Role of Haematopoietic Stem Cell Transplantation in Peripheral T-Cell Lymphoma
Simple Summary
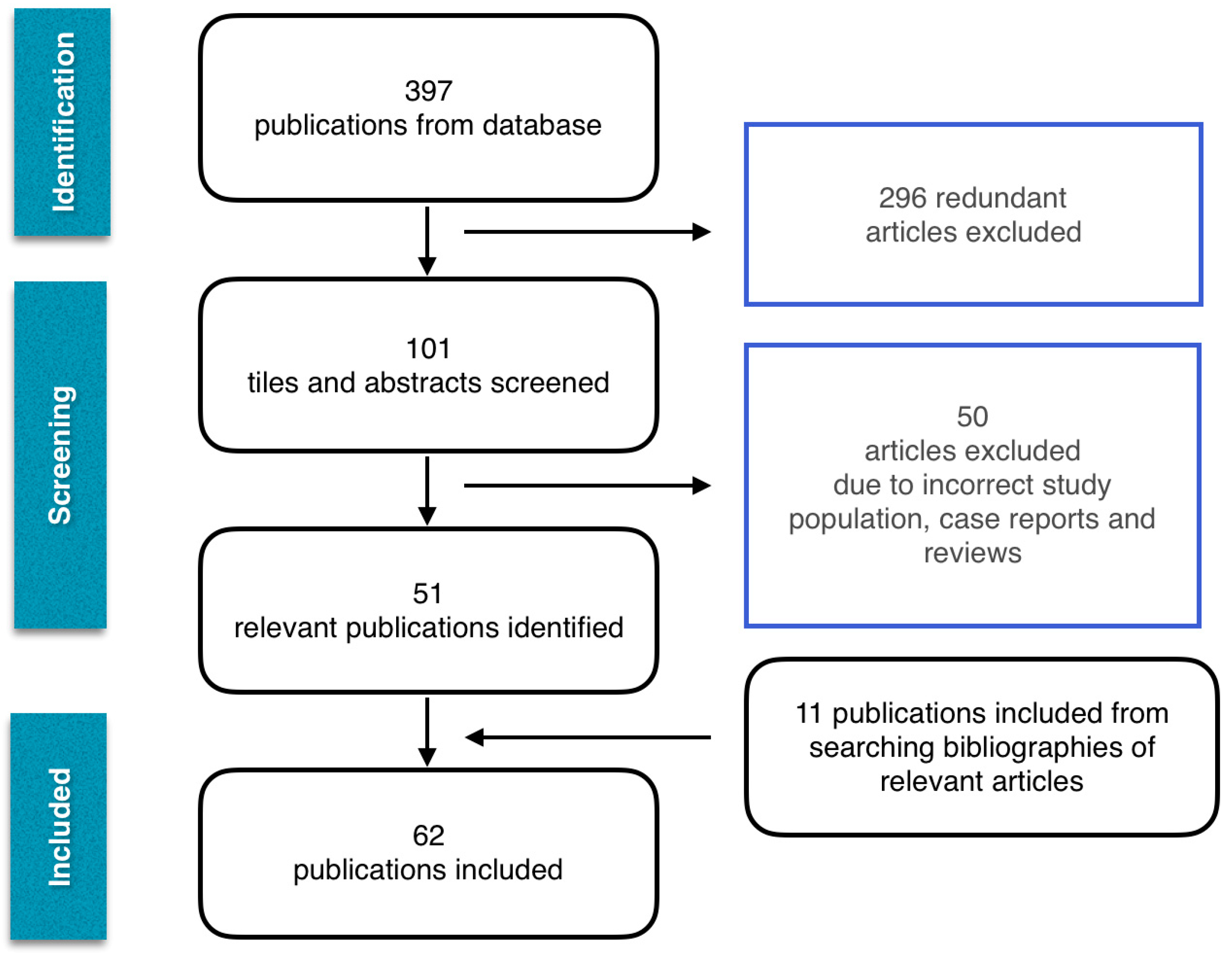
Abstract
1. Introduction
2. Methodology
3. Autologous Stem Cell Transplantation in Frontline PTCL
3.1. Prospective Data
Phase II Studies
3.2. Registry Data
3.3. Retrospective Data
4. Allogeneic Stem Cell Transplantation in Frontline PTCL
4.1. Prospective Data
4.2. Registry Data
5. Autologous Stem Cell Transplantation in Relapsed and Refractory PTCL
5.1. Registry Data
5.2. Retrospective Data
6. Allogeneic Stem Cell Transplantation in Relapsed/Refractory PTCL
6.1. Prospective Data
6.2. Registry Data
6.3. Retrospective Data
7. Comparison of National and Co-Operative Group Guidelines
8. Discussion and Recommendations
8.1. Why Explore Stem Cell Transplantation in the Management of PTCLs?
8.2. Challenges Encountered in Proceeding with Stem Cell Transplantation
8.3. Predictors of Improved Disease Outcomes Following Stem Cell Transplantation
8.4. Choice of Conditioning Regimen in AlloSCT
8.5. Haematopoietic Stem Cell Transplantation in the Frontline Management of PTCLs
8.5.1. Expected Outcome of PTCL with Standard Frontline Treatment
8.5.2. When Should a Frontline AutoSCT Be Considered?
EATL
PTCL-NOS and AITL
8.5.3. When Should a Frontline AlloSCT Be Considered?
Hepatosplenic T-Cell Lymphoma
8.5.4. Approaches to Frontline Transplantation in ALCL
ALCL, ALK-Negative and ALK-Positive High Risk
ALK-Positive Standard Risk
ALCL, ALK-Negative with DUSP 22 Rearrangement
8.6. Haematopoietic Stem Cell Transplantation in Relapsed/Refractory PTCL
8.6.1. Expected Outcome of Peripheral T-Cell Lymphoma with Salvage Treatment
8.6.2. When Should an AutoSCT Be Considered in the Salvage Setting?
PTCL-NOS, ALCL (Both ALK-Positive and Negative)
