Genetic Variants of the TERT Gene, Telomere Length, and Circulating TERT as Prognostic Markers in Rectal Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Characteristics of Rectal Cancer Patients
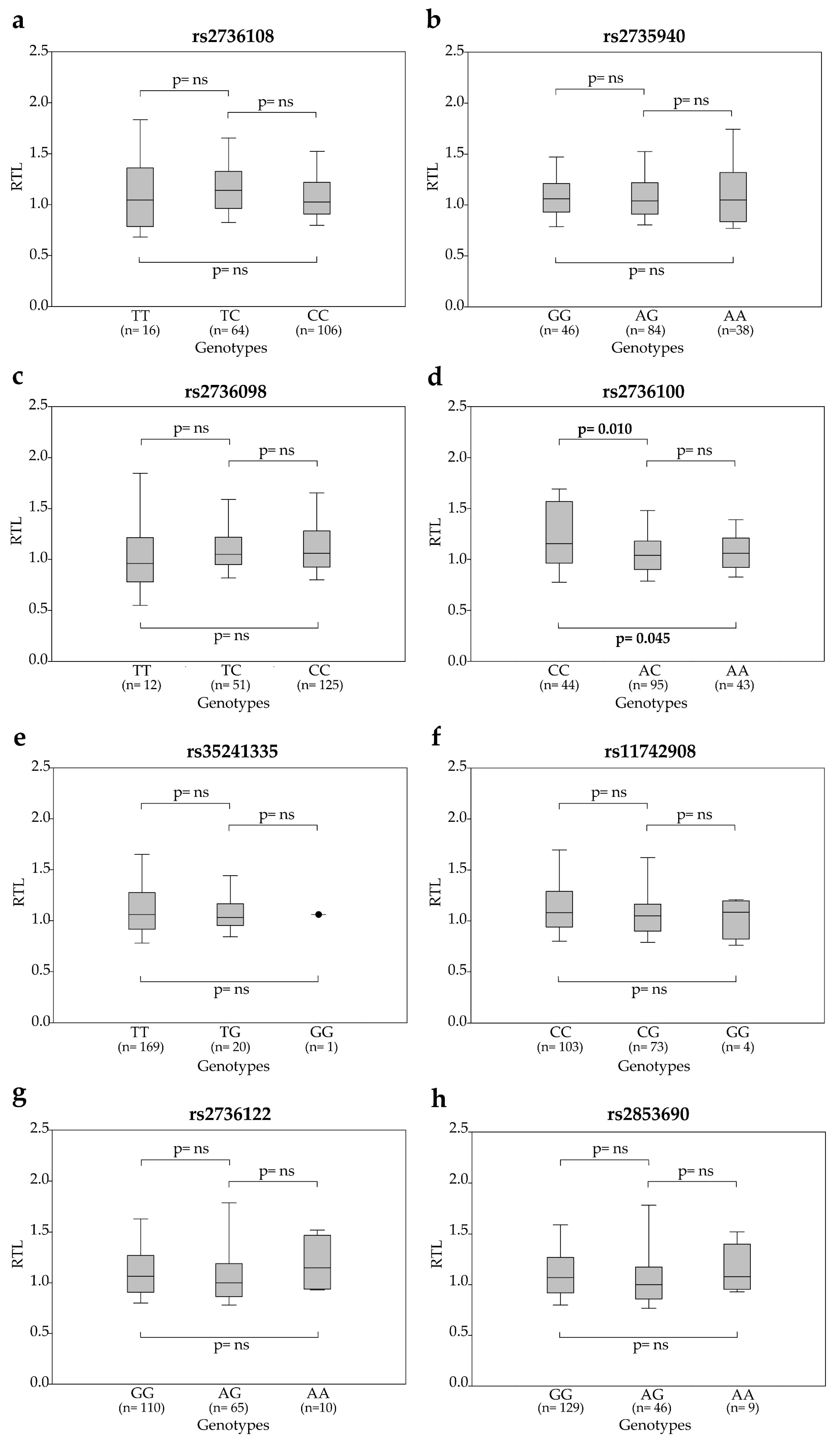
2.2. Association between SNP Genotypes and Telomere Length in Peripheral Blood Cells of Rectal Cancer Patients
2.3. Association between SNP Genotypes and Circulating TERT mRNA Levels
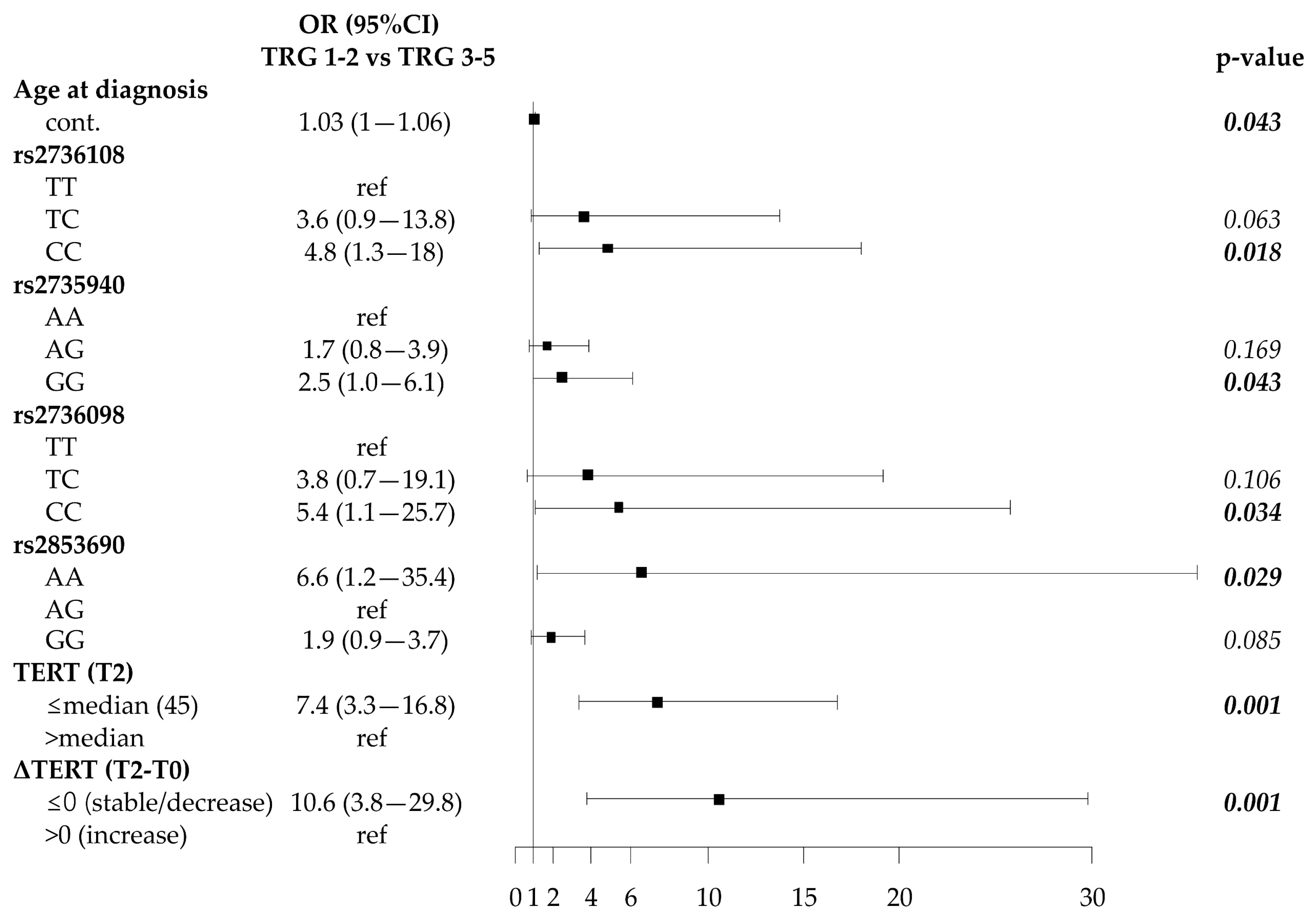
2.4. Association of SNP Genotypes, Telomere Length and Circulating Plasma TERT mRNA with Response to Neoadjuvant Therapy
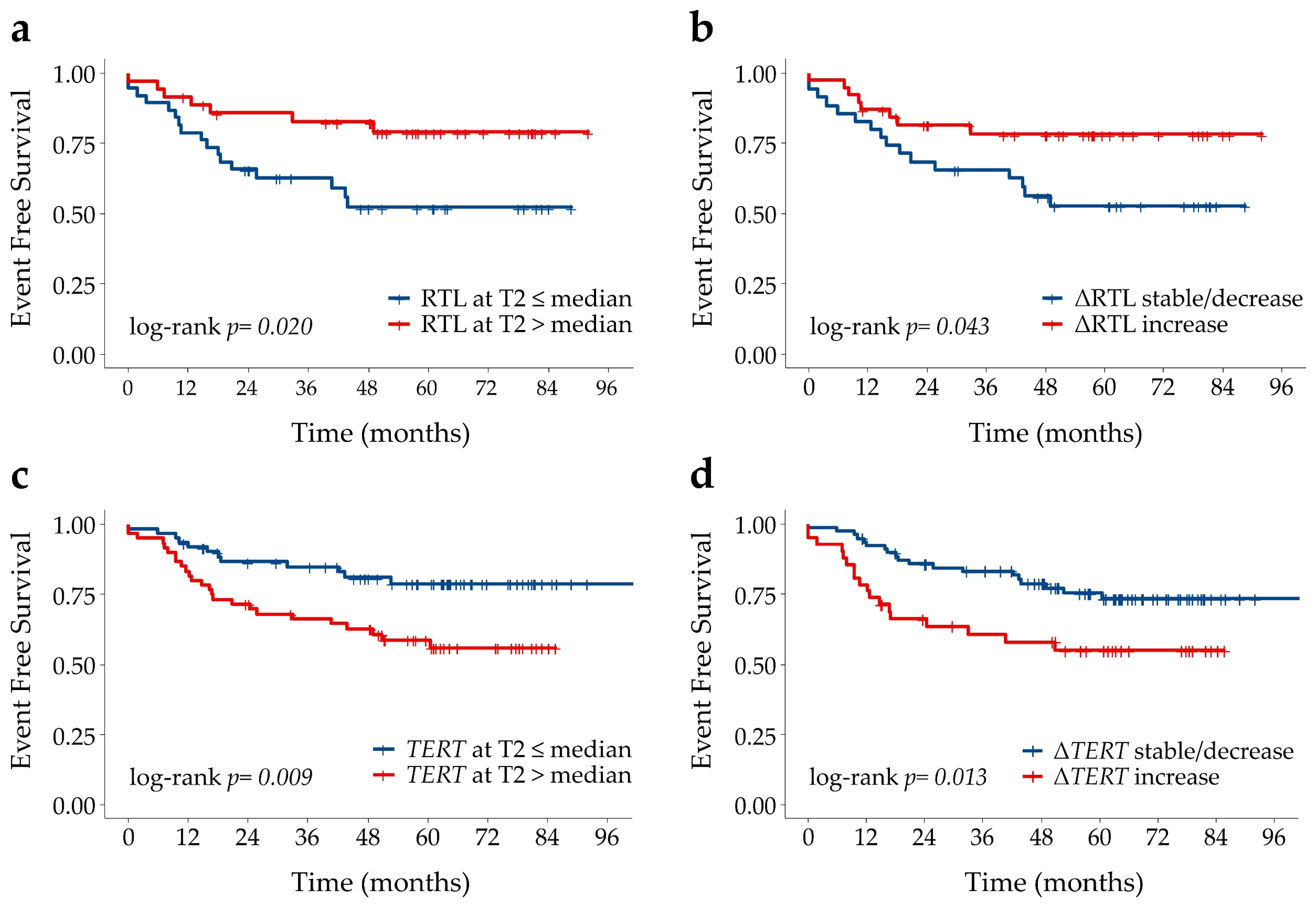
2.5. Associations of SNP Genotypes, Telomere Length and Circulating TERT mRNA with Disease Outcome
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Evaluation of Pathologic Tumor Response
4.3. DNA Extraction from Peripheral Blood Cells
4.4. Telomere Length and TERT mRNA Quantification
4.5. SNP Selection and Genotyping
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef]
- De Lange, T. Protection of mammalian telomeres. Oncogene 2002, 21, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Morin, G.B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 1989, 59, 521–589. [Google Scholar] [CrossRef]
- Nakamura, T.M.; Morin, G.B.; Chapman, K.B.; Weinrich, S.L.; Andrews, W.H.; Lingner, J.; Harley, C.B.; Cech, T.R. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997, 277, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.S.; Wen, J.; Bacchetti, S. The human telomerase catalytic subunit hTERT: Organization of the gene and characterization of the promoter. Hum. Mol. Genet. 1999, 8, 137–142. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Senescence and immortalization: Role of telomeres and telomerase. Carcinogenesis 2005, 26, 867–874. [Google Scholar] [CrossRef]
- De Martino, M.; Taus, C.; Lucca, I.; Hofbauer, S.L.; Haitel, A.; Shariat, S.F.; Klatte, T. Association of human telomerase reverse transcriptase gene polymorphisms, serum levels, and telomere length with renal cell carcinoma risk and pathology. Mol. Carcinog. 2016, 55, 1458–1466. [Google Scholar] [CrossRef]
