Validation of Prognostic Stage and Anatomic Stage in the American Joint Committee on Cancer 8th Edition for Inflammatory Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient and Staging Characteristics
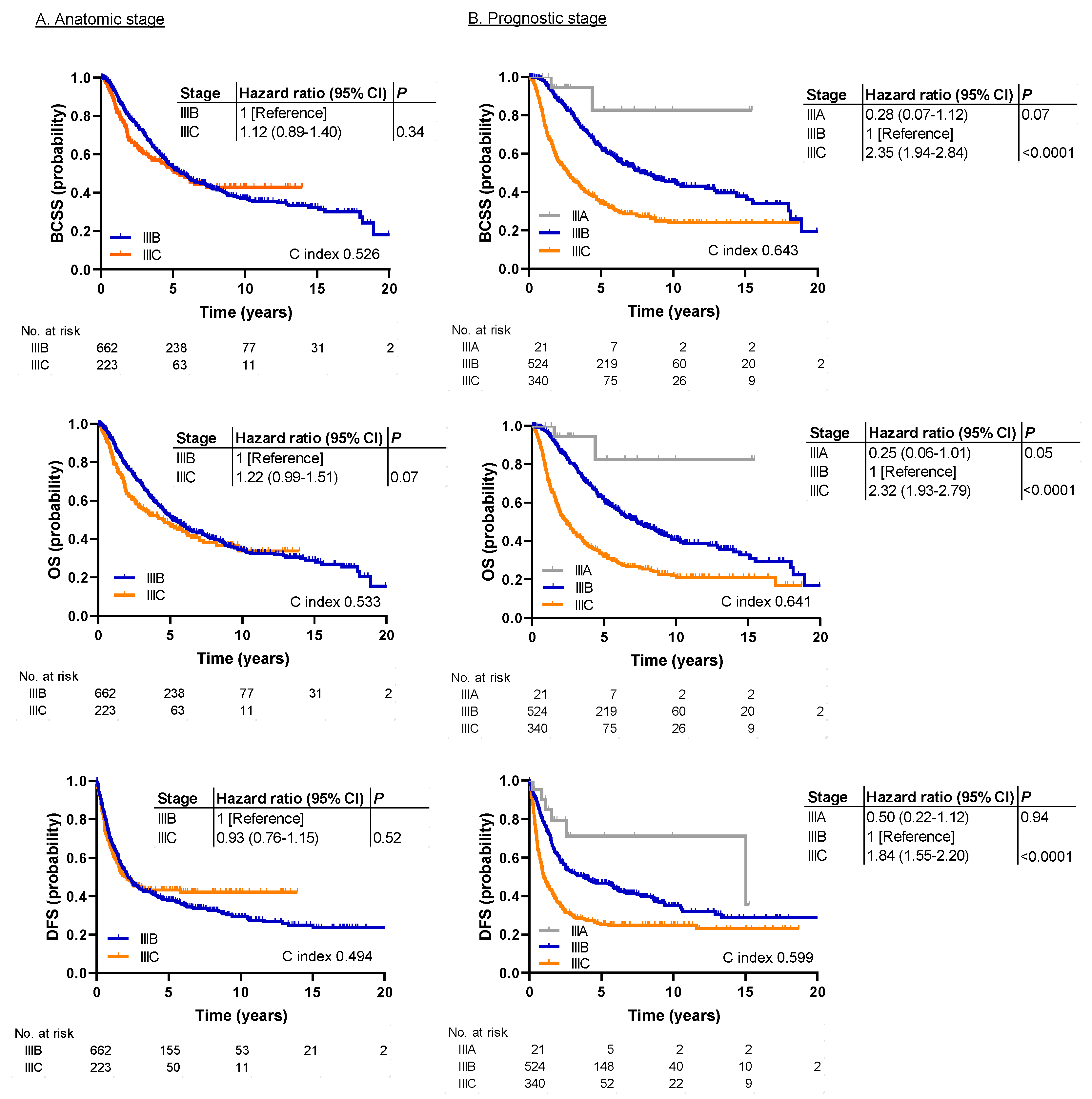
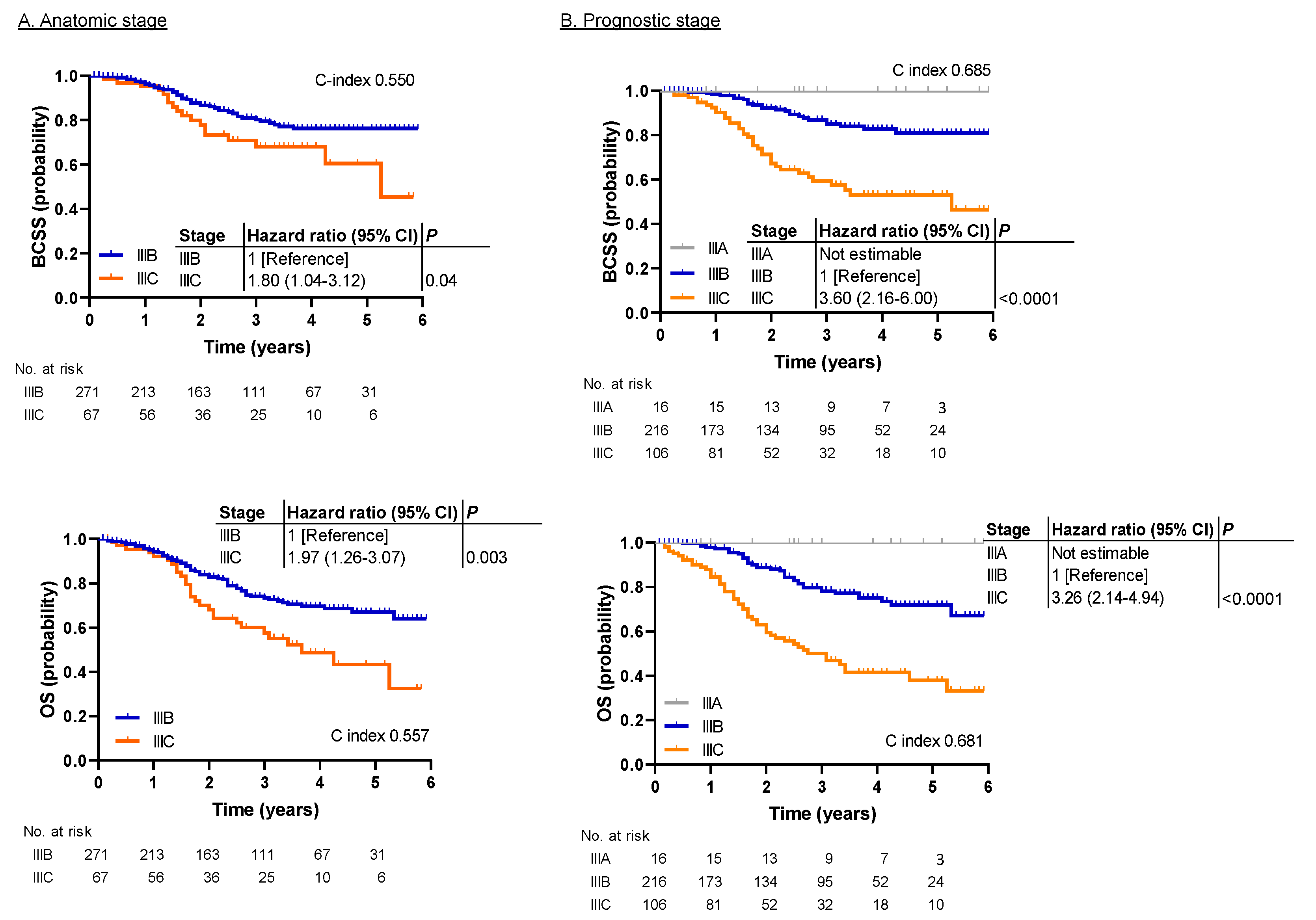
2.2. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Source
4.2. Data Collection
4.3. Survival Analysis
4.4. Statistical Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hortobagyi, G.N.; Connolly, J.L.; Edge, S.B. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Weiss, A.; Chavez-MacGregor, M.; Lichtensztajn, D.Y.; Yi, M.; Tadros, A.; Hortobagyi, G.N.; Giordano, S.H.; Hunt, K.K.; Mittendorf, E.A. Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared with the Anatomic Stage in Breast Cancer. JAMA Oncol. 2018, 4, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rahman, O. Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res. Treat. 2018, 168, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xu, L.; Ye, J.; Xin, L.; Duan, X.; Liu, Y. The Prognostic Value of the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging System in HER2- Enriched Subtype Breast Cancer, a Retrospective Analysis. Anticancer Res. 2017, 37, 4615–4621. [Google Scholar] [PubMed]
- Wang, M.; Chen, H.; Wu, K.; Ding, A.; Zhang, M.; Zhang, P. Evaluation of the prognostic stage in the 8th edition of the American Joint Committee on Cancer in locally advanced breast cancer: An analysis based on SEER 18 database. Breast 2018, 37, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Merajver, S.D.; Viens, P.; Vermeulen, P.B.; Swain, S.M.; Buchholz, T.A.; Dirix, L.Y.; Levine, P.H.; Lucci, A.; Krishnamurthy, S.; et al. International expert panel on inflammatory breast cancer: Consensus statement for standardized diagnosis and treatment. Ann. Oncol. 