Predictive Values of Blood-Based RNA Signatures for the Gemcitabine Response in Advanced Pancreatic Cancer
Simple Summary
Abstract
1. Introduction
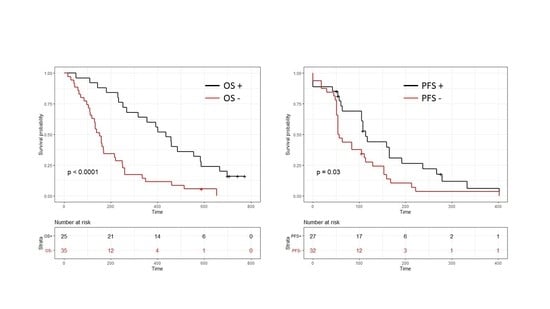
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Sample Collection
4.2. Gene Expression Analysis via qPCR
4.3. Statistical Analysis: Selection of Candidate Genes
4.4. Statistical Analysis: Gene Expression-Based (GE) Score
4.5. Statistical Analysis: Univariate and Multivariate Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neuzillet, C.; Gaujoux, S.; Williet, N.; Bachet, J.B.; Bauguion, L.; Colson Durand, L.; Conroy, T.; Dahan, L.; Gilabert, M.; Huguet, F.; et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2018, 50, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.S.; Cheung, W.Y. A Contemporary Review of the Treatment Landscape and the Role of Predictive and Prognostic Biomarkers in Pancreatic Adenocarcinoma. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1863535. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Hidalgo, M.; Alvarez, R.; Arrazubi, V.; Martinez-Galan, J.; Salgado, M.; Macarulla, T.; Carrato, A. From First Line to Sequential Treatment in the Management of Metastatic Pancreatic Cancer. J Cancer 2018, 9, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Raffenne, J.; Nicolle, R.; Puleo, F.; Le Corre, D.; Boyez, C.; Marechal, R.; Emile, J.F.; Demetter, P.; Bardier, A.; Laurent-Puig, P.; et al. hENT1 Testing in Pancreatic Ductal Adenocarcinoma: Are We Ready? A Multimodal Evaluation of hENT1 Status. Cancers 2019, 11, 1808. [Google Scholar] [CrossRef]
- Puleo, F.; Nicolle, R.; Blum, Y.; Cros, J.; Marisa, L.; Demetter, P.; Quertinmont, E.; Svrcek, M.; Elarouci, N.; Iovanna, J.; et al. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology 2018, 155, 1999–2013.e3. [Google Scholar] [CrossRef]
- Woo, S.M.; Yoon, K.A.; Hong, E.K.; Park, W.S.; Han, S.S.; Park, S.J.; Joo, J.; Park, E.Y.; Lee, J.H.; Kim, Y.H.; et al. DCK expression, a potential predictive biomarker in the adjuvant gemcitabine chemotherapy for biliary tract cancer after surgical resection: Results from a phase II study. Oncotarget 2017, 8, 81394–81404. [Google Scholar] [CrossRef]
- Porcelli, L.; Iacobazzi, R.M.; Di Fonte, R.; Serrati, S.; Intini, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Leonetti, F.; Azzariti, A.; et al. CAFs and TGF-beta Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers 2019, 11, 330. [Google Scholar] [CrossRef]
- Li, D.; Pant, S.; Ryan, D.P.; Laheru, D.; Bahary, N.; Dragovich, T.; Hosein, P.J.; Rolfe, L.; Saif, M.W.; LaValle, J.; et al. A phase II, open-label, multicenter study to evaluate the antitumor efficacy of CO-1.01 as second-line therapy for gemcitabine-refractory patients with stage IV pancreatic adenocarcinoma and negative tumor hENT1 expression. Pancreatology 2014, 14, 398–402. [Google Scholar] [CrossRef]
- Bachet, J.B.; Blons, H.F.; Hammel, P.; El Hariry, I.; Portales, F.; Mineur, L.; Metges, J.P.; Mulot, C.; Bourreau, C.; Cain, J.; et al. Circulating tumor DNA is prognostic and potentially predictive of eryaspase efficacy in second-line in patients with advanced pancreatic adenocarcinoma. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Elazezy, M.; Joosse, S.A. Techniques of using circulating tumor DNA as a liquid biopsy component in cancer management. Comput. Struct. Biotechnol. J. 2018, 16, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Deplanque, G.; Demarchi, M.; Hebbar, M.; Flynn, P.; Melichar, B.; Atkins, J.; Nowara, E.; Moye, L.; Piquemal, D.; Ritter, D.; et al. A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Honda, M.; Matsui, S.; Komori, O.; Murayama, T.; Fujiwara, T.; Mizuno, M.; Imai, Y.; Yoshimura, K.; Nasti, A.; et al. Development of novel diagnostic system for pancreatic cancer, including early stages, measuring mRNA of whole blood cells. Cancer Sci. 2019, 110, 1364–1388. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Peng, Y.; Zhu, Y.; Xu, D.; Zhu, F.; Xu, W.; Chen, Q.; Zhu, X.; Liu, T.; Hou, C.; et al. Glycolysis promotes the progression of pancreatic cancer and reduces cancer cell sensitivity to gemcitabine. Biomed. Pharm. 2020, 121, 109521. [Google Scholar] [CrossRef]