- Wei, R.; Cao, L.; Pu, H.; Wang, H.; Zheng, Y.; Niu, X.; Weng, X.; Zhang, H.; Favus, M.; Zhang, L.; et al. TERT Polymorphism rs2736100-C Is Associated with EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin. Cancer Res. 2015, 21, 5173–5180. [Google Scholar] [CrossRef]
- Sheng, X.; Tong, N.; Tao, G.; Luo, D.; Wang, M.; Fang, Y.; Li, J.; Xu, M.; Zhang, Z.; Wu, D. TERT polymorphisms modify the risk of acute lymphoblastic leukemia in Chinese children. Carcinogenesis 2013, 34, 228–235. [Google Scholar] [CrossRef]
- Beesley, J.; Pickett, H.A.; Johnatty, S.E.; Dunning, A.M.; Chen, X.; Li, J.; Lu, Y.; Rider, D.N.; Palmieri, R.T.; Stutz, M.D.; et al. Functional polymorphisms in the TERT promoter are associated with risk of serous epithelial ovarian and breast cancers. PLoS ONE 2011, 6, e24987. [Google Scholar] [CrossRef]
- Codd, V.; Nelson, C.P.; Albrecht, E.; Mangino, M.; Deelen, J.; Buxton, J.L.; Hottenga, J.J.; Fischer, K.; Esko, T.; Surakka, I.; et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 2013, 45, 422–427. [Google Scholar] [CrossRef]
- Mirabello, L.; Yu, K.; Kraft, P.; De Vivo, I.; Hunter, D.J.; Prescott, J.; Wong, J.Y.; Chatterjee, N.; Hayes, R.B.; Savage, S.A. The association of telomere length and genetic variation in telomere biology genes. Hum. Mutat. 2010, 31, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Murata, M.; Yoshida, T.; Watanabe, K.; Saito, I.; Miyaki, K.; Omae, K.; Ikeda, Y. Telomere length of normal leukocytes is affected by a functional polymorphism of hTERT. Biochem. Biophys. Res. Commun. 2006, 341, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Verdi, D.; Pooley, K.A.; Landi, M.T.; Egan, K.M.; Baird, D.M.; Prescott, J.; De Vivo, I.; Nitti, D. Telomerase reverse transcriptase locus polymorphisms and cancer risk: A field synopsis and meta-analysis. J. Natl. Cancer Inst. 2012, 104, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liu, C.; Lu, M.; Yuan, X.; Cheng, G.; Kong, F.; Lu, J.; Strååt, K.; Björkholm, M.; Ma, L.; et al. The TERT locus genotypes of rs2736100-CC/CA and rs2736098-AA predict shorter survival in renal cell carcinoma. Urol. Oncol. 