Hitting the Target but Missing the Point: Recent Progress towards Adenovirus-Based Precision Virotherapies
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Use of Targeted Therapies
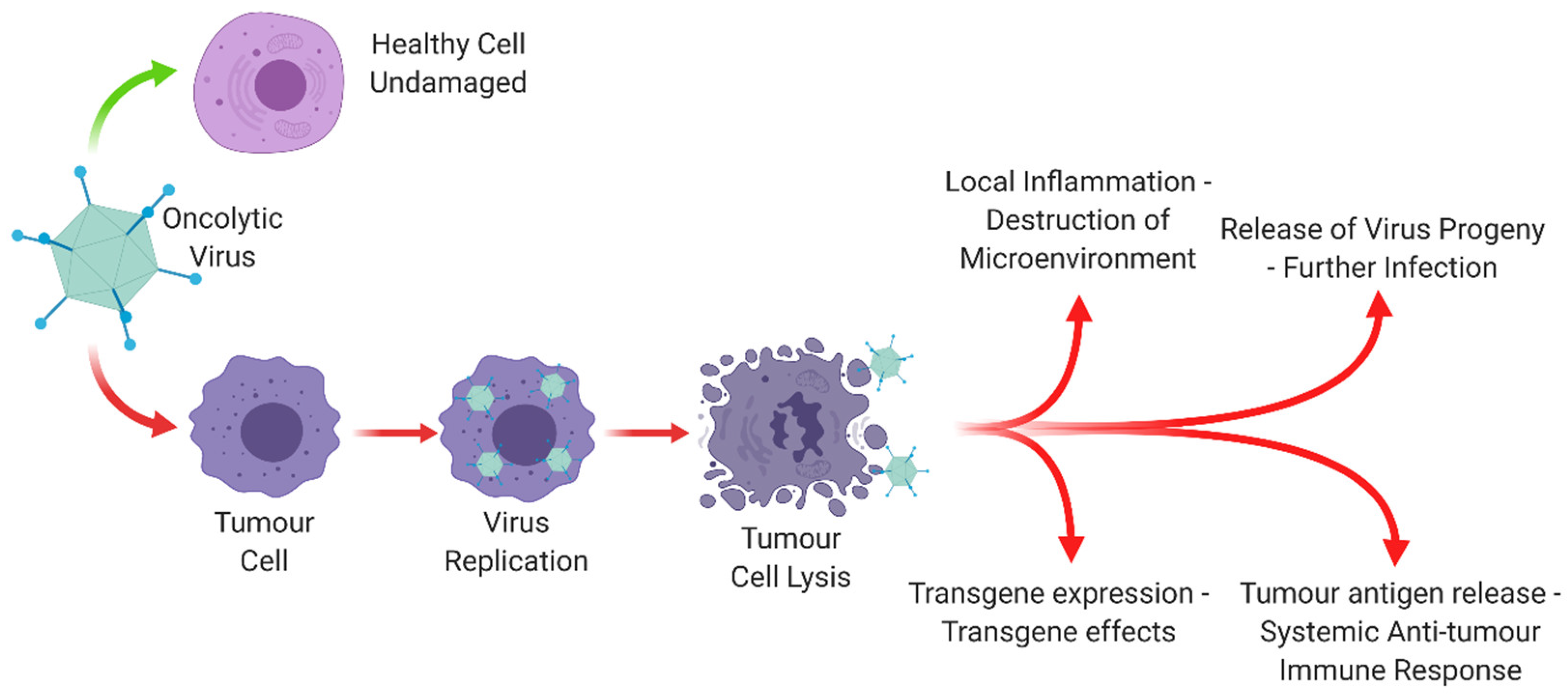
1.2. Oncolytic Viruses
1.3. Adenovirus as an Oncolytic Virotherapy
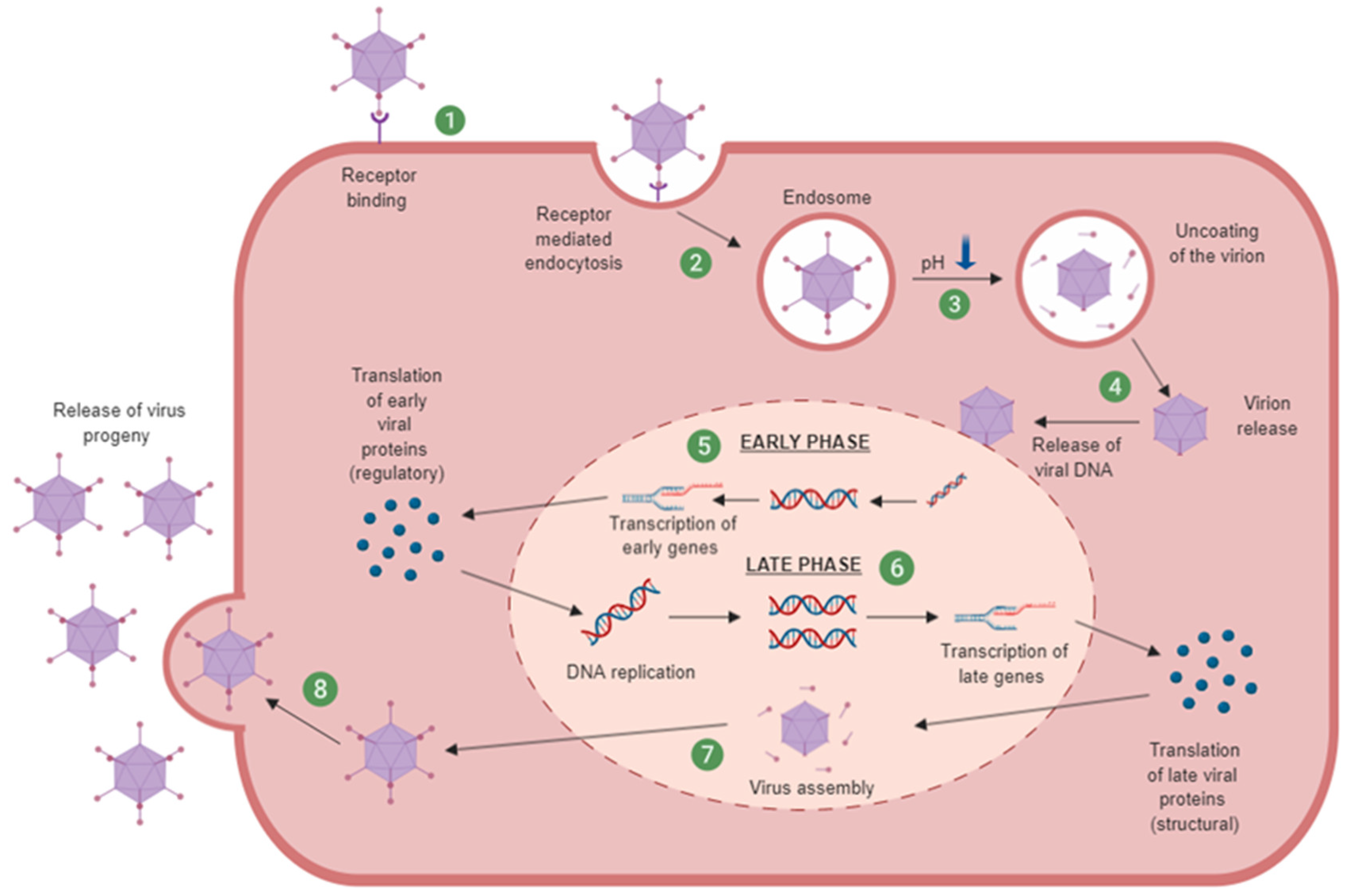
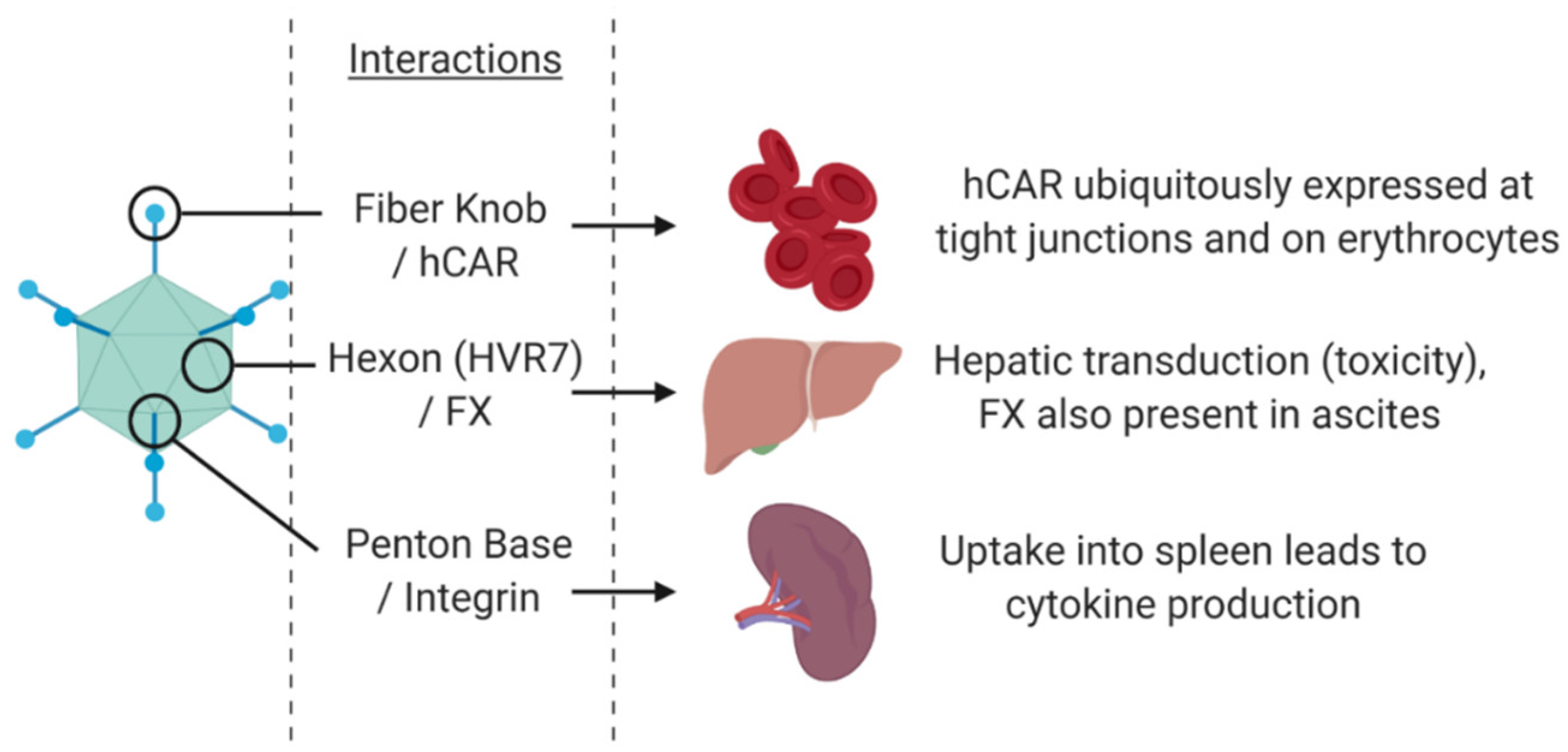
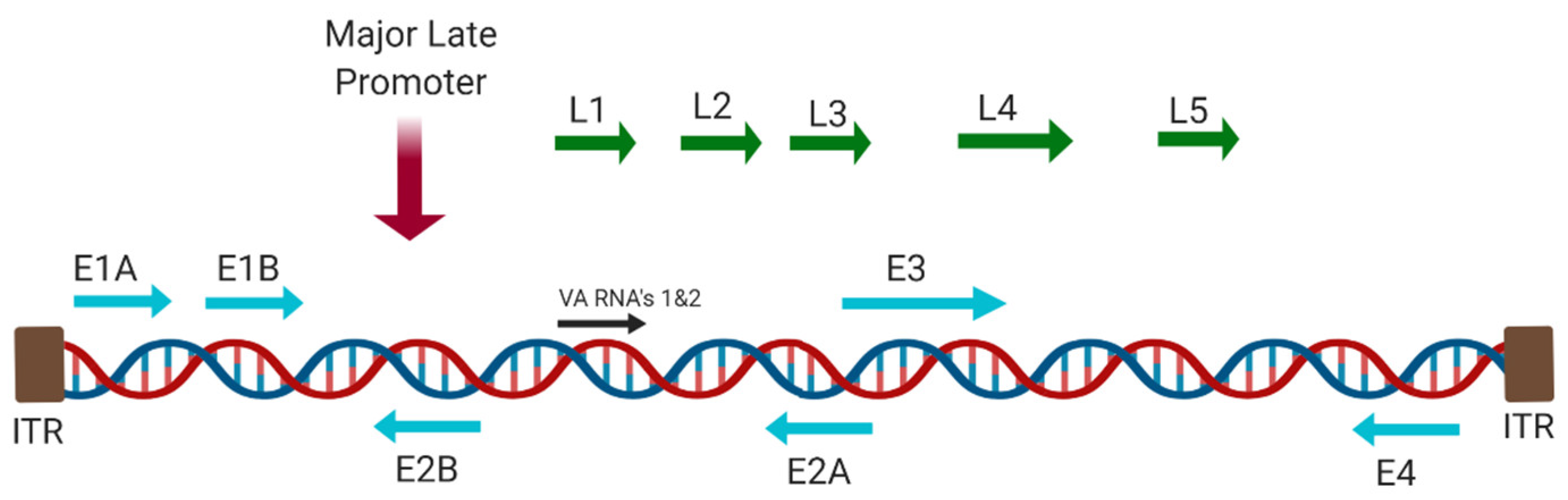
1.3.1. Adenovirus Cell Entry and Trafficking
1.3.2. Oncolytic Adenovirus
2. Genetic Engineering of Oncolytic Adenovirus
3. Current Clinical Applications of Oncolytic Adenoviral Therapies
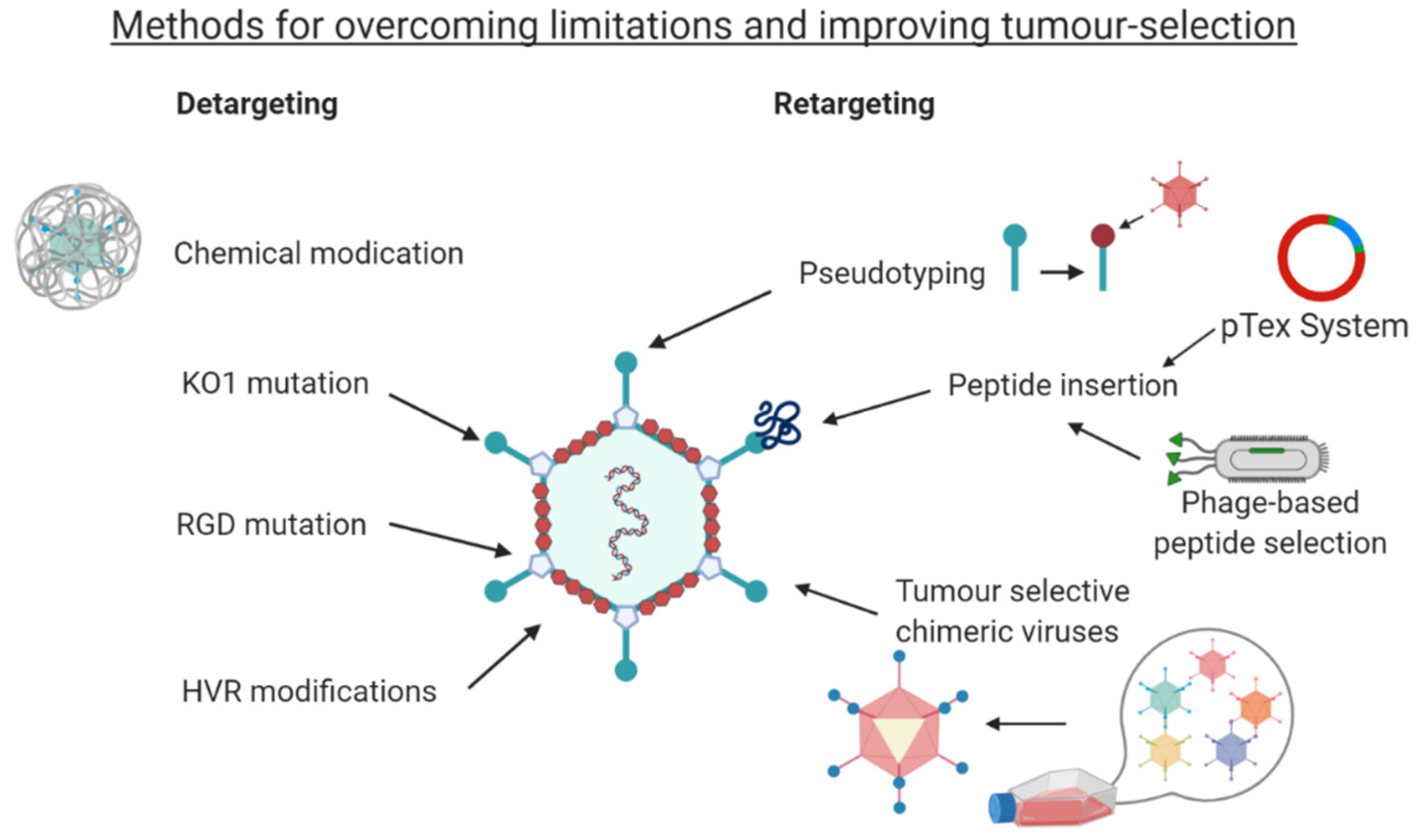
4. Detargeting Ads
5. Retargeting Strategies
5.1. Pseudotyping
5.2. Peptide Retargeting
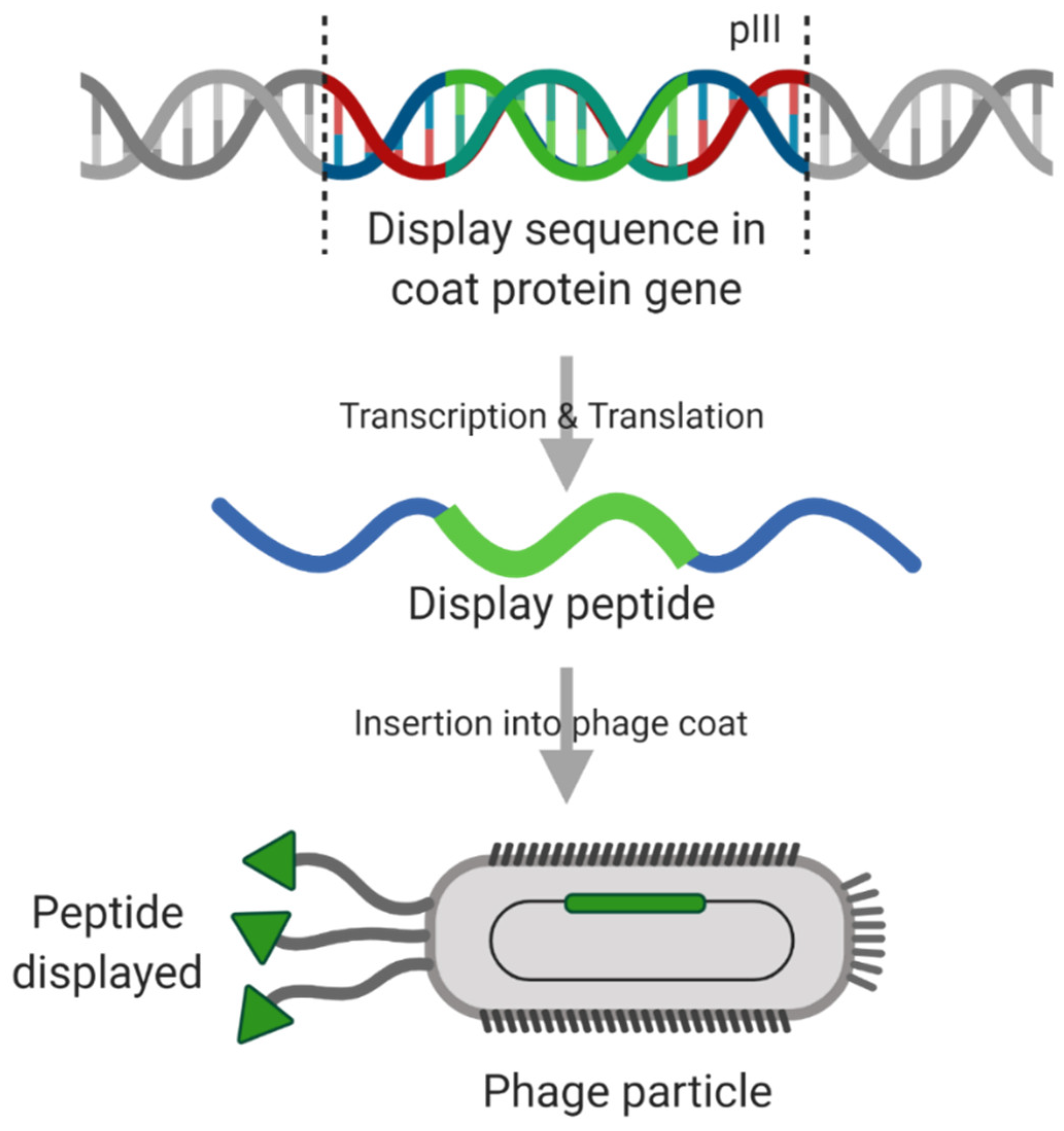
5.3. Techniques for Targeting Peptide Discovery
6. Conclusions
Funding
Conflicts of Interest
References
- Cancer Research UK. Cancer Survival Statistics|Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/survival (accessed on 23 April 2019).
- Yan, L.; Rosen, N.; Arteaga, C. Targeted cancer therapies. Chin. J. Cancer 2011, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L. Trastuzumab, an appropriate first-line single-agent therapy for HER2-overexpressing metastatic breast cancer. Breast Cancer Res. 2003, 5, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Eiger, D.; Pondé, N.F.; De Azambuja, E. Pertuzumab in HER2-positive early breast cancer: Current use and perspectives. Futur. Oncol. 2019, 15, 1823–1843. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M.; et al. Efficacy and Safety of Trastuzumab as a Single Agent in First-Line Treatment of HER2 -Overexpressing Metastatic Breast Cancer. J. Clin. Oncol. 2002, 20, 719–726. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Y.; Sun, T.; Wan, D.; Sheng, L.; Li, W.; Zhu, H.; Li, Y.; Lu, J. Overall Survival Benefit from Trastuzumab-Based Treatment in HER2-Positive Metastatic Breast Cancer: A Retrospective Analysis. Oncol. Res. Treat. 2018, 41, 450–455. [Google Scholar] [CrossRef]
- Ivy, S.P.; Wick, J.Y.; Kaufman, B.M. an overview of small-molecule inhibitors of VegFr signaling. Nat. Rev. Clin. Oncol 2009, 6, 569–579. [Google Scholar] [CrossRef]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef]