8.6.3. When Should an AlloSCT Be Considered in the Salvage Setting?
AITL
8.7. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Explanation |
| A+CHP | Brentuximab vedotin, cyclophosphamide, doxorubicin, prednisolone |
| AITL | Angioimmunoblastic T-cell lymphoma |
| aGVHD | Acute graft versus host disease |
| ALCL | Anaplastic large-cell lymphoma |
| ALK | Anaplastic lymphoma kinase |
| AlloSCT | Allogeneic stem cell transplantation |
| ALZ+CHOP | Alemtuzumab, cyclophosphamide, doxorubicin, vincristine, prednisolone |
| ASTCT | American Society for Transplantation and Cellular Therapy |
| AutoSCT | Autologous stem cell transplantation |
| BEAC | Carmustine, etoposide, cytarabine, cyclophosphamide |
| BEAM | Carmustine, etoposide, cytarabine, melphalan |
| B-NHL | B-cell non-Hodgkin lymphoma |
| cGVHD | Chronic graft versus host disease |
| CHOEP | Cyclophosphamide, doxorubicin, vincristine, etoposide, prednisolone |
| CHOP | Cyclophosphamide, doxorubicin, vincristine, prednisolone |
| CIBMTR | Centre for International Blood and Marrow Transplant Research |
| CLAEG | Cladribine, cytosine arabinoside, and etoposide with granulocyte colony-stimulating factor support |
| COMPLETE | Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment |
| CR | Complete remission |
| CR1 | First complete remission |
| DFS | Disease-free survival |
| DHAP | Dexamethasone, cisplatin, cytarabine |
| EATL | Enteropathy associated T-cell lymphoma |
| EBMT | European Society for Bone and Marrow Transplantation |
| EFS | Event-free survival |
| ESHAP | Etoposide, methylprednisolone, cytarabine, cisplatin |
| ESMO | European Society for Medical Oncology |
| GEL-TAMO | Grupo Español de Linfomas/Trasplante Autólogo de Médula Ósea |
| GOELAMS | Groupe Ouest-est d’Etude des Leucémies et Autres Maladies du Sang |
| GVHD | Graft versus host disease |
| HSCT | Haematopoietic stem cell transplantation |
| HSTCL | Hepatosplenic T-cell lymphoma |
| HyperCHidam | High-dose methotrexate given as a continuous infusion (1.6g/m2) on day 1; cyclophosphamide 300mg/m2 and high-dose cytarabine 2 g/m2 every 12 hours for 3 days |
| ICE | Ifosfamide, carboplatin, etoposide |
| IPI | International prognostic index |
| IVE | Ifosfamide, vincristine, etoposide |
| LDH | Lactate dehydrogenase |
| MAC | Myeloablative conditioning |
| MDACC | MD Anderson Cancer Centre |
| Mega-CHOP | Cyclophosphamide 2 g/m2, doxorubicin 90 mg/m2 and vincristine 1.4 mg/m2 on day 1, prednisolone 60mg/m2 days 1–5 |
| NCCN | National Comprehensive Cancer Network |
| NLG-T-01 | Nordic Lymphoma Group-T-01 |
| NMAC | Non-myeloablative conditioning |
| NRM | Non-relapse mortality |
| OS | Overall survival |
| PFS | Progression-free survival |
| PIT | Prognostic index for peripheral T-cell lymphoma |
| PR | Partial remission |
| PTCL | Peripheral T-cell lymphoma |
| PTCL-NOS | Peripheral T-cell lymphoma-not otherwise specified |
| RIC | Reduced-intensity conditioning |
| RR | Relapsed and refractory |
| TRM | Transplantation-related mortality |
References
- Schmitz, N.; Trümper, L.; Ziepert, M.; Nickelsen, M.; Ho, A.D.; Metzner, B.; Peter, N.; Loeffler, M.; Rosenwald, A.; Pfreundschuh, M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: An analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 2010, 116, 3418–3425. [Google Scholar] [CrossRef] [PubMed]
- Feugier, P.; Van Hoof, A.; Sebban, C.; Solal-Celigny, P.; Bouabdallah, R.; Fermé, C.; Christian, B.; Lepage, E.; Tilly, H.; Morschhauser, F.; et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the groupe d’etude des lymphomes de l’adulte. J. Clin. Oncol. 2005, 23, 4117–4126. [Google Scholar] [CrossRef]
- D’Amore, F.; Relander, T.; Lauritzsen, G.F.; Jantunen, E.; Hagberg, H.; Anderson, H.; Holte, H.; Österborg, A.; Merup, M.; Brown, P.; et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J. Clin. Oncol. 2012, 30, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.A.; Barrett, T.L.; Dellinger, E.P.; Krause, P.J.; Martone, W.J.; Mc Gowan, J.E.; Sweet, R.L.; Wenzel, R.P. Purpose of quality standards for infectious diseases. Clin. Infect. Dis. 1994, 18, 421. [Google Scholar] [CrossRef] [PubMed]
- Corradini, P.; Tarella, C.; Zallio, F.; Dodero, A.; Zanni, M.; Valagussa, P.; Gianni, A.M.; Rambaldi, A.; Barbui, T.; Cortelazzo, S. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia 2006, 20, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Conde, E.; Gutiérrez, A.; Arranz, R.; León, Á.; Marín, J.; Bendandi, M.; Albo, C.; Caballero, M.D. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: A prospective study from the Gel-Tamo Study Group. Eur. J. Haematol. 2007, 79, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mercadal, S.; Briones, J.; Xicoy, B.; Pedro, C.; Escoda, L.; Estany, C.; Camós, M.; Colomo, L.; Espinosa, Í.; Martínez, S.; et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann. Oncol. 2008, 19, 958–963. [Google Scholar] [CrossRef]
- Reimer, P.; Rüdiger, T.; Geissinger, E.; Weissinger, F.; Nerl, C.; Schmitz, N.; Engert, A.; Einsele, H.; Muller-Hermelink, H.K.; Wilhelm, M. Autologous stem-cell transplantation as first-line therapy in peripheral t-cell lymphomas: Results of a prospective multicenter study. J. Clin. Oncol. 2009, 27, 106–113. [Google Scholar] [CrossRef]
