Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management
Abstract
Simple Summary
Abstract
1. Introduction
2. Clinical Therapies—An Overview
3. Dietary Considerations
3.1. General Dietary Factors
3.1.1. Acidity
3.1.2. Glycemic Index
3.1.3. Cholesterol
3.2. Multifactorial Foodstuffs
3.2.1. Red Meat
3.2.2. Fish
3.2.3. Fruit and Vegetables
3.2.4. Dairy
3.2.5. Honey
3.2.6. Coffee
3.3. Specific Dietary Agents
3.3.1. Vitamin A
3.3.2. Vitamin C
3.3.3. Vitamin D
3.3.4. Vitamin E
3.3.5. Curcumin
3.3.6. Genistein
4. Nutraceuticals
4.1. Propolis
4.2. Triptolide
4.3. Cannabidiol
5. Lifestyle Factors
5.1. Obesity
5.2. Diabetes
5.3. Smoking
5.4. Alcohol
5.5. Exercise
6. A Scheme for Integration of Clinical and Complementary Approaches: Treatment Logistics and Strategy
6.1. General Conditioning
6.2. Specific Additions
- The 9 complementary agents are divided into 2 groups—C1 and C2 (Figure 19A). Each of these contains 5 agents (3 dietary, 2 nutraceutical). One agent (cannabidiol) is in both groups (to even the numbers), chosen due to the increasing all-round evidence in its favor. The mixing took into consideration their main modes of action in relation to the ‘hallmarks of cancer’, e.g., as antioxidant, anti-inflammatory/pro-immune and/or anti-angiogenic [343,344].
- One group (say C1) is taken for 2 days before the day of the chemotherapy and this is continued on the day of the treatment and for the following two days, making 5 days in total (Figure 19B).
- Then there are two days of ‘rest’ and then the cycle switches to the C2 group (Figure 19B). This continues as in (2) and then the integration switches back to the C1 agents.
- These alternating cycles (designed to increase the chance of either group working) continue for the duration of the treatment (ca. 7 weeks).
7. Future Perspectives
7.1. Quality Issues
7.2. Emerging Modalities
7.3. Molecular Mechanisms
7.4. Precision Medicine
7.5. Monitoring
7.6. Role of the Brain
7.7. Clinical Trials
8. Overall Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Glossary
| Adjuvant | supportive therapy applied after initial main treatment. |
| Akt | (protein kinase B), an enzyme that plays a role in cell metabolism, especially the insulin pathway. |
| AMPK | (5′ adenosine monophosphate-activated protein kinase), an enzyme involved in cell metabolism. |
| Analogue | a compound similar in structure to another. |
| Angiogenesis | formation of new blood vessels. |
| Apoptosis | death of cells by a pre-programmed genetic mechanism. |
| Autophagy | a mode of cell death, removing damaged proteins, organelles and pathogens. |
| Beta (β)-catenin | protein that plays a role in cell signaling and cell-cell adhesion. |
| Bioavailability | the proportion of a substance that can be absorbed by the body. |
| Biomarker | a molecule from which a biological process can be identified. |
| BMI | (body-mass index, the body weight in kg divided by the square of the body height in meters), a measure of obesity. |
| Cachexia | late-stage weakness and wasting of the body due to severe illness. |
| CA9 | (carbonic anhydrase-9), an enzyme involved in controlling the body’s acidity. |
| Case-control study | a retrospective study comparing the effect of a given measurable factor on a group of people, compared with a control (non-exposed) group. |
| Carcinogenesis | formation of cancer. |
| Caspase-3 | an enzyme involved in apoptosis. |
| Clinical trial | testing of a drug on humans prior to official approval, beginning with basic toxicity (phase I) and leading to more detailed evaluation on increasing numbers of patients (phases II–IV). |
| Cohort study | a longitudinal (time-course) study testing the effect of a certain treatment on a group of people, normally in comparison with a control (untreated) group. |