- Bourgeois-Daigneault, M.-C.; Roy, D.G.; Aitken, A.S.; El Sayes, N.; Martin, N.T.; Varette, O.; Falls, T.; St-Germain, L.E.; Pelin, A.; Lichty, B.D.; et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018, 10, eaao1641. [Google Scholar] [CrossRef]
- Chawla, S.P.; Chua, V.S.; Kim, K.; Dy, P.S.; Paz, M.K.; Angel, N.; Wang, K.; Moradkhani, A.; Quon, D.; Wong, S.; et al. The TNT protocol: A phase II study using talimogene laherparepvec (TVEC), nivolumab (N) and trabectedin (T) as first, second/third line therapy for advanced sarcoma, including desmoid tumor and chordoma. J. Clin. Oncol. 2020, 38, TPS11572. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Talimogene Laherparepvec and Pembrolizumab in Treating Patients With Stage III-IV Melanoma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02965716 (accessed on 28 October 2020).
- ClinicalTrials.gov. Ipilimumab, Nivolumab, and Talimogene Laherparepvec Before Surgery in Treating Participants With Localized, Triple-Negative or Estrogen Receptor Positive, HER2 Negative Breast Cancer-deleted-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04185311 (accessed on 28 October 2020).
- Uusi-Kerttula, H.; Davies, J.A.; Thompson, J.M.; Wongthida, P.; Evgin, L.; Shim, K.G.; Bradshaw, A.; Baker, A.T.; Rizkallah, P.J.; Jones, R.; et al. Ad5NULL-A20: A Tropism-Modified, αvβ6 Integrin-Selective Oncolytic Adenovirus for Epithelial Ovarian Cancer Therapies. Clin. Cancer Res. 2018, 24, 4215–4224. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Anguiano-Zarate, S.S.; Matchett, W.E.; Barry, M.E.; Barry, M.A. Retargeted and detargeted adenovirus for gene delivery to the muscle. Virology 2018, 514, 118–123. [Google Scholar] [CrossRef]
- Belousova, N.; Korokhov, N.; Krendelshchikova, V.; Simonenko, V.; Mikheeva, G.; Triozzi, P.L.; Aldrich, W.A.; Banerjee, P.T.; Gillies, S.D.; Curiel, D.T.; et al. Genetically Targeted Adenovirus Vector Directed to CD40-Expressing Cells. J. Virol. 2003, 77, 11367–11377. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Ruby, C.E.; Hughes, T.; Slingluff, C.L. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef]
- Kohlhapp, F.J.; Kaufman, H.L. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin. Cancer Res. 2016, 22, 1048–1054. [Google Scholar] [CrossRef]
- Farassati, F.; Yang, A.D.; Lee, P.W.K. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat. Cell Biol. 2001, 3, 745–750. [Google Scholar] [CrossRef]