- Wilhelm, M.; Smetak, M.; Reimer, P.; Geissinger, E.; Ruediger, T.; Metzner, B.; Schmitz, N.; Engert, A.; Schaefer-Eckart, K.; Birkmann, J. First-line therapy of peripheral T-cell lymphoma: Extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016, 6, e452. [Google Scholar] [CrossRef]
- Corradini, P.; Vitolo, U.; Rambaldi, A.; Miceli, R.; Patriarca, F.; Gallamini, A.; Olivieri, A.; Benedetti, F.; Todeschini, G.; Rossi, G.; et al. Intensified chemo-immunotherapy with or without stem cell transplantation in newly diagnosed patients with peripheral T-cell lymphoma. Leukemia 2014, 28, 1885–1891. [Google Scholar] [CrossRef]
- Loirat, M.; Chevallier, P.; Leux, C.; Moreau, A.; Bossard, C.; Guillaume, T.; Gastinne, T.; Delaunay, J.; Blin, N.; Mahe´, B.; et al. Upfront allogeneic stem-cell transplantation for patients with nonlocalized untreated peripheral T-cell lymphoma: An intention-to-treat analysis from a single center. Ann. Oncol. 2015, 26, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Caballero, M.D.; Gutiérrez, A.; Marín, J.; Lahuerta, J.J.; Sureda, A.; Carreras, E.; León, A.; Arranz, R.; Fernández de Sevilla, A.; et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: The GEL-TAMO experience. Ann. Oncol. 2003, 14, 1768–1775. [Google Scholar] [CrossRef]
- Ellin, F.; Landström, J.; Jerkeman, M.; Relander, T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood 2014, 124, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Horwitz, S.M.; Foss, F.M.; Pinter-Brown, L.C.; Carson, K.R.; Rosen, S.T.; Pro, B.; Hsi, E.D.; Federico, M.; Gisselbrecht, C.; et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer 2019, 125, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Kanakry, J.A.; Kasamon, Y.L.; Gocke, C.D.; Tsai, H.L.; Davis-Sproul, J.; Ghosh, N.; Symons, H.; Bolaños-Meade, J.; Gladstone, D.E.; Swinnen, L.J.; et al. Outcomes of Related Donor HLA-Identical or HLA-Haploidentical Allogeneic Blood or Marrow Transplantation for Peripheral T Cell Lymphoma. Biol. Blood Marrow Transplant. 2013, 19, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wei, J.; Xu, J.H.; Xiao, Y.; Zhang, Y.C. Autologous stem cell transplantation as the first-line treatment for peripheral T cell lymphoma: Results of a comprehensive meta-analysis. Acta Haematol. 2014, 131, 114–125. [Google Scholar] [CrossRef]
- Beitinjaneh, A.; Saliba, R.M.; Medeiros, L.J.; Turturro, F.; Rondon, G.; Korbling, M.; Fayad, L.; Fanale, M.A.; Alousi, A.M.; Anderlini, P.; et al. Comparison of survival in patients with t cell lymphoma after autologous and allogeneic stem cell transplantation as a frontline strategy or in relapsed disease. Biol. Blood Marrow Transplant. 2015, 21, 855–859. [Google Scholar] [CrossRef]
- Fossard, G.; Broussais, F.; Coelho, I.; Bailly, S.; Nicolas-Virelizier, E.; Toussaint, E.; Lancesseur, C.; Le Bras, F.; Willems, E.; Tchernonog, E.; et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: An analysis of patients from LYSA centers. Ann. Oncol. 2018, 29, 715–723. [Google Scholar] [CrossRef]
- El-Asmar, J.; Reljic, T.; Ayala, E.; Hamadani, M.; Nishihori, T.; Kumar, A.; Kharfan-Dabaja, M.A. Efficacy of High-Dose Therapy and Autologous Hematopoietic Cell Transplantation in Peripheral T Cell Lymphomas as Front-Line Consolidation or in the Relapsed/Refractory Setting: A Systematic Review/Meta-Analysis. Biol. Blood Marrow Transplant. 2016, 22, 802–814. [Google Scholar] [CrossRef]
- Kahl, C.; Leithäuser, M.; Wolff, D.; Steiner, B.; Hartung, G.; Casper, J.; Freund, M. Treatment of peripheral T-cell lymphomas (PTCL) with high-dose chemotherapy and autologous or allogeneic hematopoietic transplantation. Ann. Hematol. 2002, 81, 646–650. [Google Scholar]
- Schetelig, J.; Fetscher, S.; Reichle, A.; Berdel, W.E.; Beguin, Y.; Brunet, S.; Caballero, D.; Majolino, I.; Hagberg, H.; Johnsen, H.E.; et al. Long-term disease-free survival in patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation. Haematologica 2003, 88, 1272–1278. [Google Scholar] [PubMed]
- Jantunen, E.; Wiklund, T.; Juvonen, E.; Putkonen, M.; Lehtinen, T.; Kuittinen, O.; Franssila, K.; Söderström, K.O.; Leppä, S.; Elonen, E.; et al. Autologous stem cell transplantation in adult patients with peripheral T-cell lymphoma: A nation-wide survey. Bone Marrow Transplant. 2004, 33, 405–410. [Google Scholar] [CrossRef]
- Feyler, S.; Prince, H.M.; Pearce, R.; Towlson, K.; Nivison-Smith, I.; Schey, S.; Gibson, J.; Patton, N.; Bradstock, K.; Marks, D.I.; et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: A BSBMT and ABMTRR study. Bone Marrow Transplant. 2007, 40, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Conde, E.; Gutiérrez, A.; Arranz, R.; Gandarillas, M.; Leon, A.; Ojanguren, J.; Sureda, A.; Carrera, D.; Bendandi, M.; et al. Prolonged survival of patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation. The GELTAMO experience. Eur. J. Haematol. 2007, 78, 290–296. [Google Scholar] [CrossRef]