2019, 37, 301.e1–301.e10. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yan, C.; Du, J.; Ji, Y.; Gao, Y.; Zhu, X.; Yu, F.; Huang, T.; Dai, J.; Ma, H.; et al. Genetic variants affecting telomere length are associated with the prognosis of esophageal squamous cell carcinoma in a Chinese population. Mol. Carcinog. 2017, 56, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, J.; Bai, Y.; Wang, S.; Yin, X.; Xiang, J.; Li, X.; He, M.; Zhang, X.; Wu, T.; et al. The associations of TERT-CLPTM1L variants and TERT mRNA expression with the prognosis of early stage non-small cell lung cancer. Cancer Gene Ther. 2017, 24, 20–27. [Google Scholar] [CrossRef]
- Jannuzzi, A.T.; Karaman, E.; Oztas, E.; Yanar, H.T.; Özhan, G. Telomerase Reverse Transcriptase (TERT) Gene variations and Susceptibility of Colorectal Cancer. Genet. Test Mol. Bioma. 2015, 19, 692–697. [Google Scholar] [CrossRef]
- Hofer, P.; Baierl, A.; Bernhart, K.; Leeb, G.; Mach, K.; Micksche, M.; Gsur, A. Association of genetic variants of human telomerase with colorectal polyps and colorectal cancer risk. Mol. Carcinog. 2012, 51, E176–E182. [Google Scholar] [CrossRef]
- Kinnersley, B.; Migliorini, G.; Broderick, P.; Whiffin, N.; Dobbins, S.E.; Casey, G.; Hopper, J.; Sieber, O.; Lipton, L.; Kerr, D.J.; et al. Colon Cancer Family Registry. The TERT variant rs2736100 is associated with colorectal cancer risk. Br. J. Cancer 2012, 107, 1001–1008. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.E.; Warrier, S.K.; Lynch, A.C.; Heriot, A.G. Assessing pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A systematic review. Color. Dis. 2015, 17, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the CAO/ARO/AIO-94 trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef]
