs survival and enhances the cytotoxicity of low-dose chemotherapy in castration-refractory prostate cancer. Cell Death Dis. 2014, 5, e1210. [Google Scholar] [CrossRef]
- Martinez-Marin, D.; Jarvis, C.; Nelius, T.; De Riese, W.; Volpert, O.V.; Filleur, S. PEDF increases the tumoricidal activity of macrophages towards prostate cancer cells in vitro. PLoS ONE 2017, 12, e0174968. [Google Scholar] [CrossRef]
- Byrne, J.C.; Downes, M.R.; O’Donoghue, N.; O’Keane, C.; O’Neill, A.; Fan, Y.; Fitzpatrick, J.M.; Dunn, M.J.; Watson, R.W.G. 2D-DIGE as a strategy to identify serum markers for the progression of prostate cancer. J. Proteome Res. 2009, 8, 942–957. [Google Scholar] [CrossRef]
- Oon, S.F.; Fanning, D.M.; Fan, Y.; Boyce, S.; Murphy, T.B.; Fitzpatrick, J.M.; Watson, R.W. The identification and internal validation of a preoperative serum biomarker panel to determine extracapsular extension in patients with prostate cancer. Prostate 2012, 72, 1523–1531. [Google Scholar] [CrossRef]
- Rivera-Pérez, J.; Monter-Vera, M.d.R.; Barrientos-Alvarado, C.; Toscano-Garibay, J.D.; Cuesta-Mejías, T.; Flores-Estrada, J. Evaluation of VEGF and PEDF in prostate cancer: A preliminary study in serum and biopsies. Oncol. Lett. 2018, 15, 1072–1078. [Google Scholar]
- Ide, H.; Yamagishi, S.-i.; Lu, Y.; Sakamaki, K.; Nakajima, A.; Horiuchi, A.; Kitamura, K.; Hisasue, S.-i.; Muto, S.; Yamaguchi, R. Circulating pigment epithelium-derived factor (PEDF) is associated with pathological grade of prostate cancer. Anticancer Res. 2015, 35, 1703–1708. [Google Scholar] [CrossRef]
- Halin, S.; Wikström, P.; Rudolfsson, S.H.; Stattin, P.; Doll, J.A.; Crawford, S.E.; Bergh, A. Decreased pigment epithelium-derived factor is associated with metastatic phenotype in human and rat prostate tumors. Cancer Res. 2004, 64, 5664–5671. [Google Scholar] [CrossRef]
- Duggan, C.; de Dieu Tapsoba, J.; Wang, C.-Y.; Schubert, K.E.F.; McTiernan, A. Long-term effects of weight loss and exercise on biomarkers associated with angiogenesis. Cancer Epidemiol. Prev. Biomark. 2017, 26, 1788–1794. [Google Scholar] [CrossRef]
- Zolochevska, O.; Shearer, J.; Ellis, J.; Fokina, V.; Shah, F.; Gimble, J.M.; Figueiredo, M.L. Human adipose-derived mesenchymal stromal cell pigment epithelium–derived factor cytotherapy modifies genetic and epigenetic profiles of prostate cancer cells. Cytotherapy 2014, 16, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Halin, S.; Rudolfsson, S.H.; Doll, J.A.; Crawford, S.E.; Wikström, P.; Bergh, A. Pigment epithelium-derived factor stimulates tumor macrophage recruitment and is downregulated by the prostate tumor microenvironment. Neoplasia 2010, 12, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Cheng, S.-Q.; Ji, H.-F.; Wang, J.-S.; Xu, H.-T.; Zhang, G.-Q.; Pang, D. Evaluation of protein pigment epithelium-derived factor (PEDF) and microvessel density (MVD) as prognostic indicators in breast cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 1719–1727. [Google Scholar] [CrossRef]
- Gnerlich, J.L.; Yao, K.A.; Fitchev, P.S.; Goldschmidt, R.A.; Bond, M.C.; Cornwell, M.; Crawford, S.E. Peritumoral expression of adipokines and fatty acids in breast cancer. Ann. Surg. Oncol. 2013, 20, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-mesenchymal transition in cancer: A historical overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Fitzgerald, D.; Shreeve, S.M.; Hua, E.; Bronder, J.L.; Weil, R.J.; Davis, S.; Stark, A.M.; Merino, M.J.; Kurek, R. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 2009, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, M.B.; Dass, C.R. Regulation of MT1-MMP and MMP-2 by the serpin PEDF: A promising new target for metastatic cancer. Cell. Physiol. Biochem. 2013, 31, 487–494. [Google Scholar] [CrossRef]