| Cytokine | signaling molecule (protein) involved in cell signaling, common in the immune system. |
| Desmoplasia | dense fibrous tissue. |
| EGF | (epidermal growth factor), a protein involved in the growth, proliferation and differentiation of cells. |
| EGFR | (epidermal growth factor receptor), a protein acted upon by epidermal growth factor. |
| EMT | (epithelial-mesenchymal transition), an early event/morphological change in cancer cells becoming invasive. |
| Epigenetic | regulation of gene expression levels. |
| Flavonoid | groups of chemicals, pigments, found in plants that have anti-oxidative and anti-inflammatory properties. |
| 5-FU | (5-fluorouracil), a common anti-cancer drug (‘chemotherapy’) often used in combination with other medications. |
| FOLFIRINOX | (folinic acid, fluorouracil, irinotecan and oxaliplatin), a combination chemotherapy drug. |
| GPCR | (G protein-coupled receptor), a protein found on the cell membrane that interacts with incoming signaling molecules. |
| Hedgehog | a signaling mechanism operating in cells, especially during development, and tumorigenesis; sonic hedgehog is a main protein. |
| Hydrogel | cross-linked polymer gel that can absorb and retain water. |
| Hyperplasia | increase in the number of cells within an organ or tissue, forming a barrier. |
| IGF | (insulin-like growth factor), a protein involved in the growth, proliferation and differentiation of cells; shares the same receptor with insulin. |
| Intraperitoneal | an injection into the abdominal cavity of the body. |
| Isoform | a protein with the same function as another but differing slightly in structure. |
| Isomers | a molecule with the same formula but different structure. |
| KPC | a genetically modified mouse model of pancreatic cancer. |
| KRAS | a gene that, when mutated, can cause cells to become cancerous. |
| Macrophage | a type of cell found in the immune system, detects and destroys harmful microorganisms. |
| MAPK | (mitogen associated protein kinase), an enzyme that has a central involvement in the cancer process. |
| Meta-analysis | quantitative study that uses the results of multiple previous analyses to achieve a consensus opinion. |
| Microbiome | microorganisms, especially bacteria, that reside in parts of the body e.g., the gut. |
| Neo-adjuvant | initial therapy applied before the main treatment, e.g., chemotherapy or radiation therapy. |
| Neoplasm | new and abnormal growth of tissue, commonly leading to cancer. |
| NF-kB | (nuclear factor kappa-light-chain-enhancer of activated B cells), a protein involved in gene expression and patho/physiological regulation. |
| Notch | a protein involved in cellular signaling, especially during development, and cancer. |
| Orthotopic | something occurring in its normal location. |
| PanIN | (pancreatic intraepithelial neoplasia), a pathological indicator of pancreatic cancer and its grade. |
| PDAC | (pancreatic ductal adenocarcinoma), the most common type of pancreatic cancer. |
| PDX | (patient derived xenograft), human cancer tissue implanted and surviving in an animal model. |
| PI3K | (phosphoinositide 3-kinase), an enzyme involved in cell signaling. |
| Polyphenol | a type of plant-derived chemical with health benefits. |
| Rho-kinase | a protein/enzyme involved in cell signaling, often as an intermediary. |
| STAT3 | (signal transducer and activator of transcription 3), a protein involved in cell signaling and gene regulation. |
| T-cell | a type of lymphocyte (white blood cell) that is a central part of the immune system. |
| TGFβ | (transforming growth factor beta), a primary signaling protein involved in cellular mechanisms. |