- Rampazzo, E.; Del Bianco, P.; Bertorelle, R.; Boso, C.; Perin, A.; Spiro, G.; Bergamo, F.; Belluco, C.; Buonadonna, A.; Palazzari, E.; et al. The predictive and prognostic potential of plasma telomerase reverse transcriptase (TERT) RNA in rectal cancer patients. Br. J. Cancer 2018, 118, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, S.; Rampazzo, E.; Briarava, M.; Maretto, I.; Agostini, M.; Digito, M.; Keppel, S.; Friso, M.L.; Lonardi, S.; De Paoli, A.; et al. Telomere-specific reverse transcriptase (hTERT) and cell-free RNA in plasma as predictors of pathologic tumor response in rectal cancer patients receiving neoadjuvant chemoradiotherapy. Ann. Surg. Oncol. 2012, 19, 3089–3096. [Google Scholar] [CrossRef]
- Snetselaar, R.; van Oosterhout, M.F.M.; Grutters, J.C.; van Moorsel, C.H.M. Telomerase Reverse Transcriptase Polymorphism rs2736100: A Balancing Act between Cancer and Non-Cancer Disease, a Meta-Analysis. Front. Med. 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Shi, M.; An, C.; Yang, W.; Nie, X.; Zhang, J.; Lv, Z.; Li, J.; Zhou, L.; Du, Z.; et al. Functional evaluation of TERT-CLPTM1L genetic variants associated with susceptibility of papillary thyroid carcinoma. Sci. Rep. 2016, 6, 26037. [Google Scholar] [CrossRef]
- Zou, P.; Gu, A.; Ji, G.; Zhao, L.; Zhao, P.; Lu, A. The TERT rs2736100 polymorphism and cancer risk: A meta-analysis based on 25 case-control studies. BMC Cancer 2012, 12, 7. [Google Scholar] [CrossRef]
- Gallicchio, L.; Gadalla, S.M.; Murphy, J.D.; Simonds, N.I. The Effect of Cancer Treatments on Telomere Length: A Systematic Review of the Literature. J. Natl. Cancer Inst. 2018, 110, 1048–1058. [Google Scholar] [CrossRef]
- Diker-Cohen, T.; Uziel, O.; Szyper-Kravitz, M.; Shapira, H.; Natur, A.; Lahav, M. The effect of chemotherapy on telomere dynamics: Clinical results and possible mechanisms. Leuk. Lymphoma 2013, 54, 2023–2029. [Google Scholar] [CrossRef]