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccin. Immunother. 2018, 14, 839–846. [Google Scholar] [CrossRef]
- Shen, Y.; Nemunaitis, J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006, 13, 975–992. [Google Scholar] [CrossRef]
- Rehman, H.; Silk, A.W.; Kane, M.P.; Kaufman, H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer 2016, 4, 53. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.A.; Amatruda, T.; Menunaitis, J.J.; Chesney, J.; Puzanov, I.; Harrington, K.; Zhang, Y.; Chen, L.; et al. Durable complete responses (CR) in patients (pts) with stage IIIB-IV melanoma treated with talimogene laherparepvec (T-VEC) in OPTiM. In Proceedings of the Society for Melanoma Research 2015 Congress, San Francisco, CA USA, 18–21 November 2015; Volume 28, pp. 753–826. [Google Scholar] [CrossRef][Green Version]
- Greig, S.L. Talimogene Laherparepvec: First Global Approval. Drugs 2016, 76, 147–154. [Google Scholar] [CrossRef]
- Sun, L.; Funchain, P.; Song, J.M.; Rayman, P.; Tannenbaum, C.; Ko, J.; Mcnamara, M.; Marcela Diaz-Montero, C.; Gastman, B. Talimogene Laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III-IV melanoma: A case series. J. Immunother. Cancer 2018, 6, 36. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [PubMed]
- Russell, L.; Peng, K.W. The emerging role of oncolytic virus therapy against cancer. Chin. Clin. Oncol. 2018, 7, 16. [Google Scholar] [PubMed]
- Kuryk, L.; Møller, A.S.W.; Jaderberg, M. Abscopal effect when combining oncolytic adenovirus and checkpoint inhibitor in a humanized NOG mouse model of melanoma. J. Med. Virol. 2019, 91, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Wold, W.; Toth, K. Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr. Gene Ther. 2014, 13, 421–433. [Google Scholar]
- Phillips, M.; Stuart, J.; Rodríguez Stewart, R.; Berry, J.; Mainou, B.; Boehme, K. Current understanding of reovirus oncolysis mechanisms. Oncolytic Virother. 2018, 7, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Guse, K.; Cerullo, V.; Hemminki, A. Oncolytic vaccinia virus for the treatment of cancer. Expert Opin. Biol. Ther. 2011, 11, 595–608. [Google Scholar] [CrossRef]
- HAdV Working Group. Available online: http://hadvwg.gmu.edu/ (accessed on 30 October 2020).