2011, 22, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Hance, K.W.; Anderson, W.F.; Devesa, S.S.; Young, H.A.; Levine, P.H. Trends in Inflammatory Breast Carcinoma Incidence and Survival: The Surveillance, Epidemiology, and End Results Program at the National Cancer Institute. J. Natl. Cancer Inst. 2005, 97, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Hennessy, B.T.; Broglio, K.; Meric-Bernstam, F.; Cristofanilli, M.; Giordano, S.H.; Buchholz, T.A.; Sahin, A.; Singletary, S.E.; Buzdar, A.U.; et al. Trends for Inflammatory Breast Cancer: Is Survival Improving? Oncologist 2007, 12, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Brewer, T.M.; Liu, D.D.; Iwamoto, T.; Shen, Y.; Hsu, L.; Willey, J.S.; Gonzalez-Angulo, A.M.; Chavez-MacGregor, M.; Fouad, T.M.; et al. Long-term treatment efficacy in primary inflammatory breast cancer by hormonal receptor- and HER2-defined subtypes. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Y.; Wu, Q.; Zhu, S.; Chen, C.; Yang, W.; Wei, W.; Sun, S. Outcomes of patients with inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes: A population-based study from the SEER program. Oncotarget 2017, 8, 49370–49379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gonzalez-Angulo, A.M.; Allen, P.K.; Yu, T.K.; Woodward, W.A.; Ueno, N.T.; Lucci, A.; Krishnamurthy, S.; Gong, Y.; Bondy, M.L.; et al. Triple-Negative Subtype Predicts Poor Overall Survival and High Locoregional Relapse in Inflammatory Breast Cancer. Oncologist 2011, 16, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-G.; Zhang, W.-W.; Wang, J.; Dong, Y.; Sun, J.-Y.; Chen, Y.-X.; He, Z.-Y. Inflammatory breast cancer outcomes by breast cancer subtype: A population-based study. Future Oncol. 2019, 15, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Çakar, B.; Sürmeli, Z.; Öner, P.G.; Yelim, E.S.; Karabulut, B.; Uslu, R. The Impact of Subtype Distribution in Inflammatory Breast Cancer Outcome. Eur. J. Breast Heal. 2018, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yan, Y.; Guo, L.; Ou, H.; Hai, J.; Zhang, C.; Wu, Z.; Tang, L. Distinct outcomes in patients with different molecular subtypes of inflammatory breast cancer. Saudi Med J. 2014, 35, 1324–1330. [Google Scholar] [PubMed]