- Rodríguez, J.; Conde, E.; Gutiérrez, A.; Arranz, R.; León, Á.; Marín, J.; Bendandi, M.; Albo, C.; Calballero, M.D. The results of consolidation with autologous stem-cell transplantation in patients with peripheral T-cell lymphoma (PTCL) in first complete remission: The Spanish Lymphoma and Autologous Transplantation Group experience. Ann. Oncol. 2007, 18, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, C.; Canals, C.; Goldstone, A.; Caballero, D.; Metzner, B.; Kobbe, G.; Kolb, H.J.; Kienast, J.; Reimer, P.; Finke, J.; et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: Complete remission at transplantation is the major determinant of outcome—Lymphoma Working Party of the European Group for blood and marrow transplantation. J. Clin. Oncol. 2008, 26, 218–224. [Google Scholar] [PubMed]
- Niitsu, N.; Okamoto, M.; Nakamine, H.; Aoki, S.; Motomura, S.; Hirano, M. Clinico-pathologic features and outcome of Japanese patients with peripheral T-cell lymphomas. Hematol. Oncol. 2008, 26, 152–158. [Google Scholar] [CrossRef]
- Prochazka, V.; Faber, E.; Raida, L.; Vondrakova, J.; Kucerova, L.; Jarosovaa, M.; Indrak, K.; Papajik, T. Prolonged survival of patients with peripheral T-cell lymphoma after first-line intensive sequential chemotherapy with autologous stem cell transplantation. Biomed. Pap. 2009, 153, 63–66. [Google Scholar] [CrossRef]
- Yang, D.H.; Kim, W.S.; Kim, S.J.; Bae, S.H.; Kim, S.H.; Kim, I.H.; Yoon, S.S.; Mun, Y.C.; Shin, H.J.; Chae, Y.S.; et al. Prognostic Factors and Clinical Outcomes of High-Dose Chemotherapy followed by Autologous Stem Cell Transplantation in Patients with Peripheral T Cell Lymphoma, Unspecified: Complete Remission at Transplantation and the Prognostic Index of Peripheral T Ce. Biol. Blood Marrow Transplant. 2009, 15, 118–125. [Google Scholar] [CrossRef]
- Numata, A.; Miyamoto, T.; Ohno, Y.; Kamimura, T.; Kamezaki, K.; Tanimoto, T.; Takase, K.; Henzan, H.; Kato, K.; Takenaka, K.; et al. Long-term outcomes of autologous PBSCT for peripheral T-cell lymphoma: Retrospective analysis of the experience of the Fukuoka BMT group. Bone Marrow Transplant. 2010, 45, 311–316. [Google Scholar] [CrossRef]
- Prochazka, V.; Faber, E.; Raida, L.; Papajik, T.; Vondrakova, J.; Rusinakova, Z.; Kucerova, L.; Myslivecek, M.; Indrak, K. Long-term outcome of patients with peripheral t-cell lymphoma treated with first-line intensive chemotherapy followed by autologous stem cell transplantation. Biomed. Pap. 2011, 155, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Nademanee, A.; Palmer, J.M.; Popplewell, L.; Tsai, N.C.; Delioukina, M.; Gaal, K.; Cai, J.; Kogut, N.; Forman, S.J. High-Dose Therapy and Autologous Hematopoietic Cell Transplantation in Peripheral T Cell Lymphoma (PTCL): Analysis of Prognostic Factors. Biol. Blood Marrow Transplant. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Czyz, A.; Romejko-Jarosinska, J.; Helbig, G.; Knopinska-Posluszny, W.; Poplawska, L.; Piatkowska-Jakubas, B.; Hawrylecka, D.; Nasilowska-Adamska, B.; Dytfeld, D.; Lojko-Dankowska, A.; et al. Autologous stem cell transplantation as consolidation therapy for patients with peripheral T cell lymphoma in first remission: Long-term outcome and risk factors analysis. Ann. Hematol. 2013, 92, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Maragulia, J.C.; Moskowitz, A.; Hamlin, P.A.; Lunning, M.A.; Moskowitz, C.H.; Zelenetz, A.; Matasar, M.J.; Sauter, C.; Goldberg, J.; et al. A retrospective analysis of peripheral T-cell lymphoma treated with the intention to transplant in the first remission. Clin. Lymphoma Myeloma Leuk. 2013, 13, 664–670. [Google Scholar] [CrossRef]
- Gui, L.; Shi, Y.K.; He, X.H.; Lei, Y.H.; Zhang, H.Z.; Han, X.H.; Zhou, S.Y.; Liu, P.; Yang, J.L.; Dong, M.; et al. High-dose therapy and autologous stem cell transplantation in peripheral T-cell lymphoma: Treatment outcome and prognostic factor analysis. Int. J. Hematol. 2014, 99, 69–78. [Google Scholar] [CrossRef]
- Gritti, G.; Boschini, C.; Rossi, A.; Delaini, F.; Grassi, A.; Algarotti, A.; Micò, C.; Trezzi, R.; Gianatti, A.; Barbui, A.M.; et al. Primary treatment response rather than front line stem cell transplantation is crucial for long term outcome of peripheral T-cell lymphomas. PLoS ONE 2015, 10, e0121822. [Google Scholar] [CrossRef]
- Yam, C.; Landsburg, D.J.; Nead, K.T.; Lin, X.; Mato, A.R.; Svoboda, J.; Loren, A.W.; Frey, N.V.; Stadtmauer, E.A.; Porter, D.L.; et al. Autologous stem cell transplantation in first complete remission may not extend progression-free survival in patients with peripheral T cell lymphomas. Am. J. Hematol. 2016, 91, 672–676. [Google Scholar] [CrossRef]
- Han, X.; Zhang, W.; Zhou, D.; Ruan, J.; Duan, M.; Zhu, T.; Li, J.; Cai, H.; Cao, X.; Ouyang, M. Autologous stem cell transplantation as frontline strategy for peripheral T-cell lymphoma: A single-centre experience. J. Int. Med. Res. 2017, 45, 290–302. [Google Scholar] [CrossRef]
- Wu, M.; Wang, X.; Xie, Y.; Liu, W.; Zhang, C.; Ping, L.; Ying, Z.; Deng, L.; Zheng, W.; Lin, N.; et al. Outcome and prospective factor analysis of high-dose therapy combined with autologous peripheral blood stem cell transplantation in patients with peripheral T-cell lymphomas. Int. J. Med. Sci. 2018, 15, 867–874. [Google Scholar] [CrossRef]