- Lynch, J.P.; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science (80-) 1997, 275, 1320–1323. [Google Scholar]
- Gaggar, A.; Shayakhmetov, D.M.; Lieber, A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003, 9, 1408–1412. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.Y.; Liu, Y.; Persson, J.; Beyer, I.; Möller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.B.; et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011, 17, 96–104. [Google Scholar] [CrossRef]
- Arnberg, N.; Edlund, K.; Kidd, A.H.; Wadell, G. Adenovirus Type 37 Uses Sialic Acid as a Cellular Receptor. J. Virol. 2000, 74, 42–48. [Google Scholar] [CrossRef]
- Cupelli, K.; Stehle, T. Viral attachment strategies: The many faces of adenoviruses. Curr. Opin. Virol. 2011, 1, 84–91. [Google Scholar]
- Burmeister, W.P.; Guilligay, D.; Cusack, S.; Wadell, G.; Arnberg, N. Crystal Structure of Species D Adenovirus Fiber Knobs and Their Sialic Acid Binding Sites. J. Virol. 2004, 78, 7727–7736. [Google Scholar] [CrossRef]
- Marttila, M.; Persson, D.; Gustafsson, D.; Liszewski, M.K.; Atkinson, J.P.; Wadell, G.; Arnberg, N. CD46 Is a Cellular Receptor for All Species B Adenoviruses except Types 3 and 7. J. Virol. 2005, 79, 14429–14436. [Google Scholar]
- Stewart, P.; Chiu, C.; Huang, S.; Muir, T.; Zhao, Y.; Chait, B.; Mathias, P.; Nemerow, G. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization cell membrane permeabilization. EMBO J. 1997, 16, 1189–1198. [Google Scholar]
- Meier, O.; Greber, U.F. Adenovirus endocytosis. J. Gene Med. 2003, 5, 451–462. [Google Scholar] [CrossRef]
- Russell, W.C. Update on adenovirus and its vectors. J. Gen. Virol. 2000, 81, 2573–2604. [Google Scholar]
- Russell, W.C. Adenoviruses: Update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar]
- Hoeben, R.C.; Uil, T.G. Adenovirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a013003. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Seymour, L.W.; Fisher, K.D. Oncolytic viruses: Finally delivering. Br. J. Cancer 2016, 114, 357–361. [Google Scholar] [CrossRef]
- Cheng, P.H.; Wechman, S.L.; McMasters, K.M.; Zhou, H.S. Oncolytic replication of E1b-deleted adenoviruses. Viruses 2015, 7, 5767–5779. [Google Scholar] [CrossRef]
- Everts, B.; Van Der Poel, H.G. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. 2005, 12, 141–161. [Google Scholar] [CrossRef]
- Larson, C.; Oronsky, B.; Scicinski, J.; Fanger, G.R.; Stirn, M.; Oronsky, A.; Reid, T.R. Going viral: A review of replication-selective oncolytic adenoviruses. Oncotarget 2015, 6, 19976–19989. [Google Scholar] [CrossRef]
- Rodriguez, R.; Schuur, E.R.; Lim, H.Y.; Henderson, G.A.; Simons, J.W.; Henderson, D.R. Prostate Attenuated Replication Competent Adenovirus (ARCA) CN706: A Selective Cytotoxic for Prostate-specific Antigen-positive Prostate Cancer Cells. Cancer Res. 1997, 57, 2559–2563. [Google Scholar]
- Tanoue, K.; Shaw, A.R.; Watanabe, N.; Porter, C.; Rana, B.; Gottschalk, S.; Brenner, M.; Suzuki, M. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of Chimeric antigen receptor t cells in solid tumors. Cancer Res. 2017, 77, 2040–2051. [Google Scholar] [CrossRef]
- Dias, J.D.; Hemminki, O.; Diaconu, I.; Hirvinen, M.; Bonetti, A.; Guse, K.; Escutenaire, S.; Kanerva, A.; Pesonen, S.; Löskog, A.; et al. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012, 19, 988–998. [Google Scholar] [CrossRef]
- Cervera-Carrascon, V.; Siurala, M.; Santos, J.M.; Havunen, R.; Tähtinen, S.; Karell, P.; Sorsa, S.; Kanerva, A.; Hemminki, A. TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. Oncoimmunology 2018, 7, e1412902. [Google Scholar] [CrossRef]
- Lapteva, N.; Aldrich, M.; Weksberg, D.; Rollins, L.; Goltsova, T.; Chen, S.Y.; Huang, X.F. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J. Immunother. 2009, 32, 145–156. [Google Scholar] [CrossRef]
- Kumon, H.; Ariyoshi, Y.; Sasaki, K.; Sadahira, T.; Araki, M.; Ebara, S.; Yanai, H.; Watanabe, M.; Nasu, Y. Adenovirus vector carrying REIC/DKK-3 gene: Neoadjuvant intraprostatic injection for high-risk localized prostate cancer undergoing radical prostatectomy. Cancer Gene Ther. 2016, 23, 400. [Google Scholar] [CrossRef]
- Singh, G.; Robinson, C.M.; Dehghan, S.; Schmidt, T.; Seto, D.; Jones, M.S.; Dyer, D.W.; Chodosh, J. Overreliance on the Hexon Gene, Leading to Misclassification of Human Adenoviruses. J. Virol. 2012, 86, 4693–4695. [Google Scholar] [CrossRef]
- Pearl, T.M.; Markert, J.M.; Cassady, K.A.; Ghonime, M.G. Oncolytic Virus-Based Cytokine Expression to Improve Immune Activity in Brain and Solid Tumors. Mol. Ther. Oncolytics 2019, 13, 14–21. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Search of: Adenovirus|Cancer-List Results-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=Cancer&term=Adenovirus&cntry=&state=&city=&dist=&Search=Search (accessed on 30 April 2019).