- Alcantara, M.B.; Nemazannikova, N.; Elahy, M.; Dass, C.R. Pigment epithelium-derived factor upregulates collagen I and downregulates matrix metalloproteinase 2 in osteosarcoma cells, and colocalises to collagen I and heat shock protein 47 in fetal and adult bone. J. Pharm. Pharmacol. 2014, 66, 1586–1592. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.; Dass, C.R. Anti-chondrosarcoma effects of PEDF mediated via molecules important to apoptosis, cell cycling, adhesion and invasion. Biochem. Biophys. Res. Commun. 2010, 398, 613–618. [Google Scholar] [CrossRef]
- Seki, R.; Yamagishi, S.-i.; Matsui, T.; Yoshida, T.; Torimura, T.; Ueno, T.; Sata, M.; Okamura, T. Pigment epithelium-derived factor (PEDF) inhibits survival and proliferation of VEGF-exposed multiple myeloma cells through its anti-oxidative properties. Biochem. Biophys. Res. Commun. 2013, 431, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Dass, C.R.; Shinoda, Y.; Kawano, H.; Tanaka, S.; Choong, P.F. PEDF regulates osteoclasts via osteoprotegerin and RANKL. Biochem. Biophys. Res. Commun. 2010, 391, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Caicedo, D. The role of growth hormone on ovarian functioning and ovarian angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-h.; Wang, H.-p.; Tan, J.; Yang, M.; Mao, C.-z.; Gao, S.-f.; Li, H.; Chen, H.; Cai, W.-b. Loss of pigment epithelium-derived factor leads to ovarian oxidative damage accompanied by diminished ovarian reserve in mice. Life Sci. 2019, 216, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.M.; Diamond, M.P.; Fletcher, N.M. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 2017, 145, 595–602. [Google Scholar] [CrossRef]
- Ribaux, P.; Britan, A.; Thumann, G.; Delie, F.; Petignat, P.; Cohen, M. Malignant ascites: A source of therapeutic protein against ovarian cancer? Oncotarget 2019, 10, 5894. [Google Scholar] [CrossRef]
- Gibson, D.A.; Simitsidellis, I.; Collins, F.; Saunders, P.T. Endometrial Intracrinology: Oestrogens, androgens and endometrial disorders. Int. J. Mol. Sci. 2018, 19, 3276. [Google Scholar] [CrossRef]
- Goldberg, K.; Bar-Joseph, H.; Grossman, H.; Hasky, N.; Uri-Belapolsky, S.; Stemmer, S.M.; Chuderland, D.; Shalgi, R.; Ben-Aharon, I. Pigment epithelium–derived factor alleviates tamoxifen-induced endometrial hyperplasia. Mol. Cancer Ther. 2015, 14, 2840–2849. [Google Scholar] [CrossRef]
- Guo, T.; Gu, C.; Li, B. PEDF inhibits growth and invasiveness of endometrial cancer cells in vitro. Panminerva Med. 2012, 54, 299–304. [Google Scholar]
- Chen, Y.; Li, N.; Xu, B.; Wu, M.; Yan, X.; Zhong, L.; Cai, H.; Wang, T.; Wang, Q.; Long, F. Polymer-based nanoparticles for chemo/gene-therapy: Evaluation its therapeutic efficacy and toxicity against colorectal carcinoma. Biomed. Pharmacother. 2019, 118, 109257. [Google Scholar] [CrossRef]
- Xu, B.; Xia, S.; Wang, F.; Jin, Q.; Yu, T.; He, L.; Chen, Y.; Liu, Y.; Li, S.; Tan, X. Polymeric nanomedicine for combined gene/chemotherapy elicits enhanced tumor suppression. Mol. Pharm. 2016, 13, 663–676. [Google Scholar] [CrossRef] [PubMed]