| TRAIL | (tumor necrosis factor-related apoptosis-inducing ligand), a protein that promotes programmed cell death. |
| Transgenic | an organism that contains genetic information from another organism, often used as cancer models. |
| Wnt | (Wingless and Int-1), a signaling mechanism operating in cells, especially during development and tumorigenesis; incorporates a variety of Wnt proteins. |
| Xenograft | a tissue from one species, grafted experimentally to another (often human to mouse). |
References
- Aier, I.; Semwal, R.; Sharma, A.; Varadwaj, P.K. A systematic assessment of statistics, risk factors, and underlying features involved in pancreatic cancer. Cancer Epidemiol. 2019, 58, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, L.E.; Connor, A.A.; Gallinger, S. Molecular Events in the Natural History of Pancreatic Cancer. Trends Cancer 2017, 3, 336–346. [Google Scholar] [CrossRef]
- Lu, F.; Poruk, K.E.; Weiss, M.J. Surgery for oligometastasis of pancreatic cancer. Chin. J. Cancer Res. 2015, 27, 358–367. [Google Scholar]
- Renz, B.W.; Boeck, S.; Roeder, F.; Trumm, C.; Heinemann, V.; Werner, J. Oligometastatic Disease in Pancreatic Cancer—How to Proceed? Visc. Med. 2017, 33, 36–41. [Google Scholar] [CrossRef]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nat. Cell Biol. 2010, 467, 1114–1117. [Google Scholar] [CrossRef]
- Goral, V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pac. J. Cancer Prev. 2015, 16, 5619–5624. [Google Scholar] [CrossRef]
- Lai, E.; Puzzoni, M.; Ziranu, P.; Pretta, A.; Impera, V.; Mariani, S.; Liscia, N.; Soro, P.; Musio, F.; Persano, M.; et al. New therapeutic targets in pancreatic cancer. Cancer Treat. Rev. 2019, 81, 101926. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Zeng, L.; Chen, Y.; Lian, G.; Qian, C.; Li, J.; Xie, R.; Huang, K. New developments in the early diagnosis of pancreatic cancer. Expert Rev. Gastroenterol. Hepatol. 2016, 11, 149–156. [Google Scholar] [CrossRef]
- Birnbaum, D.J.; Bertucci, F.; Finetti, P.; Birnbaum, D.; Mamessier, E. Molecular classification as prognostic factor and guide for treatment decision of pancreatic cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2018, 1869, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Bhagwandin, V.J.; Bishop, J.M.; Wright, W.E.; Shay, J.W. The Metastatic Potential and Chemoresistance of Human Pancreatic Cancer Stem Cells. PLoS ONE 2016, 11, e0148807. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-R.; Chang, C.; Hsu, C.; Tsai, M.; Cheng, H.; Leong, M.K.; Sung, P.; Chen, J.; Weng, C.-F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2019, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Marasini, B.; Sahu, R.P. Natural Anti-Cancer Agents: Implications in Gemcitabine-Resistant Pancreatic Cancer Treatment. Mini Rev. Med. Chem. 2017, 17, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Chen, B.Z.; Lee, A.K.Y.; Chan, A.H.C.; Wu, J.C.Y.; Lin, Z. Chinese Herbal Medicine Effectively Prolongs the Overall Survival of Pancreatic Cancer Patients: A Case Series. Integr. Cancer Ther. 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Requejo, O.; De Quinto, H.G.; Rodríguez, M.C.R. Nutrition as an epigenetic factor in develops of cancer. Nutr. Hosp. 2019, 36, 53–57. [Google Scholar] [CrossRef]
- Islami, F.; Sauer, A.G.; Miller, K.D.; Siegel, R.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2017, 68, 31–54. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Shield, K.; Micallef, C.M.; Vignat, J.; Hill, C.; Rogel, A.; Menvielle, G.; Dossus, L.; Ormsby, J.-N.; Rehm, J.; et al. Cancers related to lifestyle and environmental factors in France in 2015. Eur. J. Cancer 2018, 105, 103–113. [Google Scholar] [CrossRef]
