Nerve-Sparing Systematic Lymph Node Dissection in Gynaecological Oncology: An Innovative Neuro-Anatomical and Surgical Protocol for Enhanced Functional Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
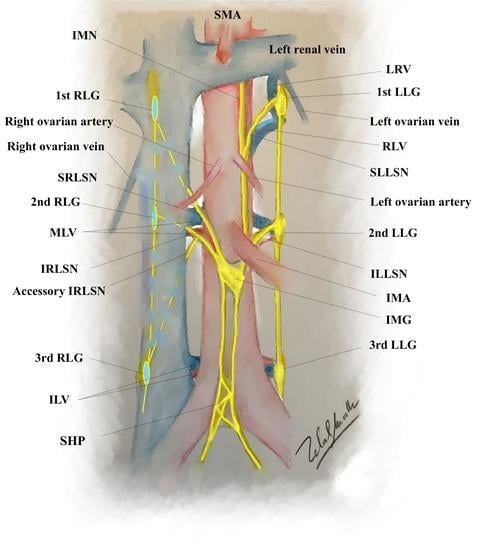
2.1. The Anatomy of Aortic Plexus
2.2. Dissection of the Right Cord of the Aortic Plexus
2.3. Dissection of the Left Cord of the Aortic Plexus
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Emons, G. Significance of Lymph Node Dissection in Gynecological Oncology. Oncol. Res. Treat. 2014, 37, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Papadia, A.; Remorgida, V.; Salom, E.M.; Ragni, N. Laparoscopic pelvic and paraaortic lymphadenectomy in gynecologic oncology. J. Am. Assoc. Gynecol. Laparosc. 2004, 11, 297–306. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Angioli, R.; Bailey, A.E.; Broach, V.; Buda, A.; Coriddi, M.R.; Dayan, J.H.; Frumovitz, M.; Kim, Y.M.; Kimmig, R.; et al. IGCS Intraoperative Technology Taskforce. Update on near infrared imaging technology: Beyond white light and the naked eye, indocyanine green and near infrared technology in the treatment of gynecologic cancers. Int. J. Gynecol. Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.-W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik, Therapie und Nachsorge maligner Ovarialtumoren, Kurzversion 4.0, 2020, AWMF-Registernummer: 032/035OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/ovarialkarzinom/ (accessed on 20 October 2020).
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Muallem, M.Z.; Jöns, T.; Seidel, N.; Sehouli, J.; Diab, Y.; Querleu, D. A Concise Paradigm on Radical Hysterectomy: The Comprehensive Anatomy of Parametrium, Paracolpium and the Pelvic Autonomic Nerve System and Its Surgical Implication. Cancers 2020, 12, 1839. [Google Scholar] [CrossRef]
- Muallem, M.Z.; Miranda, A.; Muallem, J. Nerve-sparing radical hysterectomy—Muallem technique with explanation of parametrium and paracolpium precise anatomy. Int. J. Gynecol. Cancer 2020. [Google Scholar] [CrossRef]
- Muallem, M.Z.; Armbrust, R.; Neymeyer, J.; Miranda, A.; Muallem, J. Nerve Sparing Radical Hysterectomy: Short-Term Oncologic, Surgical, and Functional Outcomes. Cancers 2020, 12, 483. [Google Scholar] [CrossRef]
- Beveridge, T.S.; Johnson, M.; Power, A.; Power, N.E.; Allman, B.L. Anatomy of the nerves and ganglia of the aortic plexus in males. J. Anat. 2015, 226, 93–103. [Google Scholar] [CrossRef]
- Beveridge, T.S.; Johnson, M.; Power, N.E.; Allman, B.L. Histological verification of the prehypogastric and ovarian ganglia confirms a bilaterally symmetrical organization of the ganglia comprising the aortic plexus in female human cadavers. J. Anat. 2016, 228, 805–811. [Google Scholar] [CrossRef]
- Clemente, C.D. Gray’s Anatomy of the Human Body, 30th ed.; Lea & Febiger: Philadelphia, PA, USA, 1985. [Google Scholar]
- Shiozawa, T.; Huebner, M.; Hirt, B.; Wallwiener, D.; Reisenauer, C. Nerve-preserving sacrocolpopexy: Anatomical study and surgical approach. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 152, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ripperda, C.M.; Jackson, L.A.; Phelan, J.N.; Carrick, K.S.; Corton, M.M. Anatomic relationships of the pelvic autonomic nervous system in female cadavers: Clinical applications to pelvic surgery. Am. J. Obstet. Gynecol. 2017, 216, 388.e1–388.e7. [Google Scholar] [CrossRef] [PubMed]