- Smith, S.M.; Burns, L.J.; Inwards, D.J.; van Besien, K.; Wiernik, P.H.; Cairo, M.S.; Le Rademacher, J.; He, W.; Hari, P.N.; Fenske, T.S.; et al. Hematopoietic cell transplantation for systemic mature T-cell non-hodgkin lymphoma. J. Clin. Oncol. 2013, 31, 3100–3109. [Google Scholar] [CrossRef]
- Le Gouill, S.; Milpied, N.; Buzyn, A.; De Latour, R.P.; Vernant, J.P.; Mohty, M.; Moles, M.P.; Bouabdallah, K.; Bulabois, C.E.; Dupuis, J.; et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: A study by the Société Française de Greffe de Moëlle et de Thérapie Cellulaire. J. Clin. Oncol. 2008, 26, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Ahn, K.W.; Litovich, C.; Ahmed, S.; Battiwalla, M.; Cohen, J.B.; Dahi, P.; Farhadfar, N.; Farooq, U.; Freytes, C.O.; et al. Allogeneic hematopoietic cell transplantation provides effective salvage despite refractory disease or failed prior autologous transplant in angioimmunoblastic T-cell lymphoma: A CIBMTR analysis. J. Hematol. Oncol. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Fukano, R.; Mori, T.; Fujita, N.; Kobayashi, R.; Mitsui, T.; Kato, K.; Suzuki, R.; Suzumiya, J.; Fukuda, T.; Shindo, M.; et al. Successful outcome with reduced-intensity condition regimen followed by allogeneic hematopoietic stem cell transplantation for relapsed or refractory anaplastic large-cell lymphoma. Int. J. Hematol. 2019, 110, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.I.; McMillan, A.; Negrin, R.S.; Horning, S.J.; Laport, G.G. Long-Term Results of Autologous Hematopoietic Cell Transplantation for Peripheral T Cell Lymphoma: The Stanford Experience. Biol. Blood Marrow Transplant. 2008, 14, 741–747. [Google Scholar] [CrossRef]
- Kewalramani, T.; Zelenetz, A.D.; Teruya-Feldstein, J.; Hamlin, P.; Yahalom, J.; Horwitz, S.; Nimer, S.D.; Moskowitz, C.H. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. Br. J. Haematol. 2006, 134, 202–207. [Google Scholar] [CrossRef]
- Kyriakou, C.; Canals, C.; Finke, J.; Kobbe, G.; Harousseau, J.L.; Kolb, H.J.; Novitzky, N.; Goldstone, A.H.; Sureda, A.; Schmitz, N. Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: A retrospective study from the lymphoma working party of the European group for blood and marrow transplantation. J. Clin. Oncol. 2009, 27, 3951–3958. [Google Scholar] [CrossRef]
- Shustov, A.R.; Gooley, T.A.; Sandmaier, B.M.; Shizuru, J.; Sorror, M.L.; Sahebi, F.; McSweeney, P.; Niederwieser, D.; Bruno, B.; Storb, R.; et al. Allogeneic haematopoietic cell transplantation after nonmyeloablative conditioning in patients with T-cell and natural killer-cell lymphomas. Br. J. Haematol. 2010, 150, 170–178. [Google Scholar] [CrossRef]
- Jacobsen, E.D.; Kim, H.T.; Ho, V.T.; Cutler, C.S.; Koreth, J.; Fisher, D.C.; Armand, P.; Alyea, E.P.; Freedman, A.S.; Soiffer, R.J.; et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann. Oncol. 2011, 22, 1608–1613. [Google Scholar] [CrossRef]
- Zain, J.; Palmer, J.M.; Delioukina, M.; Thomas, S.; Tsai, N.C.; Nademanee, A.; Popplewell, L.; Gaal, K.; Senitzer, D.; Kogut, N.; et al. Allogeneic hematopoietic cell transplant for peripheral T-cell non-Hodgkin lymphoma results in long-term disease control. Leuk. Lymphoma 2011, 52, 1463–1473. [Google Scholar] [CrossRef]
- Delioukina, M.; Zain, J.; Palmer, J.M.; Tsai, N.; Thomas, S.; Forman, S. Reduced-intensity allogeneic hematopoietic cell transplantation using fludarabine-melphalan conditioning for treatment of mature T-cell lymphomas. Bone Marrow Transplant. 2012, 47, 65–72. [Google Scholar] [CrossRef]
- Dodero, A.; Spina, F.; Narni, F.; Patriarca, F.; Cavattoni, I.; Benedetti, F.; Ciceri, F.; Baronciani, D.; Scimè, R.; Pogliani, E.; et al. Allogeneic transplantation following a reduced-intensity conditioning regimen in relapsed/refractory peripheral T-cell lymphomas: Long-term remissions and response to donor lymphocyte infusions support the role of a graft-versus-lymphoma effect. Leukemia 2012, 26, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.D.; Chou, J.F.; Horwitz, S.; Teruya-Feldstein, J.; Barker, J.N.; Boulad, F.; Castro-Malaspina, H.; Giralt, S.; Jakubowski, A.A.; Koehne, G.; et al. Long-term survival in patients with peripheral T-cell non-Hodgkin lymphomas after allogeneic hematopoietic stem cell transplant. Leuk. Lymphoma 2012, 53, 1124–1129. [Google Scholar] [CrossRef]
- Czajczynska, A.; Günther, A.; Repp, R.; Humpe, A.; Schub, N.; Raff, T.; Nickelsen, M.; Schrauder, A.; Schrappe, M.; Kneba, M.; et al. Allogeneic stem cell transplantation with BEAM and alemtuzumab conditioning immediately after remission induction has curative potential in advanced T-cell non-hodgkin’s lymphoma. Biol. Blood Marrow Transplant. 2013, 19, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, Y.; Wang, Q.; Guo, L.; Jin, Z.; Fu, Z.; Han, Y.; Sun, A.; Liu, W.; Ruan, J.; et al. Outcome of Allogeneic and Autologous Hematopoietic Cell Transplantation for High-Risk Peripheral T Cell Lymphomas: A Retrospective Analysis From a Chinese Center. Biol. Blood Marrow Transplant. 2017, 23, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Rohlfing, S.; Dietrich, S.; Witzens-Harig, M.; Hegenbart, U.; Schönland, S.; Luft, T.; Ho, A.D.; Dreger, P. The impact of stem cell transplantation on the natural course of peripheral T-cell lymphoma: A real-world experience. Ann. Hematol. 2018, 97, 1241–1250. [Google Scholar] [CrossRef]