- Djamgoz, M.; Plant, J. Beat Cancer: How to Regain Control of Your Health and Your Life; Ebury Publishing: London, UK, 2014. [Google Scholar]
- Drozdoff, L.; Klein, E.; Kiechle, M.; Paepke, D. Use of biologically-based complementary medicine in breast and gynecological cancer patients during systemic therapy. BMC Complement. Altern. Med. 2018, 18, 259. [Google Scholar] [CrossRef] [PubMed]
- Shalom-Sharabi, I.; Frenkel, M.; Caspi, O.; Bar-Sela, G.; Toledano, M.; Samuels, N.; Schiff, E.; Ben-Arye, E. Integrative Oncology in Supportive Cancer Care in Israel. Integr. Cancer Ther. 2018, 17, 697–706. [Google Scholar] [CrossRef]
- Keene, M.R.; Heslop, I.M.; Sabesan, S.S.; Glass, B.D. Complementary and alternative medicine use in cancer: A systematic review. Complement. Ther. Clin. Pract. 2019, 35, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.E.; Latte-Naor, S. Integrative Oncology: The Role of Complementary Medicine in Supportive Cancer Care. In The MASCC Textbook of Cancer Supportive Care and Survivorship; Olver, I., Ed.; Springer: Cham, Germany, 2018. [Google Scholar] [CrossRef]
- Block, K.; Block, P.B.; Gyllenhaal, C. Integrative Treatment for Colorectal Cancer: A Comprehensive Approach. J. Altern. Complement. Med. 2018, 24, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I. An Integrative Approach to Prostate Cancer. J. Altern. Complement. Med. 2018, 24, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Maindet, C.; Burnod, A.; Minello, C.; George, B.; Allano, G.; Lemaire, A. Strategies of complementary and integrative therapies in cancer-related pain—Attaining exhaustive cancer pain management. Support. Care Cancer 2019, 27, 3119–3132. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Vardy, J.L.; Oh, B.; Trejo, M.J. Integration of complementary and alternative medicine into cancer-specific supportive care programs in Australia: A scoping study. Asia Pac. J. Clin. Oncol. 2016, 13, 6–12. [Google Scholar] [CrossRef]
- Smith, C.A.; Hunter, J.; Delaney, G.; Ussher, J.M.; Templeman, K.; Grant, S.; Oyston, E. Integrative oncology and complementary medicine cancer services in Australia: Findings from a national cross-sectional survey. BMC Complement. Altern. Med. 2018, 18, 289. [Google Scholar] [CrossRef]
- Fremd, C.; Hack, C.C.; Schneeweiss, A.; Rauch, G.; Wallwiener, D.; Brucker, S.Y.; Taran, F.-A.; Hartkopf, A.; Overkamp, F.; Tesch, H.; et al. Use of complementary and integrative medicine among German breast cancer patients: Predictors and implications for patient care within the PRAEGNANT study network. Arch. Gynecol. Obstet. 2017, 295, 1239–1245. [Google Scholar] [CrossRef]
- Ben-Arye, E.; Lev, E.; Schiff, E. Complementary medicine oncology research in the Middle-East: Shifting from traditional to integrative cancer care. Eur. J. Integr. Med. 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Isherwood, J.; Arshad, A.; Chung, W.Y.; Runau, F.; Cooke, J.; Pollard, C.; Howells, L.; Fishwick, J.; Thompson, J.; Metcalfe, M.; et al. Myeloid derived suppressor cells are reduced and T regulatory cells stabilised in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega 3. Ann. Transl. Med. 2020, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.S.; Cullen, J.J. Treating pancreatic cancer: More antioxidants more problems? Expert Rev. Gastroenterol. Hepatol. 2018, 12, 849–851. [Google Scholar] [CrossRef]
- Springett, G.M.; Husain, K.; Neuger, A.; Centeno, B.; Chen, D.-T.; Hutchinson, T.Z.; Lush, R.M.; Sebti, S.; Malafa, M. A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E δ-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine 2015, 2, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2010, 68, 157–164. [Google Scholar] [CrossRef]