- Garg, M.B.; Lincz, L.F.; Adler, K.; Scorgie, F.E.; Ackland, S.P.; Sakoff, J.A. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: A multivariate analysis. Br. J. Cancer 2012, 107, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Ozkaynak, M.F.; Drullinsky, P.; Sandoval, C.; Tugal, O.; Jayabose, S.; Moore, M.A. Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia 1998, 12, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, Z. Telomere Length as a Prognostic Factor for Overall Survival in Colorectal Cancer Patients. Cell Physiol. Biochem. 2016, 38, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Barczak, W.; Sobecka, A.; Golusinski, P.; Masternak, M.M.; Rubis, B.; Suchorska, W.M.; Golusinski, W. hTERT gene knockdown enhances response to radio- and chemotherapy in head and neck cancer cell lines through a DNA damage pathway modification. Sci. Rep. 2018, 8, 5949. [Google Scholar] [CrossRef]
- Giunco, S.; Zangrossi, M.; Dal Pozzolo, F.; Celeghin, A.; Ballin, G.; Petrara, M.R.; Amin, A.; Argenton, F.; Godinho Ferreira, M.; De Rossi, A. Anti-Proliferative and Pro-Apoptotic Effects of Short-Term Inhibition of Telomerase In Vivo and in Human Malignant B Cells Xenografted in Zebrafish. Cancers 2020, 12, 2052. [Google Scholar] [CrossRef]
- Ségal-Bendirdjian, E.; Geli, V. Non-canonical Roles of Telomerase: Unraveling the Imbroglio. Front. Cell Dev. Biol. 2019, 7, 332. [Google Scholar] [CrossRef]
- Martínez, P.; Blasco, M.A. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Cancer 2011, 11, 161–176. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Mandard, A.M.; Dalibard, F.; Mandard, J.C.; Marnay, J.; Henry-Amar, M.; Petiot, J.F.; Roussel, A.; Jacob, J.H.; Segol, P.; Samama, G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Giunco, S.; Rampazzo, E.; Brutti, M.; Spinato, G.; Menegaldo, A.; Stellin, M.; Mantovani, M.; Bandolin, L.; Rossi, M.; et al. TERT promoter hotspot mutations and their relationship with TERT levels and telomere erosion in patients with head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 381–389. [Google Scholar] [CrossRef]
- Gianesin, K.; Noguera-Julian, A.; Zanchetta, M.; Del Bianco, P.; Petrara, M.R.; Freguja, R.; Rampon, O.; Fortuny, C.; Camós, M.; Mozzo, E.; et al. Premature aging and immune senescence in HIV-infected children. AIDS 2016, 30, 1363–1373. [Google Scholar] [CrossRef]