- Jiang, H.; Gomez-Manzano, C.; Lang, F.; Alemany, R.; Fueyo, J. Oncolytic Adenovirus: Preclinical and Clinical Studies in Patients with Human Malignant Gliomas. Curr. Gene Ther. 2009, 9, 422–427. [Google Scholar] [CrossRef]
- Freytag, S.O.; Movsas, B.; Stricker, H. Clinical Trials of Oncolytic Adenovirus-Mediated Gene Therapy. Mol. Ther. 2016, 24, S205. [Google Scholar] [CrossRef]
- Abbink, P.; Lemckert, A.A.C.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative Seroprevalence and Immunogenicity of Six Rare Serotype Recombinant Adenovirus Vaccine Vectors from Subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef]
- Dakin, R.S.; Parker, A.L.; Delles, C.; Nicklin, S.A.; Baker, A.H. Efficient Transduction of Primary Vascular Cells by the Rare Adenovirus Serotype 49 Vector. Hum. Gene Ther. 2015, 26, 312–319. [Google Scholar] [CrossRef]
- Parker, A.L.; Waddington, S.N.; Buckley, S.M.K.; Custers, J.; Havenga, M.J.E.; van Rooijen, N.; Goudsmit, J.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Effect of Neutralizing Sera on Factor X-Mediated Adenovirus Serotype 5 Gene Transfer. J. Virol. 2009, 83, 479–483. [Google Scholar] [CrossRef]
- Alemany, R. Oncolytic Adenoviruses in Cancer Treatment. Biomedicines 2014, 2, 36–49. [Google Scholar] [CrossRef]
- Carlisle, R.C.; Di, Y.; Cerny, A.M.; Sonnen, A.F.P.; Sim, R.B.; Green, N.K.; Subr, V.; Ulbrich, K.; Gilbert, R.J.C.; Fisher, K.D.; et al. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood 2009, 113, 1909–1918. [Google Scholar] [CrossRef]
- Smith, T.; Idamakanti, N.; Kylefjord, H.; Rollence, M.; King, L.; Kaloss, M.; Kaleko, M.; Stevenson, S.C. In Vivo Hepatic Adenoviral Gene Delivery Occurs Independently of the Coxsackievirus–Adenovirus Receptor. Mol. Ther. 2002, 5, 770–779. [Google Scholar] [CrossRef]
- Schlipp, A.; Schinner, C.; Spindler, V.; Vielmuth, F.; Gehmlich, K.; Syrris, P.; McKenna, W.J.; Dendorfer, A.; Hartlieb, E.; Waschke, J. Desmoglein-2 interaction is crucial for cardiomyocyte cohesion and function. Cardiovasc. Res. 2014, 104, 245–257. [Google Scholar] [CrossRef]
- Nava, P.; Hopkins, A.N.; Laukoetter, M.G.; Green, K.J.; Parkos, C.A.; Nusrat, A. A role of Desmoglein 2 in intestinal epithelial apoptosis. FASEB J. 2007, 21, A192. [Google Scholar] [CrossRef]
- Baker, A.T.; Mundy, R.M.; Davies, J.A.; Rizkallah, P.J.; Parker, A.L. Human adenovirus type 26 uses sialic acid-bearing glycans as a primary cell entry receptor. Sci. Adv. 2019, 5, eaax3567. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Waddington, S.N.; Nicol, C.G.; Shayakhmetov, D.M.; Buckley, S.M.; Denby, L.; Kemball-Cook, G.; Ni, S.; Lieber, A.; McVey, J.H.; et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 2006, 108, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Waddington, S.N.; McVey, J.H.; Bhella, D.; Parker, A.L.; Barker, K.; Atoda, H.; Pink, R.; Buckley, S.M.K.; Greig, J.A.; Denby, L.; et al. Adenovirus Serotype 5 Hexon Mediates Liver Gene Transfer. Cell 2008, 132, 397–409. [Google Scholar] [CrossRef]
- Stewart, P.L.; Nemerow, G.R. Cell integrins: Commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007, 15, 500–507. [Google Scholar] [CrossRef]
- Nemerow, G.R. A new link between virus cell entry and inflammation: Adenovirus interaction with integrins induces specific proinflammatory responses. Mol. Ther. 2009, 17, 1490–1491. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Miao, E.A.; Iwakura, Y.; Murali-Krishna, K.; Aderem, A.; Flavell, R.A.; Papayannopoulou, T.; Shayakhmetov, D.M. Virus Binding to a Plasma Membrane Receptor Triggers Interleukin-1α-Mediated Proinflammatory Macrophage Response In Vivo. Immunity 2009, 31, 110–121. [Google Scholar] [CrossRef]
- Bradshaw, A.C.; Coughlan, L.; Miller, A.M.; Alba, R.; Van Rooijen, N.; Nicklin, S.A.; Baker, A.H. Biodistribution and inflammatory profiles of novel penton and hexon double-mutant serotype 5 adenoviruses. J. Control. Release 2012, 164, 394–402. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Armendáriz-Borunda, J.; Bastidas-Ramírez, B.E.; Sandoval-Rodríguez, A.; González-Cuevas, J.; Gómez-Meda, B.; García-Bañuelos, J. Production of first generation adenoviral vectors for preclinical protocols: Amplification, purification and functional titration. JBIOSC 2011, 112, 415–421. [Google Scholar] [CrossRef]
- Rowe, D.T.; Branton, P.E.; Graham, F.L. The kinetics of synthesis of early viral proteins in KB cells infected with wild-type and transformation-defective host-range mutants of human adenovirus type 5. J. Gen. Virol. 1984, 65, 585–597. [Google Scholar] [CrossRef]
- Saha, B.; Parks, R.J. Human adenovirus type 5 vectors deleted of early region 1 (E1) undergo limited expression of early replicative E2 proteins and DNA replication in non-permissive cells. PLoS ONE 2017, 12, e0181012. [Google Scholar] [CrossRef]
- Kovesdi, I.; Hedley, S.J. Adenoviral producer cells. Viruses 2010, 2, 1681. [Google Scholar] [CrossRef]
- Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977, 36, 59–72. [Google Scholar] [CrossRef]
- Fallaux, F.J.; Bout, A.; Van Der Velde, I.; Van Den Wollenberg, D.J.M.; Hehir, K.M.; Keegan, J.; Auger, C.; Cramer, S.J.; Van Ormondt, H.; Van Der Eb, A.J.; et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 1998, 9, 1909–1917. [Google Scholar] [CrossRef]
- Suzuki, K.; Alemany, R.; Yamamoto, M.; Curiel, D.T.; Program, G.T. The Presence of the Adenovirus E3 Region Improves the Oncolytic Potency of Conditionally Replicative Adenoviruses 1. Clin. Cancer Res. 2002, 8, 3348–3359. [Google Scholar]
- Gorziglia, M.I.; Lapcevich, C.; Roy, S.; Kang, Q.; Kadan, M.; Wu, V.; Pechan, P.; Kaleko, M. Generation of an Adenovirus Vector Lacking E1, E2a, E3, and All of E4 except Open Reading Frame 3. J. Virol. 1999, 73, 6048–6055. [Google Scholar] [CrossRef]
- Alba, R.; Bosch, A.; Chillon, M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005, 12, S18–S27. [Google Scholar] [CrossRef]
- Wang, W.; Ji, W.; Hu, H.; Ma, J.; Li, X.; Mei, W.; Xu, Y.; Hu, H.; Yan, Y.; Song, Q.; et al. Survivin promoter-regulated oncolytic adenovirus with Hsp70 gene exerts effective antitumor efficacy in gastric cancer immunotherapy. Oncotarget 2014, 5, 150–160. [Google Scholar] [CrossRef]
- Wirth, T.; Zender, L.; Schulte, B.; Mundt, B.; Plentz, R.; Rudolph, K.L.; Manns, M.; Kubicka, S.; Kuhnel, F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer (Cancer Research (2003) 63 (3181-3188)). Cancer Res. 2018, 78, 6027. [Google Scholar]
- Berns, K.I.; Giraud, C. Adenovirus and Adeno-Associated Virus as Vectors for Gene Therapy. Ann. N. Y. Acad. Sci. 1995, 772, 95–104. [Google Scholar] [PubMed]
- Suzuki, S.; Kofune, H.; Uozumi, K.; Yoshimitsu, M.; Arima, N.; Ishitsuka, K.; Ueno, S.I.; Kosai, K.I. A survivin-responsive, conditionally replicating adenovirus induces potent cytocidal effects in adult T-cell leukemia/lymphoma. BMC Cancer 2019, 19, 516. [Google Scholar]