- Muallem, M.Z.; Diab, Y.; Sehouli, J.; Fujii, S. Nerve-sparing radical hysterectomy: Steps to standardize surgical technique. Int. J. Gynecol. Cancer 2019, 29, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Haroun, H.S.W. Clinical anatomy of the splanchnic nerves. MOJ Anat. Physiol. 2018, 5, 87–90. [Google Scholar] [CrossRef]
- Nagar, H. The role of lymphadenectomy in ovarian epithelial cancer. In Ovarian Cancer—From Pathogenesis to Treatment; InTechopen: Londeon, UK, 2018. [Google Scholar] [CrossRef][Green Version]
- Fastrez, M.; Goffin, F.; Vergote, I.; Vandromme, J.; Petit, P.; Leunen, K.; Degueldre, M. Multi-center experience of robot- assisted laparoscopic para-aortic lymphadenectomy for staging of locally advanced cervical carcinoma. Acta Obstet. Gynecol. Scand. 2013, 92, 895–901. [Google Scholar] [CrossRef]
- Vandeperre, A.; Van Limbergen, E.; Leunen, K.; Moerman, P.; Amant, F.; Vergote, I. Para-aortic lymph node metastases in locally advanced cervical cancer: Comparison between surgical staging and imaging. Gynecol. Oncol. 2015, 138, 299–303. [Google Scholar] [CrossRef]
- Gouy, S.; Morice, P.; Narducci, F.; Uzan, C.; Gilmore, J.; Kolesnikov-Gauthier, H.; Querleu, D.; Haie-Meder, C.; Leblanc, E. Nodal-staging surgery for locally advanced cervical cancer in the era of PET. Lancet Oncol. 2012, 13, e212–e220. [Google Scholar] [CrossRef]
- Uzan, C.; Souadka, A.; Gouy, S.; Debaere, T.; Duclos, J.; Lumbroso, J.; Haie-Meder, C.; Morice, P. Analysis of morbidity and clinical implications of laparoscopic para-aortic lymphadenectomy in a continuous series of 98 patients with advanced-stage cervical cancer and negative PET-CT imaging in the para-aortic area. Oncologist 2011, 16, 1021–1027. [Google Scholar] [CrossRef]
- Loverix, L.; Salihi, R.R.; Van Nieuwenhuysen, E.; Concin, N.; Han, S.; van Gorp, T.; Vergote, I. Para-aortic lymph node surgical staging in locally-advanced cervical cancer: comparison between robotic versus conventional laparoscopy. Int. J. Gynecol. Cancer 2020, 30, 466–472. [Google Scholar] [CrossRef]
- Marnitz-Schulze, S.; Tsunoda, A.; Martus, P.; Vieira, M.V.; Affonso, R.; Nunes, J.S.; Budach, V.; Schneider, A.; Hertel, H.; Mustea, A.; et al. Uterus-11 study: A randomized clinical trial on surgical staging versus CT-staging prior to primary chemoradiation in patients with FIGO 2009 stages IIb-IVa cervical cancer. Int. J. Gynecol. Cancer 2019, 29. [Google Scholar] [CrossRef]
- Andersen, B.L. Quality of life for women with gynecologic cancer. Curr. Opin. Obstet. Gynecol. 1995, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; Woods, X.A.; Copeland, L.J. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J. Consult. Clin. Psychol. 1997, 65, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Carmack Taylor, C.L.; Basen-Engquist, K.; Shinn, E.H.; Bodurka, D.C. Predictors of sexual functioning in ovarian cancer patients. J. Clin. Oncol. 2004, 22, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.T.; Groenvold, M.; Klee, M.C.; Thranov, I.; Petersen, M.A.; Machin, D. Early-stage cervical carcinoma, radical hysterectomy, and sexual function. A longitudinal study. Cancer 2004, 100, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.M.; Pieterse, Q.D.; van Lonkhuijzen, L.R.C.W.; Trimbos, B.J.B.M.Z.; Creutzberg, C.L.; Kenter, G.G.; de Kroon, C.D.; Ter Kuile, M.M. A Controlled Study on Vaginal Blood Flow during Sexual Arousal among Early-Stage Cervical Cancer Survivors Treated with Conventional Radical or Nerve-Sparing Surgery with or without Radiotherapy. Int. J. Gynecol. Cancer 2017, 27, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Maas, K.; Moriya, Y.; Kenter, G.; Trimbos, B.; van de Velde, C. A plea for preservation of the pelvic autonomic nerves. Lancet 1999, 354, 772–773. [Google Scholar] [CrossRef]