- Rampazzo, E.; Bertorelle, R.; Serra, L.; Terrin, L.; Candiotto, C.; Pucciarelli, S.; Del Bianco, P.; Nitti, D.; De Rossi, A. Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br. J. Cancer 2010, 102, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef] [PubMed]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]


| Characteristics | Total | |
|---|---|---|
| (n = 194) | ||
| Age at diagnosis (years) | Median (IQR) | 65 (58–72) |
| Gender | M | 138 (71.1%) |
| F | 56 (28.9%) | |
| Distance from anal verge(cm) | Median (IQR) | 5 (3–8) |
| missing | 9 | |
| CEA, ng/mL (T0) | Median (IQR) | 2.0 (1.2–3.5) |
| Missing | 45 | |
| Grading | G1 | 12 (7.7%) |
| G2 | 132 (84.6%) | |
| G3 | 12 (7.7%) | |
| Missing | 38 | |
| cTNM | I | 6 (3.2%) |
| II | 21 (11.1%) | |
| III | 159 (84.1%) | |
| IV | 3 (1.6%) | |
| Missing | 5 | |
| Total RT dose | <50.4 Gy | 22 (12.4%) |
| 50.4 Gy | 89 (50.0%) | |
| >50.4 Gy | 67 (37.6%) | |
| Missing | 16 | |
| Fluoropyrimide | Alone | 93 (50.8%) |
| +other drugs | 84 (45.9%) | |
| No | 6 (3.3%) | |
| Missing | 11 | |
| Interval between CRT and surgery (months) | Median (IQR) | 1.9 (1.7–2.2) |
| Interval between diagnosis and Surgery (months) | Median (IQR) | 3.5 (3.1–3.9) |
| Variable Pre-CRT | Genotype | OR TRG 1-2 vs TRG 3-5 (95% CI) | p-Value |
| Age at diagnosis (years) | cont. | 1.03 (1.0─1.07) | 0.052 |
| rs2735940 | AA | ns | |
| AG | |||
| GG | |||
| rs2736098 | TT | ns | |
| TC | |||
| CC | |||
| rs2736108 | TT | Ref | |
| TC | 3.4 (0.8─14.4) | 0.102 | |
| CC | 4.6 (1.1─19.1) | 0.034 | |
| rs2853690 | GG | 2.7 (1.2-6.3) | 0.017 |
| AG | Ref | ||
| AA | 9.7 (1.6─57.0) | 0.012 | |
| AA/GG | 3.0 (1.3─6.9) | 0.008 | |
| Variable Post-CRT | Genotype/Characteristics | OR TRG 1-2 vs TRG 3-5 (95% CI) | p-Value |
| Age at diagnosis (years) | cont. | ns | |
| rs2735940 | AA | ns | |
| AG | |||
| GG | |||
| rs2736098 | TT | ns | |
| TC | |||
| CC | |||
| rs2736108 | TT | ns | |
| TC | |||
| CC | |||