- Doloff, J.C.; Waxman, D.J.; Jounaidi, Y. Human Telomerase Reverse Transcriptase Promoter-Driven Oncolytic Adenovirus with E1B-19 kDa and E1B-55 kDa Gene Deletions. Hum. Gene Ther. 2008, 19, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.Y.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.X.; Levin, V.A.; Yung, W.K.A.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hulin-Curtis, S.L.; Davies, J.A.; Jones, R.; Hudson, E.; Hanna, L.; Chester, J.D.; Parker, A.L. Histone deacetylase inhibitor trichostatin A sensitises cisplatinresistant ovarian cancer cells to oncolytic adenovirus. Oncotarget 2018, 9, 26328–26341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Konecny, G.E.; Winterhoff, B.; Kolarova, T.; Qi, J.; Manivong, K.; Dering, J.; Yang, G.; Chalukya, M.; Wang, H.J.; Anderson, L.; et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin. Cancer Res. 2011, 17, 1591–1602. [Google Scholar]
- Sellers, W.R.; Kaelin, W.G. Role of the retinoblastoma protein in the pathogenesis of human cancer. J. Clin. Oncol. 1997, 15, 3301–3312. [Google Scholar] [CrossRef]
- Gros, A.; Martínez-Quintanilla, J.; Puig, C.; Guedan, S.; Molleví, D.G.; Alemany, R.; Cascallo, M. Bioselection of a gain of function mutation that enhances adenovirus 5 release and improves its antitumoral potency. Cancer Res. 2008, 68, 8928–8937. [Google Scholar]
- Dong, W.; Van Ginkel, J.W.H.; Au, K.Y.; Alemany, R.; Meulenberg, J.J.M.; Van Beusechem, V.W. ORCA-010, a novel potency-enhanced oncolytic adenovirus, exerts strong antitumor activity in preclinical models. Hum. Gene Ther. 2014, 25, 897–904. [Google Scholar]
- Ganly, I.; Kirn, D.; Eckhardt, S.G.; Rodriguez, G.I.; Soutar, D.S.; Otto, R.; Robertson, A.G.; Park, O.; Gulley, M.L.; Heise, C.; et al. A Phase I Study of Onyx-015, an E1B Attenuated Adenovirus, Administered Intratumorally to Patients with Recurrent Head and Neck Cancer. Clin. Cancer Res. 2000, 6, 798. [Google Scholar] [PubMed]
- Garber, K. China approves world’s first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006, 98, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Ries, S.; Korn, W.M. ONYX-015: Mechanisms of action and clinical potential of a replication-selective adenovirus. Br. J. Cancer 2002, 86, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Vousden, K.H. P53 mutations in cancer. Nat. Cell Biol. 2013, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, C.C.; Johnson, L.; Bagus, B.; Choi, S.; Nicholas, C.; Shen, A.; Boyle, L.; Pandey, K.; Soria, C.; Kunich, J.; et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell 2004, 6, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.S.; Hecht, J.R.; Chan, E. Talimogene laherparepvec: Review of its mechanism of action and clinical efficacy and safety. Immunotherapy 2019, 11, 705–723. [Google Scholar] [CrossRef] [PubMed]
- Kirn, D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): Results of Phase I and II trials. Expert Opin. Biol. Ther. 2001, 1, 525–538. [Google Scholar] [CrossRef]
- Ram, Z.; Culver, K.W.; Oshiro, E.M.; Viola, J.J.; Devroom, H.L.; Otto, E.; Long, Z.; Chiang, Y.; Mcgarrity, G.J.; Muul, L.M.; et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat. Med. 1997, 3, 1354–1361. [Google Scholar] [CrossRef]
- Sterman, D.H.; Treat, J.; Litzky, L.A.; Amin, K.M.; Coonrod, L.; Molnar-Kimber, K.; Recio, A.; Knox, L.; Wilson, J.M.; Albelda, S.M.; et al. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: Results of a phase I clinical trial in malignant mesothelioma. Hum. Gene Ther. 1998, 9, 1083–1092. [Google Scholar] [CrossRef]
- Dhar, D.; Spencer, J.F.; Toth, K.; Wold, W.S.M. Pre-existing immunity and passive immunity to adenovirus 5 prevents toxicity caused by an oncolytic adenovirus vector in the syrian hamster model. Mol. Ther. 2009, 17, 1724–1732. [Google Scholar] [CrossRef]
- Belousova, N.; Mikheeva, G.; Xiong, C.; Stagg, L.J.; Gagea, M.; Fox, P.S.; Bassett, R.L.; Ladbury, J.E.; Braun, M.B.; Stehle, T.; et al. Native and engineered tropism of vectors derived from a rare species D adenovirus serotype 43. Oncotarget 2016, 7, 53414–53429. [Google Scholar] [CrossRef] [PubMed]
- Greig, J.A.; Buckley, S.M.K.; Waddington, S.N.; Parker, A.L.; Bhella, D.; Pink, R.; Rahim, A.A.; Morita, T.; Nicklin, S.A.; McVey, J.H.; et al. Influence of coagulation factor X on in vitro and in vivo gene delivery by adenovirus (Ad) 5, Ad35, and chimeric Ad5/Ad35 vectors. Mol. Ther. 2009, 17, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Bradshaw, A.C.; Parker, A.L.; Bhella, D.; Waddington, S.N.; Nicklin, S.A.; Van Rooijen, N.; Custers, J.; Goudsmit, J.; Barouch, D.H.; et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: Effect of mutagenesis on FX interactions and gene transfer. Blood 2009, 114, 965–971. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, W.; Ehrhardt, A. Expanding the spectrum of adenoviral vectors for cancer therapy. Cancers (Basel) 2020, 12, 1139. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Gao, J.; Schaffarczyk, L.; Janz, S.; Ehrke-Schulz, E.; Dittmar, T.; Ehrhardt, A.; Zhang, W. Spectrum-wide exploration of human adenoviruses for breast cancer therapy. Cancers (Basel) 2020, 12, 1403. [Google Scholar] [CrossRef]
- Kanerva, A.; Wang, M.; Bauerschmitz, G.J.; Lam, J.T.; Desmond, R.A.; Bhoola, S.M.; Barnes, M.N.; Alvarez, R.D.; Siegal, G.P.; Curiel, D.T.; et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol. Ther. 2002, 5, 695–704. [Google Scholar] [CrossRef]
- Krasnykh, V.N.; Mikheeva, G.V.; Douglas, J.T.; Curiel, D.T. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 1996, 70, 6839–6846. [Google Scholar] [CrossRef]
- Kuhn, I.; Harden, P.; Bauzon, M.; Chartier, C.; Nye, J.; Thorne, S.; Reid, T.; Ni, S.; Lieber, A.; Fisher, K.; et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS ONE 2008, 3, e2409. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Salazar, R.; Duran, I.; Osman-Garcia, I.; Paz-Ares, L.; Bozada, J.M.; Boni, V.; Blanc, C.; Seymour, L.; Beadle, J.; et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J. Immunother. Cancer 2017, 5, 71. [Google Scholar] [CrossRef]
- McNeish, I.A.; Oza, A.M.; Coleman, R.L.; Scott, C.L.; Konecny, G.E.; Tinker, A.; O’Malley, D.M.; Brenton, J.; Kristeleit, R.S.; Bell-McGuinn, K.; et al. Results of ARIEL2: A Phase 2 trial to prospectively identify ovarian cancer patients likely to respond to rucaparib using tumor genetic analysis. J. Clin. Oncol. 2015, 33, 5508. [Google Scholar] [CrossRef]
- Dyer, A.; Di, Y.; Calderon, H.; Illingworth, S.; Kueberuwa, G.; Tedcastle, A.; Jakeman, P.; Chia, S.L.; Brown, A.; Silva, M.A.; et al. Oncolytic Group B Adenovirus Enadenotucirev Mediates Non-apoptotic Cell Death with Membrane Disruption and Release of Inflammatory Mediators. Mol. Ther. Oncolytics 2017, 4, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.J.; Shieh, J.T.C.; Pickles, R.J.; Okegawa, T.; Hsieh, J.T.; Bergelson, J.M. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 2001, 98, 15191–15196. [Google Scholar] [CrossRef] [PubMed]
- Korn, W.M.; Macal, M.; Christian, C.; Lacher, M.D.; McMillan, A.; Rauen, K.A.; Warren, R.S.; Ferrell, L. Expression of the coxsackievirus- and adenovirus receptor in gastrointestinal cancer correlates with tumor differentiation. Cancer Gene Ther. 2006, 13, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.D.; Rauen, K.A.; Ramamurthy, M.; Dodson, J.L.; De Marzo, A.M.; Putzi, M.J.; Schoenberg, M.P.; Rodriguez, R. Integrin αv and coxsackie adenovirus receptor expression in clinical bladder cancer. Urology 2002, 60, 531–536. [Google Scholar] [CrossRef]