- Löhr, J.-M.; Karimi, M.; Omazic, B.; Kartalis, N.; Verbeke, C.S.; Berkenstam, A.; Frödin, J.-E.; Löhr, J.-M. A phase I dose escalation trial of AXP107-11, a novel multi-component crystalline form of genistein, in combination with gemcitabine in chemotherapy-naive patients with unresectable pancreatic cancer. Pancreatology 2016, 16, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, C.A.F.; Cardoso, A.C.F.; Parker, N.; Ngo-Huang, A.; Petzel, M.Q.; Kim, M.P.; Fogelman, D.; Romero, S.G.; Wang, H.; Park, M.; et al. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Maggino, L.; Vollmer, C.M. Recent Advances in Pancreatic Cancer Surgery. Curr. Treat. Options Gastroenterol. 2017, 15, 520–537. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Bundred, J.; Marc, O.S.; Jiao, L.R.; Manas, D.; Abu Hilal, M.; White, S.A. Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 6–14. [Google Scholar] [CrossRef]
- Foley, K.; Kim, V.; Jaffee, E.; Zheng, L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016, 381, 244–251. [Google Scholar] [CrossRef]
- Karakas, Y.; Lacin, S.; Yalcin, S. Recent advances in the management of pancreatic adenocarcinoma. Expert Rev. Anticancer Ther. 2017, 18, 51–62. [Google Scholar] [CrossRef]
- Goess, R.; Friess, H. A look at the progress of treating pancreatic cancer over the past 20 years. Expert Rev. Anticancer Ther. 2018, 18, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Motoi, F.; Unno, M. Neoadjuvant treatment for resectable pancreatic adenocarcinoma: What is the best protocol? Ann. Gastroenterol. Surg. 2020, 4, 100–108. [Google Scholar] [CrossRef]
- Singh, R.R.; O’Reilly, E.M. New Treatment Strategies for Metastatic Pancreatic Ductal Adenocarcinoma. Drugs 2020, 80, 647–669. [Google Scholar] [CrossRef] [PubMed]
- Janssen, Q.P.; Buettner, S.; Suker, M.; Beumer, B.R.; Addeo, P.; Bachellier, P.; Bahary, N.; Bekaii-Saab, T.; Bali, M.A.; Besselink, M.G.; et al. Neoadjuvant FOLFIRINOX in Patients with Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, A.; Kumar, A.; Rayala, R.; Hindi, R.M.; Adhikary, A.; Wnuk, S.F.; Sevilla, M.D. One-Electron Oxidation of Gemcitabine and Analogs: Mechanism of Formation of C3′ and C2′ Sugar Radicals. J. Am. Chem. Soc. 2014, 136, 15646–15653. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, S.; Li, H.; Duan, Q.; Zhang, Z.; Shen, Q.; Wang, C.; Yin, T. ROS/KRAS/AMPK Signaling Contributes to Gemcitabine-Induced Stem-like Cell Properties in Pancreatic Cancer. Mol. Ther. Oncolytics 2019, 14, 299–312. [Google Scholar] [CrossRef]
- Sarvepalli, D.; Rashid, M.U.; Rahman, A.U.; Ullah, W.; Hussain, I.; Hasan, B.; Jehanzeb, S.; Khan, A.K.; Jain, A.G.; Khetpal, N.; et al. Gemcitabine: A Review of Chemoresistance in Pancreatic Cancer. Crit. Rev. Oncog. 2019, 24, 199–212. [Google Scholar] [CrossRef]
- Manji, G.A.; Olive, K.P.; Saenger, Y.M.; Oberstein, P. Current and Emerging Therapies in Metastatic Pancreatic Cancer. Clin. Cancer Res. 2017, 23, 1670–1678. [Google Scholar] [CrossRef]
- Saluja, A.K.; Dudeja, V.; Banerjee, S. Evolution of novel therapeutic options for pancreatic cancer. Curr. Opin. Gastroenterol. 2016, 32, 401–407. [Google Scholar] [CrossRef]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. nab-Paclitaxel Plus Gemcitabine for Metastatic Pancreatic Cancer: Long-Term Survival from a Phase III Trial. J. Natl. Cancer Inst. 2015, 107, dju413. [Google Scholar] [CrossRef]