- Rueth, N.M.; Lin, H.Y.; Bedrosian, I.; Shaitelman, S.F.; Ueno, N.T.; Shen, Y.; Babiera, G. Underuse of Trimodality Treatment Affects Survival for Patients with Inflammatory Breast Cancer: An Analysis of Treatment and Survival Trends From the National Cancer Database. J. Clin. Oncol. 2014, 32, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.; Jack, A.; Parkin, D.M. International Classification of Diseases for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College Of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, N.; Moran, M.S.; Su, P.; Haffty, B.G.; Yang, Q. Borderline ER-Positive Primary Breast Cancer Gains No Significant Survival Benefit From Endocrine Therapy: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | MDA Cohort (n = 885) | SEER Cohort (n = 338) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age at diagnosis in years | ||||
| Median (range) | 50 (19–96) | 57 (25–93) | ||
| <40 | 161 | 18.2 | 34 | 10.1 |
| 40–49 | 255 | 28.8 | 60 | 17.8 |
| 50–59 | 299 | 33.8 | 104 | 30.8 |
| 60–69 | 132 | 14.9 | 78 | 23.1 |
| ≥70 | 38 | 4.3 | 62 | 18.3 |
| Race | ||||
| White | 679 | 76.7 | 279 | 82.5 |
| Black | 78 | 8.8 | 33 | 9.8 |
| Other | 128 | 14.5 | 26 | 7.7 |
| Tumor grade | ||||
| 1 | 8 | 0.9 | 9 | 2.7 |
| 2 | 183 | 20.7 | 105 | 31.1 |
| 3 | 694 | 78.4 | 224 | 66.3 |
| ER status | ||||
| Positive | 418 | 47.2 | 185 | 54.7 |
| Negative | 467 | 52.8 | 153 | 45.3 |
| PR status | ||||
| Positive | 306 | 34.6 | 145 | 42.9 |
| Negative | 579 | 65.4 | 193 | 57.1 |
| HER2 status | ||||
| Positive | 325 | 36.7 | 128 | 37.9 |
| Negative | 560 | 63.3 | 210 | 62.1 |
| Subtype | ||||
| HR+/HER2− | 311 | 35.1 | 127 | 37.6 |
| HR+/HER2+ | 137 | 15.5 | 70 | 20.7 |
| HR−/HER2+ | 188 | 21.2 | 58 | 17.2 |
| HR−/HER2− | 249 | 28.1 | 83 | 24.6 |
| Lymph node stage | ||||
| N0 | 136 | 15.4 | 58 | 17.2 |
| N1 | 436 | 49.3 | 140 | 41.4 |
| N2 | 86 | 9.7 | 73 | 21.6 |
| N3 | 227 | 25.6 | 67 | 19.8 |
| AJCC anatomic stage * | ||||
| IIIB | 662 | 74.8 | 271 | 80.2 |
| IIIC | 223 | 25.2 | 67 | 19.8 |
| AJCC prognostic stage * | ||||
| IIIA | 21 | 2.4 | 16 | 4.7 |
| IIIB | 524 | 59.2 | 216 | 63.9 |