- He, S.-S.; Wu, Q.-J.; Gong, C.Y.; Luo, S.-T.; Zhang, S.; Li, M.; Lu, L.; Wei, Y.-Q.; Yang, L. Enhanced efficacy of combination therapy with adeno-associated virus-delivered pigment epithelium-derived factor and cisplatin in a mouse model of Lewis lung carcinoma. Mol. Med. Rep. 2014, 9, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.T.; Dass, C.R.; Larson, I.; Choong, P.F.; Dunstan, D.E. A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy. Biomaterials 2009, 30, 4815–4823. [Google Scholar] [CrossRef] [PubMed]

| Tissue | Cell Type | PEDF Role in Tissue | Sex Hormones and PEDF Expression | Study Type | References |
|---|---|---|---|---|---|
| Breast | BC cells | Blocks ERE signalling in BC and may prevent endocrine treatment resistance. | E2 ↓↓ PEDF | In vitro | [22] |
| Ovary | OSE cells | Regulates cell growth by blocking E2-mediated proliferation. | E2 ↓↓ PEDF | In vitro | [18] |
| OC cells | |||||
| GCs | Regulates follicular angiogenesis. | E2 ↓↓ PEDF | In vitro | [19] | |
| HCG ↓↓ PEDF | |||||
| P4 ↓↓ PEDF | |||||
| Endometrium | ECCs | Regulates endometrial angiogenesis during reproductive cycles. | E2 ↓↓ PEDF | In vitro | [20] |
| In vivo | |||||
| Clinical | |||||
| PPECs | P4 ↑↑ PEDF | ||||
| ESFs | Inhibits proliferation of normal and malignant endometrium. | E2 ↓↓ PEDF | In vitro | [23] | |
| EECs | |||||
| ECCs | P4 ↑↑ PEDF | In vivo | |||
| Prostate | PSCs | Prevents stromal vasculature and epithelial tissue growth. | DHT ↓↓ PEDF | In vitro | [21] |
| Tumour Type | PEDF Anti-Tumour Role | Pathways Involved | Effectors | References |
|---|---|---|---|---|
| Breast | Anti-proliferative ↓↓ Chemoresistance | RET/Akt | ER-α | [22] |
| Anti-angiogenic | HIF-1α/α-SMA | Unknown | [24] | |
| ↓↓ Invasiveness | ERK/Akt, NF-κB, FAK | E-cadherin, MMP-2, MMP-9, MMP-14, vimentin, Snail, fibronectin, uPAR | [25,26,27] | |
| Pro-apoptotic | PPAR-γ | Fas, MAX, caspases | [17] | |
| Prostate | Anti-metastatic | PAI-2, Caveolin-1, PPAR-γ/NF- κB | uPA, proteases, Il-8 | [28,29,30] |
| Anti-angiogenic | ||||
| Anti-proliferative | ||||
| Pro-apoptotic | LR/JNK/PPAR-γ/FasL | Caspases | [31,32] | |
| ↑↑ Chemosensitivity | Il-8/CXCR1 | Macrophages | [33,34] | |
| ↓↓ Invasiveness | ||||
| Anti-proliferative | ||||
| ↑↑ Anti-tumour immune response | Unknown | PEDF-R, CD-47, ATP5B | [35] | |
| Ovarian | Anti-proliferative | Unknown | Unknown | [18] |
| Pro-apoptotic | ||||
| Endometrial | Pro-apoptotic | PI3K/Akt/mTOR, JNK | VEGF, MMPs, ER-α, c-Myc | [23] |
| Anti-metastatic | ||||
| Anti-angiogenic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brook, N.; Brook, E.; Dass, C.R.; Chan, A.; Dharmarajan, A. Pigment Epithelium-Derived Factor and Sex Hormone-Responsive Cancers. Cancers 2020, 12, 3483. https://doi.org/10.3390/cancers12113483
Brook N, Brook E, Dass CR, Chan A, Dharmarajan A. Pigment Epithelium-Derived Factor and Sex Hormone-Responsive Cancers. Cancers. 2020; 12(11):3483. https://doi.org/10.3390/cancers12113483
Chicago/Turabian StyleBrook, Naomi, Emily Brook, Crispin R. Dass, Arlene Chan, and Arun Dharmarajan. 2020. "Pigment Epithelium-Derived Factor and Sex Hormone-Responsive Cancers" Cancers 12, no. 11: 3483. https://doi.org/10.3390/cancers12113483
APA StyleBrook, N., Brook, E., Dass, C. R., Chan, A., & Dharmarajan, A. (2020). Pigment Epithelium-Derived Factor and Sex Hormone-Responsive Cancers. Cancers, 12(11), 3483. https://doi.org/10.3390/cancers12113483