- Meston, C.M.; Frohlich, P.F. The neurobiology of sexual function. Arch. Gen. Psychiatry 2000, 57, 1012–1030. [Google Scholar] [CrossRef]
- Basson, R. A model of women’s sexual arousal. J. Sex Marital Ther. 2002, 28, 1–10. [Google Scholar] [CrossRef]
- Laan, E.; Everaerd, W.; van der Velde, J.; Geer, J.H. Determinants of subjective experience of sexual arousal in women: Feedback from genital arousal and erotic stimulus content. Psychophysiology 1995, 32, 444–451. [Google Scholar] [CrossRef]
- Lorenz, T.A.; Harte, C.B.; Hamilton, L.D.; Meston, C.M. Evidence for a curvilinear relationship between sympathetic nervous system activation and women’s physiological sexual arousal. Psychophysiology 2012, 49, 111–117. [Google Scholar] [CrossRef]
- Meston, C.M. Sympathetic nervous system activity and female sexual arousal. Am. J. Cardiol. 2000, 86, 30F–34F. [Google Scholar] [CrossRef]
- Hasenburg, A.; Sehouli, J.; Lampe, B.; Reuss, A.; Schmalfeld, B.; Belau, A.K.; Bossart, M.; Mahner, S.; Hillemanns, P.; Petry, U.; et al. LION-PAW (lymphadenectomy in ovarian neoplasm) sexual function assessment: A prospective sub-study of the LION trial. Int. J. Gynecol. Cancer 2020, 30, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Muallem, M.Z. (Department of Gynecology with Center for Oncological Surgery, Charité—Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Virchow Campus Clinic, Charité Medical University, 13353 Berlin, Germany). Personal communication, 2020.
- Muallem, M.Z.; Sehouli, J.; Miranda, A.; Richter, R.; Almuheimid, J. Total retroperitoneal en bloc resection of multivisceral-peritoneal packet (TROMP operation): A novel surgical technique for advanced ovarian cancerInternational. J. Gynecol. Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Muallem, M.Z. Nerve Sparing Aortic Lymph Node Dissection. Available online: https://www.youtube.com/watch?v=qGqJ6NcdWz4 (accessed on 20 October 2020).
- Muallem, M.Z. Available online: https://www.youtube.com/watch?v=jI5jEivNJuc (accessed on 20 October 2020).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muallem, M.Z.; Diab, Y.; Jöns, T.; Sehouli, J.; Muallem, J. Nerve-Sparing Systematic Lymph Node Dissection in Gynaecological Oncology: An Innovative Neuro-Anatomical and Surgical Protocol for Enhanced Functional Outcomes. Cancers 2020, 12, 3473. https://doi.org/10.3390/cancers12113473
Muallem MZ, Diab Y, Jöns T, Sehouli J, Muallem J. Nerve-Sparing Systematic Lymph Node Dissection in Gynaecological Oncology: An Innovative Neuro-Anatomical and Surgical Protocol for Enhanced Functional Outcomes. Cancers. 2020; 12(11):3473. https://doi.org/10.3390/cancers12113473
Chicago/Turabian StyleMuallem, Mustafa Zelal, Yasser Diab, Thomas Jöns, Jalid Sehouli, and Jumana Muallem. 2020. "Nerve-Sparing Systematic Lymph Node Dissection in Gynaecological Oncology: An Innovative Neuro-Anatomical and Surgical Protocol for Enhanced Functional Outcomes" Cancers 12, no. 11: 3473. https://doi.org/10.3390/cancers12113473
APA StyleMuallem, M. Z., Diab, Y., Jöns, T., Sehouli, J., & Muallem, J. (2020). Nerve-Sparing Systematic Lymph Node Dissection in Gynaecological Oncology: An Innovative Neuro-Anatomical and Surgical Protocol for Enhanced Functional Outcomes. Cancers, 12(11), 3473. https://doi.org/10.3390/cancers12113473