- Fenocchio, E.; Filippi, R.; Lombardi, P.; Quarà, V.; Milanesio, M.; Aimar, G.; Leone, F.; Aglietta, M. Is There a Standard Adjuvant Therapy for Resected Pancreatic Cancer? Cancers 2019, 11, 1547. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.M.; Hidalgo, M.; Alvarez, R.; Arrazubi, V.; Martínez-Galán, J.; Salgado, M.; Macarulla, T.; Carrato, A. From First Line to Sequential Treatment in the Management of Metastatic Pancreatic Cancer. J. Cancer 2018, 9, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Yegya-Raman, N.; Shah, M.M.; Grandhi, M.S.; Poplin, E.; August, D.A.; Kennedy, T.J.; Malhotra, U.; Spencer, K.R.; Carpizo, D.R.; Jabbour, S.K. Adjuvant therapeutic strategies for resectable pancreatic adenocarcinoma. Ann. Pancreat. Cancer 2018, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Bardeesy, N. Pancreatic Cancer Metabolism: Breaking It Down to Build It Back Up. Cancer Discov. 2015, 5, 1247–1261. [Google Scholar] [CrossRef]
- Vennin, C.; Chin, V.T.; Warren, S.C.; Lucas, M.C.; Herrmann, D.; Magenau, A.; Melenec, P.; Walters, S.N.; Del Monte-Nieto, G.; Conway, J.R.W.; et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci. Transl. Med. 2017, 9, eaai8504. [Google Scholar] [CrossRef]
- Mishra, K.P.; Meher, P.K. Radiation oxidative stress in cancer induction and prevention. J. Radiat. Cancer Res. 2017, 8, 44. [Google Scholar] [CrossRef]
- Palta, M.; Godfrey, D.; Goodman, K.A.; Hoffe, S.; Dawson, L.A.; Dessert, D.; Hall, W.A.; Herman, J.M.; Khorana, A.A.; Merchant, N.; et al. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2019, 9, 322–332. [Google Scholar] [CrossRef]
- Dong, W.; Cai, Z.; Pang, J.; Wang, J.; Tang, N.; Zhang, W.; Wang, F.; Xie, Z.; Lin, F.; Chang, X.; et al. Radiotherapy Enhancement for Human Pancreatic Carcinoma Using a Peptide-Gold Nanoparticle Hybrid. J. Biomed. Nanotechnol. 2020, 16, 352–363. [Google Scholar] [CrossRef]
- Jin, L.; Shi, N.; Ruan, S.; Hou, B.; Zou, Y.; Zou, X.; Jin, H.; Jian, Z. The role of intraoperative radiation therapy in resectable pancreatic cancer: A systematic review and meta-analysis. Radiat. Oncol. 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Coveler, A.L. Pancreatic cancer: Optimizing treatment options, new, and emerging targeted therapies. Drug Des. Dev. Ther. 2015, 9, 3529–3545. [Google Scholar] [CrossRef]
- Dreyer, S.B.; Chang, D.K.; Bailey, P.; Biankin, A.V. Pancreatic Cancer Genomes: Implications for Clinical Management and Therapeutic Development. Clin. Cancer Res. 2017, 23, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Chiramel, J.; Backen, A.; Pihlak, R.; Lamarca, A.; Frizziero, M.; Tariq, N.-U.-A.; Hubner, R.A.; Valle, J.W.; Amir, E.; McNamara, M.G. Targeting the Epidermal Growth Factor Receptor in Addition to Chemotherapy in Patients with Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2017, 18, 909. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Djamgoz, M.B.A. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat. Rev. 2018, 62, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Ireland, L.; Santos, A.; Ahmed, M.S.; Rainer, C.; Nielsen, S.R.; Quaranta, V.; Weyer-Czernilofsky, U.; Engle, D.D.; Perez-Mancera, P.A.; Coupland, S.E.; et al. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factors. Cancer Res. 2016, 76, 6851–6863. [Google Scholar] [CrossRef] [PubMed]
- Pishvaian, M.J.; Brody, J.R. Therapeutic Implications of Molecular Subtyping for Pancreatic Cancer. Oncology 2017, 31, 168. [Google Scholar]
- Ko, A.H.; LoConte, N.; Tempero, M.A.; Walker, E.J.; Kelley, R.K.; Lewis, S.; Chang, W.-C.; Kantoff, E.; Vannier, M.W.; Catenacci, D.V.; et al. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas 2016, 45, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 2848–2854. [Google Scholar] [CrossRef]
- Marshall, H.T.; Djamgoz, M.B.A. Immuno-Oncology: Emerging Targets and Combination Therapies. Front. Oncol. 2018, 8, 315. [Google Scholar] [CrossRef]
- Sunami, Y.; Kleeff, J. Immunotherapy of pancreatic cancer. Prog. Mol. Biol. Transl. Sci. 2019, 164, 189–216. [Google Scholar] [CrossRef]
- Kotteas, E.A.; Saif, M.W.; Syrigos, K. Immunotherapy for pancreatic cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1795–1805. [Google Scholar] [CrossRef]