- Corradini, P.; Dodero, A.; Zallio, F.; Caracciolo, D.; Casini, M.; Bregni, M.; Narni, F.; Patriarca, F.; Boccadoro, M.; Benedetti, F.; et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin’s lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J. Clin. Oncol. 2004, 22, 2172–2176. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in OncologyTM. T-Cell Lymphoma (Version 1.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf (accessed on 13 August 2020). [CrossRef]
- Kharfan-Dabaja, M.A.; Kumar, A.; Ayala, E.; Hamadani, M.; Reimer, P.; Gisselbrecht, C.; d’Amore, F.; Jantunen, E.; Ishida, T.; Bazarbachi, A.; et al. Clinical Practice Recommendations on Indication and Timing of Hematopoietic Cell Transplantation in Mature T Cell and NK/T Cell Lymphomas: An International Collaborative Effort on Behalf of the Guidelines Committee of the American Society for Blood and Ma. Biol. Blood Marrow Transplant. 2017, 23, 1826–1838. [Google Scholar] [CrossRef]
- d’Amore, F.; Gaulard, P.; Trümper, L.; Corradini, P.; Kim, W.S.; Specht, L.; Bjerregaard Pedersen, M.; Ladetto, M. Peripheral T-cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v108–v115. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef]
- Chihara, D.; Fanale, M.A.; Miranda, R.N.; Noorani, M.; Westin, J.R.; Nastoupil, L.J.; Hagemeister, F.B.; Fayad, L.E.; Romaguera, J.E.; Samaniego, F.; et al. The survival outcome of patients with relapsed/refractory peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma. Br. J. Haematol. 2017, 176, 750–758. [Google Scholar] [CrossRef]
- Bellei, M.; Foss, F.M.; Shustov, A.R.; Horwitz, S.M.; Marcheselli, L.; Kim, W.S.; Cabrera, M.E.; Dlouhy, I.; Nagler, A.; Advani, R.H.; et al. The outcome of peripheral t-cell lymphoma patients failing first-line therapy: A report from the prospective, international t-cell project. Haematologica 2018, 103, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- d’Amore, F.; Leppä, S.; da Silva, M.G.; Relander, T.; Lauritzsen, G.F.; Brown, P.D.N.; Pezzutto, A.; Doorduijn, J.K.; Weidmann, E.; van Gelder, M.; et al. Final Analysis of the Front-Line Phase III Randomized ACT-1 Trial in Younger Patients with Systemic Peripheral T-Cell Lymphoma Treated with CHOP Chemotherapy with or without Alemtuzumab and Consolidated By Autologous Hematopoietic Stem Cell Transplant. Blood 2018, 132, 998. [Google Scholar] [CrossRef]
- Wulf, G.G.; Altmann, B.; Ziepert, M.; D’Amore, F.; Held, G.; Greil, R.; Tournilhac, O.; Relander, T.; Viardot, A.; Wilhelm, M.; et al. Alemtuzumab plus CHOP versus CHOP in elderly patients with peripheral T-cell lymphoma: The DSHNHL2006-1B/ACT-2 trial. Leukemia 2020, 1–3. [Google Scholar] [CrossRef]
- Sieniawski, M.; Angamuthu, N.; Boyd, K.; Chasty, R.; Davies, J.; Forsyth, P.; Jack, F.; Lyons, S.; Mounter, P.; Revell, P.; et al. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood 2010, 115, 3664–3670. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.H.; Lannon, M.M.; Lopes, A.; Chadwick, H.; Jones, G.; Sieniawski, M.; Davies, A.; Wood, K.; Clifton-Hadley, L.; Smith, P.; et al. High-dose chemotherapy and autologous stem cell transplantation in enteropathy-associated and other aggressive T-cell lymphomas: A UK NCRI/Cancer Research UK Phase II Study. Bone Marrow Transplant. 2019, 54, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, E.; Boumendil, A.; Finel, H.; Luan, J.J.; Johnson, P.; Rambaldi, A.; Haynes, A.; Duchosal, M.A.; Bethge, W.; Biron, P.; et al. Autologous stem cell transplantation for enteropathy-associated T-cell lymphoma: A retrospective study by the EBMT. Blood 2013, 121, 2529–2532. [Google Scholar] [CrossRef]
- Nijeboer, P.; de Baaij, L.R.; Visser, O.; Witte, B.I.; Cillessen, S.A.G.M.; Mulder, C.J.; Bouma, G. Treatment response in enteropathy associated T-cell lymphoma; survival in a large multicenter cohort. Am. J. Hematol. 2015, 90, 493–498. [Google Scholar] [CrossRef]
- Tanase, A.; Schmitz, N.; Stein, H.; Boumendil, A.; Finel, H.; Castagna, L.; Blaise, D.; Milpied, N.; Sucak, G.; Sureda, A.; et al. Allogeneic and autologous stem cell transplantation for hepatosplenic T-cell lymphoma: A retrospective study of the EBMT Lymphoma Working Party. Leukemia 2015, 29, 686–688. [Google Scholar] [CrossRef]
- Rashidi, A.; Cashen, A.F. Outcomes of allogeneic stem cell transplantation in hepatosplenic T-cell lymphoma. Blood Cancer J. 2015, 5, e318. [Google Scholar] [CrossRef]
- Deconinck, E.; Lamy, T.; Foussard, C.; Gaillard, F.; Delwail, V.; Colombat, P.; Casassus, P.; Lemevel, A.; Brion, A.; Milpied, N. Autologous stem cell transplantation for anaplastic large- cell lymphomas: Results of a prospective trial. Br. J. Haematol. 2000, 109, 736–742. [Google Scholar] [CrossRef]