- Rauen, K.A.; Sudilovsky, D.; Le, J.L.; Chew, K.L.; Hann, B.; Weinberg, V.; Schmitt, L.D.; Mccormick, F. Expression of the Coxsackie Adenovirus Receptor in Normal Prostate and in Primary and Metastatic Prostate Carcinoma: Potential Relevance to Gene Therapy 1. Cancer Res. 2002, 62, 3812–3818. [Google Scholar] [PubMed]
- Nicklin, S.A.; Von Seggern, D.J.; Work, L.M.; Pek, D.C.K.; Dominiczak, A.F.; Nemerow, G.R.; Baker, A.H. Ablating adenovirus type 5 fiber-CAR binding and HI loop insertion of the SIGYPLP peptide generate an endothelial cell-selective adenovirus. Mol. Ther. 2001, 4, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Tzeng, E.; Shears Ii, L.L.; Roelvink, P.W.; Li, Y.; Lee, G.M.; Brough, D.E.; Lizonova, A.; Kovesdi, I. Increased In Vitro and In Vivo Gene Transfer by Adenovirus Vectors Containing Chimeric Fiber Proteins. J. Virol. 1997, 71, 8221–8229. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Davies, J.; Marlow, G.; Uusi-Kerttula, H.; Seaton, G.; Piggott, L.; Clarkson, R.; Chester, J. Ad5 (NULL)-A20-a precision virotherapy that efficiently and selectively targets αvβ6 positive cancers following intravenous administration. Br. J. Cancer 2019, 121, 16. [Google Scholar]
- Nakamura, T.; Sato, K.; Hamada, H. Reduction of Natural Adenovirus Tropism to the Liver by both Ablation of Fiber-Coxsackievirus and Adenovirus Receptor Interaction and Use of Replaceable Short Fiber. J. Virol. 2003, 77, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Gall, J.G.D.; Falck-Pedersen, E. Subgroup B and F Fiber Chimeras Eliminate Normal Adenovirus Type 5 Vector Transduction In Vitro and In Vivo. J. Virol. 2003, 77, 1039–1048. [Google Scholar] [CrossRef][Green Version]
- Kashentseva, E.A.; Douglas, J.T.; Zinn, K.R.; Curiel, D.T.; Dmitriev, I.P. Targeting of Adenovirus Serotype 5 Pseudotyped with Short Fiber from Serotype 41 to c-erbB2-Positive Cells using Bispecific Single-Chain Diabody. J. Mol. Biol. 2009, 388, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Hofherr, S.E.; Shashkova, E.V.; Weaver, E.A.; Khare, R.; Barry, M.A. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 2008, 16, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, F.; Kochanek, S. Modification of adenovirus gene transfer vectors with synthetic polymers: A scientific review and technical guide. Mol. Ther. 2008, 16, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, P.H.; Kim, S.W.; Yun, C.O. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials 2012, 33, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, J.K.; Nettelbeck, D.M. Virus chimeras for gene therapy, vaccination, and oncolysis: Adenoviruses and beyond. Trends Mol. Med. 2012, 18, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Mei, Y.-F. Transduction and Oncolytic Profile of a Potent Replication-Competent Adenovirus 11p Vector (RCAd11pGFP) in Colon Carcinoma Cells. PLoS ONE 2011, 6, e17532. [Google Scholar] [CrossRef]
- Rein, D.T.; Volkmer, A.; Beyer, I.M.; Curiel, D.T.; Janni, W.; Dragoi, A.; Hess, A.P.; Maass, N.; Baldus, S.E.; Bauerschmitz, G.; et al. Treatment of chemotherapy resistant ovarian cancer with a MDR1 targeted oncolytic adenovirus. Gynecol. Oncol. 2011, 123, 138–146. [Google Scholar] [CrossRef]
- Nakayama, M.; Both, G.W.; Banizs, B.; Tsuruta, Y.; Yamamoto, S.; Kawakami, Y.; Douglas, J.T.; Tani, K.; Curiel, D.T.; Glasgow, J.N. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology 2006, 350, 103–115. [Google Scholar] [CrossRef]
- Nicklin, S.A.; Wu, E.; Nemerow, G.R.; Baker, A.H. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol. Ther. 2005, 12, 384–393. [Google Scholar] [CrossRef]
- Shayakhmetov, D.M.; Lieber, A. Dependence of Adenovirus Infectivity on Length of the Fiber Shaft Domain. J. Virol. 2000, 74, 10274–10286. [Google Scholar] [CrossRef]
- Xial, D.; Henry2, L.J.; Gerard2, R.D.; Eisenhoferl, J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure 1994, 2, 1259–1270. [Google Scholar]
- Hemminki, A.; Wang, M.; Desmond, R.A.; Strong, T.V.; Alvarez, R.D.; Curiel, D.T. Serum and ascites neutralizing antibodies in ovarian cancer patients treated with intraperitoneal adenoviral gene therapy. Hum. Gene Ther. 2002, 13, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, J.; Wakimoto, H. Preclinical And Clinical Development Of Oncolytic Adenovirus For The Treatment Of Malignant Glioma. Oncolytic Virotherapy 2019, 8, 27–37. [Google Scholar] [CrossRef] [PubMed]
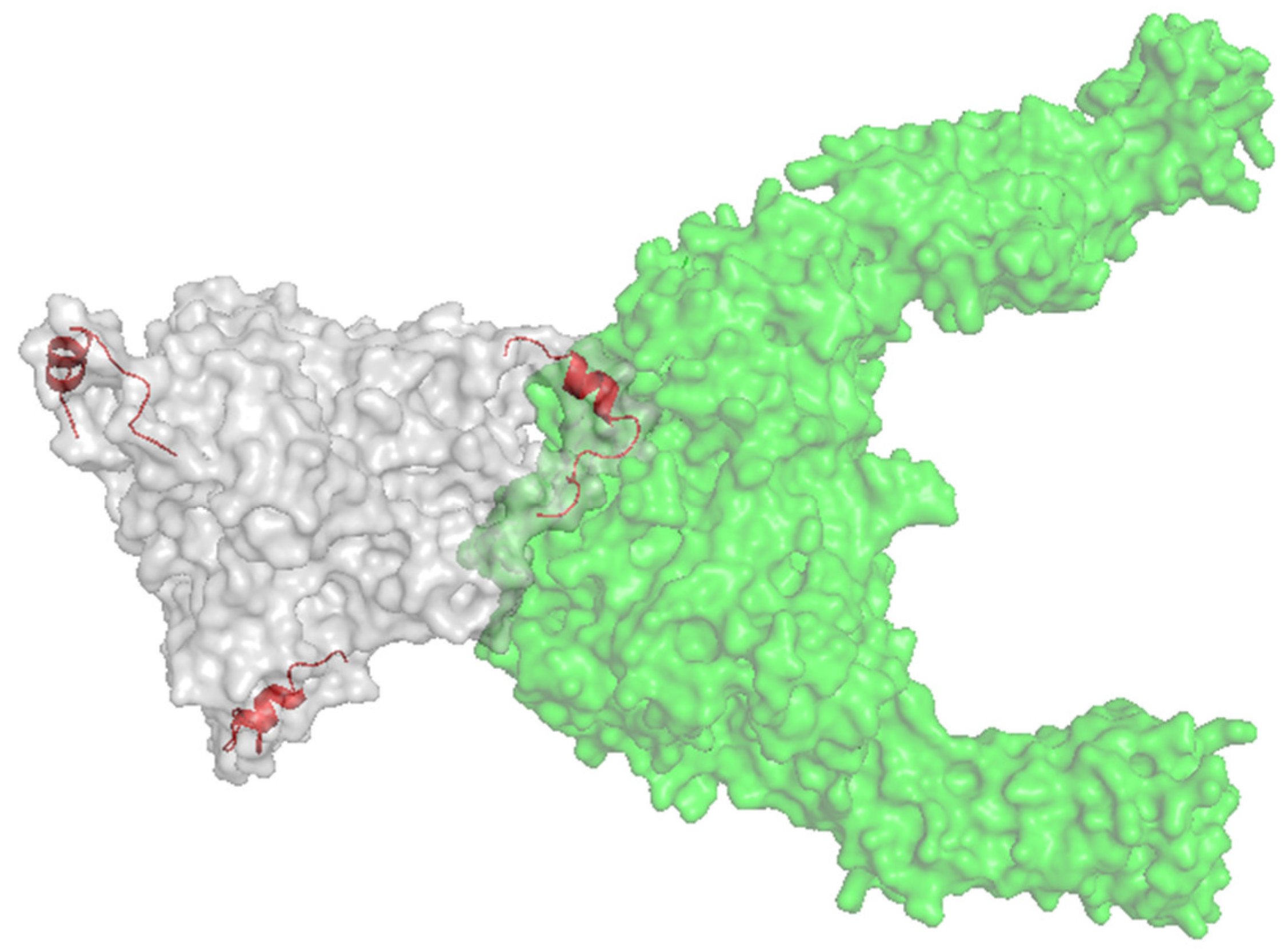
- Krasnykh, V.; Dmitriev, I.; Mikheeva, G.; Miller, C.R.; Belousova, N.; Curiel, D.T. Characterization of an Adenovirus Vector Containing a Heterologous Peptide Epitope in the HI Loop of the Fiber Knob. J. Virol. 1998, 72, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.; Uusi-Kerttula, H.; Ma, J.; Degg, B.P.; Parker, A.L.; Baker, A.H. Retargeting adenovirus serotype 48 fiber knob domain by peptide incorporation. Hum. Gene Ther. 2014, 25, 385–394. [Google Scholar] [CrossRef]
- Uusi-Kerttula, H.; Davies, J.; Coughlan, L.; Hulin-Curtis, S.; Jones, R.; Hanna, L.; Chester, J.D.; Parker, A.L. Pseudotyped αvβ6 integrin-targeted adenovirus vectors for ovarian cancer therapies. Oncotarget 2016, 7, 27926–27937. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Henning, P.; Myhre, S.; Wikman, M.; Uil, T.G.; Friedman, M.; Andersson, K.M.E.; Hong, S.S.; Hoeben, R.C.; Habib, N.A.; et al. Adenovirus 5 vector genetically re-targeted by an Affibody molecule with specificity for tumor antigen HER2/neu. Cancer Gene Ther. 2007, 14, 468–479. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Kraaij, R.; Leadley, R.M.; De Ridder, C.M.A.; Van Weerden, W.M.; Van Schie, K.A.J.; Van Der Kroeg, M.; Hoeben, R.C.; Maitland, N.J.; Lindholm, L. A transductionally retargeted adenoviral vector for virotherapy of her2/neu-expressing prostate cancer. Hum. Gene Ther. 2012, 23, 70–82. [Google Scholar] [CrossRef]
- Whilding, L.M.; Parente-Pereira, A.C.; Zabinski, T.; Davies, D.M.; Petrovic, R.M.G.; Kao, Y.V.; Saxena, S.A.; Romain, A.; Costa-Guerra, J.A.; Violette, S.; et al. Targeting of Aberrant αvβ6 Integrin Expression in Solid Tumors Using Chimeric Antigen Receptor-Engineered T Cells. Mol. Ther. 2017, 25, 2427. [Google Scholar] [CrossRef]
- Ahmed, N.; Riley, C.; Rice, G.E.; Quinn, M.A.; Baker, M.S. αvβ6 integrin-A marker for the malignant potential of epithelial ovarian cancer. J. Histochem. Cytochem. 2002, 50, 1371–1379. [Google Scholar] [CrossRef]
- Reader, C.S.; Vallath, S.; Steele, C.W.; Haider, S.; Brentnall, A.; Desai, A.; Moore, K.M.; Jamieson, N.B.; Chang, D.; Bailey, P.; et al. The integrin αvβ6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J. Pathol. 2019, 249, 332–342. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, F.; Wang, B.; Liu, S.; Niu, W.; Liu, E.; Peng, C.; Wang, J.; Gao, H.; Liang, B.; et al. Interleukin-8 promotes cell migration through integrin αvβ6 upregulation in colorectal cancer. Cancer Lett. 2014, 354, 245–253. [Google Scholar] [CrossRef]