- Jia, Y.; Xie, J. Promising molecular mechanisms responsible for gemcitabine resistance in cancer. Genes Dis. 2015, 2, 299–306. [Google Scholar] [CrossRef]
- Chen, M.; Xue, X.; Wang, F.; An, Y.; Tang, D.; Xu, Y.; Wang, H.; Yuan, Z.; Gao, W.; Wei, J.; et al. Expression and promoter methylation analysis of ATP-binding cassette genes in pancreatic cancer. Oncol. Rep. 2012, 27, 265–269. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via theelastic net. J. R Stat. Soc. B 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Tibshirani, R. The lasso method for variable selection in the Cox model. Stat. Med. 1997, 16, 385–395. [Google Scholar] [CrossRef]
- Hoerl, A.; Kennard, R. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Mellert, H.; Foreman, T.; Jackson, L.; Maar, D.; Thurston, S.; Koch, K.; Weaver, A.; Cooper, S.; Dupuis, N.; Sathyanarayana, U.G.; et al. Development and Clinical Utility of a Blood-Based Test Service for the Rapid Identification of Actionable Mutations in Non-Small Cell Lung Carcinoma. J. Mol. Diagn. 2017, 19, 404–416. [Google Scholar] [CrossRef]

| Analyze | Univariate | Multivariate | ||
|---|---|---|---|---|
| Result | HR (95% CI for HR) | p-Value | HR (95% CI for HR) | p-Value |
| GE score OS + prediction | 0.31 (0.17–0.56) | 0.000095 | 0.39 (0.21–0.7) | 0.002 |
| CA 19–9 (U/mL) | 1 (1–1) | 0.28 | ||
| Albumin (g/L) | 0.97 (0.94–1) | 0.16 | ||
| QLQ-C30 | 1 (1–1) | 0.0027 | 1.02 (1.0–1.04) | 0.015 |
| Body mass index | 0.98 (0.92–1) | 0.5 | ||
| ECOG PS | 1.8 (0.96–3.2) | 0.067 | ||
| Monocyte count (per µL) | 2 (0.9–4.6) | 0.09 | ||
| Tumor localization | ||||
| Head | 0.86 (0.51–1.5) | 0.59 | ||
| Body | 1.1 (0.62–1.9) | 0.81 | ||
| Tail | 1.5 (0.85–2.7) | 0.16 | ||
| Clinical stage | 0.34 (0.17–0.68) | 0.0024 | 0.41 (0.2–0.83) | 0.014 |
| Analyze | Univariate | Multivariate | ||
|---|---|---|---|---|
| Result | HR (95% CI for HR) | p-Value | HR (95% CI for HR) | p-Value |
| GE score PFS + prediction | 0.55 (0.32–0.95) | 0.032 | 0.5 (0.28–0.9) | 0.025 |
| CA 19–9 (U/mL) | 1 (1–1) | 0.47 | ||
| Albumin (g/L) | 1 (0.96–1) | 0.81 | ||
| QLQ-C30 | 1 (1–1) | 0.038 | 1.02 (1.0–1.04) | 0.026 |
| Body mass index | 0.99 (0.93–1.1) | 0.76 | ||
| ECOG PS | 2 (1–3.8) | 0.045 | 1.6 (0.8–3.1) | 0.17 |
| Monocyte count (per µL) | 0.73 (0.29–1.8) | 0.49 | ||
| Tumor localization | ||||
| head | 1.2 (0.69–2.1) | 0.52 | ||
| body | 1.1 (0.64–1.9) | 0.7 | ||
| tail | 1.5 (0.84–2.8) | 0.16 | ||
| Clinical classification | 0.7 (0.35–1.4) | 0.32 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piquemal, D.; Noguier, F.; Pierrat, F.; Bruno, R.; Cros, J. Predictive Values of Blood-Based RNA Signatures for the Gemcitabine Response in Advanced Pancreatic Cancer. Cancers 2020, 12, 3204. https://doi.org/10.3390/cancers12113204
Piquemal D, Noguier F, Pierrat F, Bruno R, Cros J. Predictive Values of Blood-Based RNA Signatures for the Gemcitabine Response in Advanced Pancreatic Cancer. Cancers. 2020; 12(11):3204. https://doi.org/10.3390/cancers12113204
Chicago/Turabian StylePiquemal, David, Florian Noguier, Fabien Pierrat, Roman Bruno, and Jerome Cros. 2020. "Predictive Values of Blood-Based RNA Signatures for the Gemcitabine Response in Advanced Pancreatic Cancer" Cancers 12, no. 11: 3204. https://doi.org/10.3390/cancers12113204
APA StylePiquemal, D., Noguier, F., Pierrat, F., Bruno, R., & Cros, J. (2020). Predictive Values of Blood-Based RNA Signatures for the Gemcitabine Response in Advanced Pancreatic Cancer. Cancers, 12(11), 3204. https://doi.org/10.3390/cancers12113204