| Levels of evidence | |
| I | Evidence from at least one properly randomized, controlled trial |
| II | Evidence from at least one well designed clinical trial without randomization, from cohort or case-control analytic studies, from multiple time-series studies or from dramatic results in uncontrolled experiments |
| III | Evidence from opinions or respected authorities, based on clinical experience, descriptive studies or reports of expert committees |
| Grades of recommendation | |
| A | Good evidence to support a recommendation for use |
| B | Moderate evidence to support a recommendation for use |
| C | Poor evidence to support recommendation |
| D | Moderate evidence to support a recommendation against use |
| E | Good evidence to support a recommendation against use |
| Study | Year | Cases (n) | PTCL Subtypes (n = ALCL) | ITT Analysis (n = Failed to Proceed to HSCT) | % Proceeded to HSCT | Response Prior to AutoSCT | Survival | TRM % |
|---|---|---|---|---|---|---|---|---|
| Autologous Stem Cell Transplantation | ||||||||
| Corradini et al. [5] | 2006 | 62 | All subtypes including ALCL (ALK+ n = 19 ALK− n = 4) | Yes (n = 16) | 74 | CR: 66% PR: 16% | DFS: 55% at 12 years OS: 30% at 12 years | 4.8 |
| Rodgriguez et al. [6] | 2007 | 26 | All subtypes including ALCL (ALK− n = 8) | No (n = 7) | 73 | CR: 65% PR: 8% | PFS: 53% at 3 years OS: 73% at 3 years | 0 |
| Mercadal et al. [7] | 2008 | 41 | All subtypes including ALCL (ALK+ n = 1) | No (n = 7) | 41 | CR: 49% PR: 10% | PFS: 30% at 4 years OS: 39% at 4 years | - |
| Reimer et al. [8] | 2009 | 83 | All subtypes including ALCL (ALK− n = 13) | Yes (n = 28) | 66 | CR: 39% PR: 40% | OS: 48% at 3 years | 3.6 |
| d’Amore et al. [3] | 2012 | 166 | All subtypes excluding ALCL, ALK+ (ALK− n = 31) | Yes (n = 51) | 72 | CR: 51% PR: 30% | PFS: 44% at 5 years OS: 51% at 5 years | 4 |
| Wilhelm et al. [9] | 2016 | 111 | All subtypes including ALCL (ALK− n = 16) | Yes (n = 36) | 68 | CR: 62% PR: 20% | PFS: 39% at 5 years OS: 44% at 5 years | 3.6 |
| Allogeneic stem cell transplantation | ||||||||
| Corradini et al. [10] | 2014 | 61 | All subtypes excluding ALCL, ALK+ (ALK− n = 12) | No (n = 24) | alloSCT 38 | CR: 87% PR: 13% | PFS: 69% at 4 years OS: 69% at 4 years | 13 |
| autoSCT 23 | CR: 71% PR: 29% | PFS: 70% at 4 years OS: 92% at 4 years | ||||||
| Loirat et al. [11] | 2014 | 49 | All subtypes excluding ALCL, ALK+ (ALK− n = 7) | Yes (n = 13) | 60 | CR: 41.5% PR: 58.5% | PFS: 62.5% at 2 years OS: 72.5% at 2 years | 8.2 |
| Autologous Stem Cell Transplantation | ||||||
|---|---|---|---|---|---|---|
| Study | Year | Cases (n) | PTCL Subtypes (n = ALCL) | Median Follow-Up (Years) | Survival | TRM (%) |
| Rodriguez et al. [12] (GEL-TAMO) | 2003 | 37 | All subtypes including ALCL | 37 | DFS: 79% at 5 years OS: 80% at 5 years | 8 |
| Ellin et al. [13] (Swedish) | 2014 | 128 | All subtypes including ALCL (ALK− n = 24) | 8.1 | PFS: 41% at 5 years OS: 48% at 5 years | - |
| Park et al. [14] (COMPLETE) | 2019 | 119 (autoSCT n = 36) | All subtypes including ALCL (ALK− n = 42) | 2.8 | OS: 87.6% at 2 year Improved PFS and OS for AITL PFS: 68.8% at 2 years OS: 93.3% at 2 years | - |
| Allogeneic Stem Cell Transplantation | ||||||
| Kanakry et al. [15] (Johns Hopkins Hospital) | 2013 | 44 (MAC 20 RIC 24) | All subtypes ALCL, (ALK+ n = 2 ALCL, ALK− n = 5 ALCL, ALKu n = 3) | 3.9 | PFS: 40% at 2 years OS: 43% at 2 years | MAC 10 RIC 8 |
| Study | Year | Cases (n) | PTCL Subtypes | Median Follow-Up (Months) | Overall Survival |
|---|---|---|---|---|---|
| Khal et al. [20] | 2002 | 10 | All subtypes | 13.3 | 58% at 1 year |
| Schetelig et al. [21] | 2003 | 29 | AITL | 60 | 44% at 5 years |
| Rodriguez et al. [12] | 2003 | 35 | All subtypes | 37.5 | 37% at 5 years |
| Jantunen et al. [22] | 2004 | 18 | All subtypes | 24 | 63% at 5 years |
| Feyler et al. [23] | 2007 | 64 | All subtypes | 37 | 53% at 3 years |
| Rodriguez et al. [24] | 2007 | 19 | AITL | 25 | 60% at 3 years |
| Rodriguez et al. [25] | 2007 | 74 | All subtypes | 67 | 68% at 5 years |
| Kyriakou et al. [26] | 2008 | 146 | AITL | 31 | 59% at 4 years |
| Niitsu et al. [27] | 2008 | 10 | All subtypes | 72 | 60% at 5 years |
| Prochazka et al. [28] | 2009 | 18 | All subtypes | 25.7 | 71% at 2 years |
| Yang et al. [29] | 2009 | 64 | PTCL-NOS | 29.7 | 53% at 3 years |
| Numata et al. [30] | 2010 | 39 | All subtypes | 78 | 62.2% at 5 years |
| Prochazka et al. [31] | 2011 | 29 | All subtypes | 55.1 | 65% at 2 years |
| Nademanee et al. [32] | 2011 | 12 | All subtypes | 33.6 | 92% at 5 years |
| Czyz et al. [33] | 2012 | 12 | ALCL, ALK+ excluded | 53 | 77.2% at 5 years |
| Mehta et al. [34] | 2013 | 34 | ALCL, ALK+ excluded | 4 years | 67.4% at 4 years |
| Gui et al. [35] | 2014 | 18 | All subtypes | 113.5 | 89% at 5 years |
| Beitinjaneh et al. [17] | 2015 | 47 | All subtypes | 35 | 76% at 4 years |
| Gritti et al. [36] | 2015 | 21 | All subtypes | 5.61 years | 82% at 3 years |
| Yam et al. [37] | 2016 | 20 | ALCL, ALK+ excluded | 23.1 | 72% at 3 years |