- Hausner, S.H.; DiCara, D.; Marik, J.; Marshall, J.F.; Sutcliffe, J.L. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: Generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin αvβ6 expression with positron emission tomography. Cancer Res. 2007, 67, 7833–7840. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.M.; Desai, A.; de Luxán Delgado, B.; Trabulo, S.M.D.; Reader, C.; Brown, N.F.; Murray, E.R.; Brentnall, A.; Howard, P.; Masterson, L.; et al. Integrin αvβ6-specific therapy for pancreatic cancer developed from foot-and-mouth-disease virus. Theranostics 2020, 10, 2930–2942. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Parker, A.L.; Clarke, C.; Duffy, M.R.; Alba, R.; Nicklin, S.A.; Bakerk, A.H. Retargeting FX-binding-ablated HAdV-5 to vascular cells by inclusion of the RGD-4C peptide in hexon hypervariable region 7 and the HI loop. J. Gen. Virol. 2016, 97, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Vigne, E.; Mahfouz, I.; Dedieu, J.-F.; Brie, A.; Perricaudet, M.; Yeh, P. RGD Inclusion in the Hexon Monomer Provides Adenovirus Type 5-Based Vectors with a Fiber Knob-Independent Pathway for Infection. J. Virol. 1999, 73, 5156–5161. [Google Scholar] [CrossRef]
- Hulin-Curtis, S.L.; Davies, J.A.; Nestić, D.; Bates, E.A.; Baker, A.T.; Cunliffe, T.G.; Majhen, D.; Chester, J.D.; Parker, A.L. Identification of folate receptor α (FRα) binding oligopeptides and their evaluation for targeted virotherapy applications. Cancer Gene Ther. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Maruta, F.; Parker, A.L.; Fisher, K.D.; Hallissey, M.T.; Ismail, T.; Rowlands, D.C.; Chandler, L.A.; Kerr, D.J.; Seymour, L.W. Identification of FGF receptor-binding peptides for cancer gene therapy. Cancer Gene Ther. 2002, 9, 543–552. [Google Scholar] [CrossRef]
- Uusi-Kerttula, H.; Legut, M.; Davies, J.; Jones, R.; Hudson, E.; Hanna, L.; Stanton, R.J.; Chester, J.D.; Parker, A.L. Incorporation of Peptides Targeting EGFR and FGFR1 into the Adenoviral Fiber Knob Domain and Their Evaluation as Targeted Cancer Therapies. Hum. Gene Ther. 2015, 26, 320–329. [Google Scholar] [CrossRef]
- Ehrlich, G.K.; Berthold, W.; Bailon, P. Phage Display Technology: Affinity Selection by Biopanning. In Affinity Chromatography; Humana Press: Totowa, NJ, USA, 2000; Volume 147, pp. 195–208. [Google Scholar]
- Li, Z.; Zhao, R.; Wu, X.; Sun, Y.; Yao, M.; Li, J.; Xu, Y.; Gu, J. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J. 2005, 19, 1978–1985. [Google Scholar] [CrossRef]
- Urbanelli, L.; Ronchini, C.; Fontana, L.; Menard, S.; Orlandi, R.; Monaci, P. Targeted gene transduction of mammalian cells expressing the HER2/neu receptor by filamentous phage 1 1Edited by J. Karn. J. Mol. Biol. 2001, 313, 965–976. [Google Scholar] [CrossRef]
- Senol, S.; Ceyran, A.B.; Aydin, A.; Zemheri, E.; Ozkanli, S.; Kösemetin, D.; Sehitoglu, I.; Akalin, I. Folate receptor α expression and significance in endometrioid endometrium carcinoma and endometrial hyperplasia. Int. J. Clin. Exp. Pathol. 2015, 8, 5633–5641. [Google Scholar]
- Lupold, S.E.; Kudrolli, T.A.; Chowdhury, W.H.; Wu, P.; Rodriguez, R. A novel method for generating and screening peptides and libraries displayed on adenovirus fiber. Nucleic Acids Res. 2007, 35, 138. [Google Scholar] [CrossRef]
- Brown, K.C. Peptidic tumor targeting agents: The road from phage display peptide selections to clinical applications. Curr. Pharm. Des. 2010, 16, 1040–1054. [Google Scholar] [CrossRef]

| Biologic | Synonyms | Adenovirus genome | Modifications | Targeting | NCI Identifier |
|---|---|---|---|---|---|
| GM-CSF-encoding Oncolytic Adenovirus CGTG-102 | ONCOS-102 | Adenovirus serotype 5/3 (capsid-modified) | Ad5 capsid protein replaced with Ad3 knob domain. Granulocyte-macrophage colony stimulating factor (GM-CSF) | Selective replication in Rb/p16 defective cells. Ad3 receptors. | C98287 |
| OX40L-expressing Oncolytic Adenovirus DNX-2440 | Oncolytic Adenovirus Armed with OX40L DNX-2440 | Adenovirus serotype 5 | Expresses OX40 ligand (OX40L). ∆24 mutation | Selective replication in Rb/p16 defective cells | C160192 |
| Oncolytic Adenovirus ORCA-010 | Modified Ad5 ORCA-010 | Ad5/3 | ∆24 mutation. RGD-4C motif. T1 mutation in E3/19K gene | Selective replication in Rb/p16 defective cells. T1 mutation enhances Ad5 release, Ad3 receptors | C168607 |
| Oncolytic Adenovirus ORCA-010 | |||||
| Oncolytic Adenovirus Encoding GM-CSF | CG0070 | Adenovirus serotype 5 | E2F-1 promotor. Granulocyte-macrophage colony stimulating factor (GM-CSF) in E3 region | Selective replication in Rb/p16 defective cells | C48412 |
| Delolimogene Mupadenorepvec | Double-armed TMZ-CD40L/4-1BBL Oncolytic Ad5/35 Adenovirus LOAd703 | Adenovirus serotype 5 with L5 segment of fiber replaced with Ad35 fiber | Expresses trimerized CD40 ligand. ∆24 mutation in E1A | Targets CD46. Selective replication in Rb/p16 defective cells | C148462 |
| Oncolytic Adenovirus ICOVIR5-infected Autologous Mesenchymal Stem Cells | LOAd 703 | Wildtype human adenovirus 5 | RGD-4C motif allows integrin binding. ∆24 in E1A prevents Rb complex and transition into S phase | Bone marrow-derived MSCs target and deliver adenovirus to tumour | C107160 |
| Tasadenoturev | DNX-2401 | Adenovirus serotype 5 | RGD-4C motif allows integrin binding. ∆24 in E1A prevents Rb complex and transition into S phase | CAR independent. Selective replication in Rb/p16 defective cells | C74067 |
| (Oncolytic Adenovirus) Ad5-∆24RGD | |||||
| Oncolytic Adenovirus Ad5-DNX-2401 | |||||
| Tasadenoturev-infected Allogeneic Bone Marrow-derived Mesenchymal Stem Cells | Ad5-DNX-2401-infected Allogeneic Bone Marrow Mesenchymal Stem Cells | Ad5-DNX-2401 | RGD-4C motif, ∆24 in E1A prevents Rb complex and transition into S phase | Bone marrow-derived MSCs target and deliver adenovirus to tumour | C159798 |
| (Allogeneic) BM-hMSC-∆24 | |||||
| (Allogeneic) BM-hMSC-∆24-RGD | |||||
| Ad5-yCD/mutTKSR39rep-hIL12 | Oncolytic Adenovirus Ad5-yCD/mutTKSR39rep-hIL12 | Adenovirus serotype 5 | Encodes murine interleukin-12 (IL-12) gene in E3 region and a suicide fusion gene (yCD/HSV-1 TKSR39) in E1 region | E1B55K deletion | C123930 |
| Enadenotucirev | ColoAd-1 | Chimeric Oncolytic Adenovirus Ad3/Ad11p | Deletions in E3 Region (2444 bp) and E4 Region (24 bp) and 197 Non-homologous nucleotides in the E2B Region | Not fully understood | C113786 |
| EnAd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunliffe, T.G.; Bates, E.A.; Parker, A.L. Hitting the Target but Missing the Point: Recent Progress towards Adenovirus-Based Precision Virotherapies. Cancers 2020, 12, 3327. https://doi.org/10.3390/cancers12113327
Cunliffe TG, Bates EA, Parker AL. Hitting the Target but Missing the Point: Recent Progress towards Adenovirus-Based Precision Virotherapies. Cancers. 2020; 12(11):3327. https://doi.org/10.3390/cancers12113327
Chicago/Turabian StyleCunliffe, Tabitha G., Emily A. Bates, and Alan L. Parker. 2020. "Hitting the Target but Missing the Point: Recent Progress towards Adenovirus-Based Precision Virotherapies" Cancers 12, no. 11: 3327. https://doi.org/10.3390/cancers12113327
APA StyleCunliffe, T. G., Bates, E. A., & Parker, A. L. (2020). Hitting the Target but Missing the Point: Recent Progress towards Adenovirus-Based Precision Virotherapies. Cancers, 12(11), 3327. https://doi.org/10.3390/cancers12113327