| IIIC | 340 | 38.4 | 106 | 31.4 |
| Chemotherapy | ||||
| Yes | 863 | 97.5 | Not known | |
| Neoadjuvant | 834 | 94.3 | Not known | |
| Adjuvant | 29 | 3.3 | Not known | |
| No | 22 | 2.5 | Not known | |
| Radiation therapy | ||||
| Yes | 736 | 83.2 | Not known | |
| No | 149 | 16.8 | Not known | |
| Treatment | ||||
| Chemotherapy + surgery + radiation | 724 | 81.8 | Not known | |
| Chemotherapy + surgery | 139 | 15.7 | Not known | |
| Surgery + radiation | 12 | 1.4 | Not known | |
| Surgery alone | 10 | 1.1 | Not known | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kida, K.; Hess, K.R.; Lim, B.; Iwase, T.; Chainitikun, S.; Valero, V.; Lucci, A.; Le-Petross, H.C.; Woodward, W.A.; Krishnamurthy, S.; et al. Validation of Prognostic Stage and Anatomic Stage in the American Joint Committee on Cancer 8th Edition for Inflammatory Breast Cancer. Cancers 2020, 12, 3105. https://doi.org/10.3390/cancers12113105
Kida K, Hess KR, Lim B, Iwase T, Chainitikun S, Valero V, Lucci A, Le-Petross HC, Woodward WA, Krishnamurthy S, et al. Validation of Prognostic Stage and Anatomic Stage in the American Joint Committee on Cancer 8th Edition for Inflammatory Breast Cancer. Cancers. 2020; 12(11):3105. https://doi.org/10.3390/cancers12113105
Chicago/Turabian StyleKida, Kumiko, Kenneth R. Hess, Bora Lim, Toshiaki Iwase, Sudpreeda Chainitikun, Vicente Valero, Anthony Lucci, Huong Carisa Le-Petross, Wendy A. Woodward, Savitri Krishnamurthy, and et al. 2020. "Validation of Prognostic Stage and Anatomic Stage in the American Joint Committee on Cancer 8th Edition for Inflammatory Breast Cancer" Cancers 12, no. 11: 3105. https://doi.org/10.3390/cancers12113105
APA StyleKida, K., Hess, K. R., Lim, B., Iwase, T., Chainitikun, S., Valero, V., Lucci, A., Le-Petross, H. C., Woodward, W. A., Krishnamurthy, S., Hortobagyi, G. N., Tripathy, D., & Ueno, N. T. (2020). Validation of Prognostic Stage and Anatomic Stage in the American Joint Committee on Cancer 8th Edition for Inflammatory Breast Cancer. Cancers, 12(11), 3105. https://doi.org/10.3390/cancers12113105