| Han et al. [38] | 2017 | 52 | All subtypes | 34 | 71.1% at 5 years |
| Wu et al. [39] | 2018 | 47 | All subtypes | 23.6 | 89.8% at 2 years |
| Fossard et al. [18] | 2018 | 269 | All subtypes | 4.5 years | 59.2% at 5 years |
| Autologous Stem Cell Transplantation | ||||||
|---|---|---|---|---|---|---|
| Study | Year | Cases (n) | PTCL Subtypes (n = ALCL) | Median Follow-Up (Months) | Survival | TRM (%) |
| Rodriguez et al. [12] (GEL-TAMO) | 2003 | 78 | All subtypes including ALCL | 37 | DFS: 49% at 5 years OS: 45% at 5 years | 8 (37 months) |
| Smith et al. [40] (CIBMTR) | 2013 | 75 | All subtypes including ALCL (n = 39) | 73 | PFS: 41% at 3 years OS: 53% at 3 years | 6 (3 year) |
| Allogeneic stem cell transplantation | ||||||
| Le Gouill et al. [41] (French) | 2008 | 77 | All subtypes including ALCL (n = 27) | 43 | EFS: 53% at 5 years OS: 57% at 5 years | 32 (1 year) |
| Smith et al. [40] (CIBMTR) | 2013 | 126 | All subtypes including ALCL (n = 51) | 49 | PFS: 47% at 3 years OS: 46% at 3 years | 34 (3 year) |
| Epperla et al. [42] (CIBMTR) | 2019 | 249 | AITL | 49 | PFS: 49% at 4 years OS: 56% at 4 years | 19 (1 year) |
| Fukano et al. [43] (Japanese) | 2019 | 38 (RIC 8 MAC 30) | ALCL | 72 | OS: at 5 years RIC 100%, MAC 49% EFS: at 5 years RIC 88%, MAC 43% | RIC 0 MAC 25.9 (5 year) |
| Autologous Stem Cell Transplantation | ||||||
|---|---|---|---|---|---|---|
| Study | Year | Cases (n) | PTCL Subtypes (n = ALCL) | Median Follow-Up (Months) | Survival | TRM (%) |
| Kewalramani et al. [45] | 2006 | 24 | All subtypes excluding ALCL, ALK+ (ALCL, ALK− n = 4) | 6 years | PFS: 24% at 5 years OS: 33% at 5 years | - |
| Chen et al. [44] | 2008 | 32 | All subtypes including ALCL (n = 13) | - | PFS: 12% at 5 years OS: 40% at 5 years | 4 |
| Nademanee et al. [32] | 2011 | 55 | All subtypes including ALCL, ALK+ | 33.6 | PFS: 32% at 5 years OS: 45% at 5 years | 0 |
| Czyz et al. [33] | 2012 | 46 | All subtypes including ALCL, ALK+ | 53 | PFS: 51.7% at 5 years OS: 56.8% at 5 years | - |
| Beitinjaneh et al. [17] | 2015 | 76 autoSCT 41 alloSCT 35 | All subtypes excluding ALCL, ALK+ (ALCL, ALK− n = 28) | - | autoSCT OS: 50% at 4 years alloSCT OS: 36% at 4 years PFS: NSD | - |
| El-Asma et al. [19] (meta-analysis) | 2016 | 581 | All subtypes including ALCL | - | PFS: 36% OS: 47% | 10 |
| Wu et al. [39] | 2018 | 32 | All subtypes including ALCL | 23.6 | PFS: 32.9% at 2 years OS: 50.5% at 2 years | - |
| Allogeneic Stem Cell Transplantation | ||||||
| Kyriakou et al. [46] | 2009 | 45 | AITL | 29 | PFS: 54% at 3 years OS: 64% at 3 years | 25% (1 year) |
| Shustov et al. [47] | 2020 | 17 | All subtypes including ALCL | 3.3 years | PFS: 53% at 3 years OS: 59% at 3 years | 19% (3 year) |
| Jacobsen et al. [48] | 2011 | 52 | All subtypes including ALCL | 49 | Nodal PTCL PFS: 45% at 3 years OS: 52% at 3 years Extranodal PTCL PFS: 6% at 3 years OS: 23% at 3 years | 27 (3 year) |
| Zain et al. [49] | 2011 | 37 | All subtypes including ALCL and CTCL (ALCL n = 6) | 20.3 | PFS: 46.7% at 5years OS: 52.2% at 5 years | 28.9% (5 years) |
| Delioukina et al. [50] | 2012 | 27 | All subtypes including ALCL and CTCL | 36 | PFS: 47% at 2 years OS: 55% at 2 years | 22% (2 years) |
| Dodero et al. [51] | 2012 | 52 | All subtypes including ALCL (n = 11) | 67 | PFS: 40% at 5years OS: 50% at 5 years | 12% (5 years) |
| Golberg et al. [52] | 2012 | 34 (n = CR1) | All subtypes including ALCL (n = 8) | 45 | PFS: 50% at 2 years OS: 61% at 2 years | 18 |
| Czajczynska et al. [53] | 2013 | 24 | All subtypes including ALCL (n = 4) | 44.8 | OS 42.4% t 3 years | 25 |
| Huang et al. [54] | 2017 | 67 (autoSCT 43 alloSCT 24) | All subtypes excluding ALCL, ALK+ (ALCL, ALK− n = 19) | 27 | autoSCT PFS: 49% at 5 years OS: 57% at 5 years alloSCT PFS: 54% at 5 years OS: 55% at 5 years | 18 (alloSCT) 7 (autoSCT) |
| Rohlfing et al. [55] | 2018 | 117 (RR 91) Underwent SCT n = 38 (autoSCT 7 alloSCT 31) | All subtypes including ALCL (n = 27) | 5.8 years | autoSCT OS: 10 months alloSCT OS: 52% at 5 years | 23 (alloSCT) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeyakoon, C.; van der Weyden, C.; Harrop, S.; Khot, A.; Dickinson, M.; Yannakou, C.K.; Prince, H.M. Role of Haematopoietic Stem Cell Transplantation in Peripheral T-Cell Lymphoma. Cancers 2020, 12, 3125. https://doi.org/10.3390/cancers12113125
Abeyakoon C, van der Weyden C, Harrop S, Khot A, Dickinson M, Yannakou CK, Prince HM. Role of Haematopoietic Stem Cell Transplantation in Peripheral T-Cell Lymphoma. Cancers. 2020; 12(11):3125. https://doi.org/10.3390/cancers12113125
Chicago/Turabian StyleAbeyakoon, Chathuri, Carrie van der Weyden, Sean Harrop, Amit Khot, Michael Dickinson, Costas K. Yannakou, and H. Miles Prince. 2020. "Role of Haematopoietic Stem Cell Transplantation in Peripheral T-Cell Lymphoma" Cancers 12, no. 11: 3125. https://doi.org/10.3390/cancers12113125
APA StyleAbeyakoon, C., van der Weyden, C., Harrop, S., Khot, A., Dickinson, M., Yannakou, C. K., & Prince, H. M. (2020). Role of Haematopoietic Stem Cell Transplantation in Peripheral T-Cell Lymphoma. Cancers, 12(11), 3125. https://doi.org/10.3390/cancers12113125