| rs2853690 | GG | ns | |
| AG | |||
| AA | |||
| TERT (T2) | ≤median (45) | 5.8 (1.9─17.8) | 0.002 |
| >median | Ref | ||
| ΔTERT (T2-T0) | ≤0 (stable/decrease) | 5.3 (1.4─19.4) | 0.012 |
| ˃0 (increase) | Ref |
| Covariate | Characteristics | HR (95%CI) | p-Value |
|---|---|---|---|
| RTL (T2) | ≤median (1.14) | Ref | |
| >median | 0.4 (0.1–0.9) | 0.024 | |
| ΔRTL (T2-T0) | ≤0 (stable/decrease) | ns | |
| >0 (increase) | |||
| TERT (T2) | ≤median (45) | 0.3 (0.1–0.8) | 0.015 |
| >median | Ref | ||
| ΔTERT (T2-T0) | ≤0 (stable/decrease) | ns | |
| >0 (increase) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampazzo, E.; Cecchin, E.; Del Bianco, P.; Menin, C.; Spolverato, G.; Giunco, S.; Lonardi, S.; Malacrida, S.; De Paoli, A.; Toffoli, G.; et al. Genetic Variants of the TERT Gene, Telomere Length, and Circulating TERT as Prognostic Markers in Rectal Cancer Patients. Cancers 2020, 12, 3115. https://doi.org/10.3390/cancers12113115
Rampazzo E, Cecchin E, Del Bianco P, Menin C, Spolverato G, Giunco S, Lonardi S, Malacrida S, De Paoli A, Toffoli G, et al. Genetic Variants of the TERT Gene, Telomere Length, and Circulating TERT as Prognostic Markers in Rectal Cancer Patients. Cancers. 2020; 12(11):3115. https://doi.org/10.3390/cancers12113115
Chicago/Turabian StyleRampazzo, Enrica, Erika Cecchin, Paola Del Bianco, Chiara Menin, Gaya Spolverato, Silvia Giunco, Sara Lonardi, Sandro Malacrida, Antonino De Paoli, Giuseppe Toffoli, and et al. 2020. "Genetic Variants of the TERT Gene, Telomere Length, and Circulating TERT as Prognostic Markers in Rectal Cancer Patients" Cancers 12, no. 11: 3115. https://doi.org/10.3390/cancers12113115
APA StyleRampazzo, E., Cecchin, E., Del Bianco, P., Menin, C., Spolverato, G., Giunco, S., Lonardi, S., Malacrida, S., De Paoli, A., Toffoli, G., Pucciarelli, S., & De Rossi, A. (2020). Genetic Variants of the TERT Gene, Telomere Length, and Circulating TERT as Prognostic Markers in Rectal Cancer Patients. Cancers, 12(11), 3115. https://doi.org/10.3390/cancers12113115