- Dalgleish, A.G.; Stebbing, J.; Adamson, D.J.; Arif, S.S.; Bidoli, P.; Chang, D.; Cheeseman, S.; Diaz-Beveridge, R.; Fernandez-Martos, C.; Glynne-Jones, R.; et al. Randomised, open-label, phase II study of gemcitabine with and without IMM-101 for advanced pancreatic cancer. Br. J. Cancer 2016, 115, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- McCormick, K.A.; Coveler, A.L.; Rossi, G.R.; Vahanian, N.N.; Link, C.; Chiorean, E.G. Pancreatic cancer: Update on immunotherapies and algenpantucel-L. Hum. Vaccines Immunother. 2015, 12, 563–575. [Google Scholar] [CrossRef]
- Johnson, B.A.; Yarchoan, M.; Lee, V.; Laheru, D.A.; Jaffee, E.M. Strategies for Increasing Pancreatic Tumor Immunogenicity. Clin. Cancer Res. 2017, 23, 1656–1669. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.E.; Berry, A.A.; Strutt, J.P.; Gerrard, D.T.; Hanley, N.A. Human pancreas development. Development 2015, 142, 3126–3137. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-Y.; Shu, L.; Shen, S.-S.; Chen, X.; Zhang, X.-Y. Dietary Patterns and Pancreatic Cancer Risk: A Meta-Analysis. Nutrients 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Weisbeck, A.; Jansen, R.J. Nutrients and the Pancreas: An Epigenetic Perspective. Nutrients 2017, 9, 283. [Google Scholar] [CrossRef]
- Lohse, I.; Wildermuth, E.; Brothers, S.P. Naturally occurring compounds as pancreatic cancer therapeutics. Oncotarget 2018, 9, 35448–35457. [Google Scholar] [CrossRef][Green Version]
- Azimi, H.; Khakshur, A.A.; Abdollahi, M.; Rahimi, R. Potential New Pharmacological Agents Derived from Medicinal Plants for the Treatment of Pancreatic Cancer. Pancreas 2015, 44, 11–15. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, W.; Shao, L.; Zhong, D.; Wu, Y.; Cai, J. Association between intake of antioxidants and pancreatic cancer risk: A meta-analysis. Int. J. Food Sci. Nutr. 2016, 67, 744–753. [Google Scholar] [CrossRef]
- Gordon-Dseagu, V.L.Z.; Thompson, F.E.; Subar, A.F.; Ruder, E.H.; Thiébaut, A.C.M.; Potischman, N.; Stolzenberg-Solomon, R. A Cohort Study of Adolescent and Midlife Diet and Pancreatic Cancer Risk in the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2017, 186, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.M.; Jeung, H.C.; Lee, I.J.; Park, J.S.; Song, M.; Lee, D.K.; Lee, S.-M. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 2019, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.A.; MacKenzie, G.G. Pancreatic cancer: A critical review of dietary risk. Nutr. Res. 2018, 52, 1–13. [Google Scholar] [CrossRef]
- Goldstein, I.; Rivlin, N.; Shoshana, O.-Y.; Ezra, O.; Madar, S.; Goldfinger, N.; Rotter, V. Chemotherapeutic agents induce the expression and activity of their clearing enzyme CYP3A4 by activating p53. Carcinogenesis 2012, 34, 190–198. [Google Scholar] [CrossRef]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Serpe, L.; Canaparo, R.; Dell’Osso, B.; Galentino, R.; De Michele, S.; Dina, C.Z.; Porta, M.; et al. Food Bioactive Compounds and Their Interference in Drug Pharmacokinetic/Pharmacodynamic Profiles. Pharmaceutics 2018, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Knott, S.R.V.; Wagenblast, E.; Khan, S.; Kim, S.Y.; Soto, M.; Wagner, M.; Turgeon, M.-O.; Fish, L.; Erard, N.; Gable, A.L.; et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nat. Cell Biol. 2018, 554, 378–381. [Google Scholar] [CrossRef]
- Ali, M.; Alam, S.P.; Kumar, S.; Anupam Kumar, R.; Kumar, A. Does blood pH change in cancer patients. Int. J. Curr. Res. 2016, 8, 29543–29544. [Google Scholar]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Lee, S.-H.; McIntyre, D.; Honess, D.; Hulikova, A.; Pacheco-Torres, J.; Cerdán, S.; Swietach, P.; Harris, A.L.; Griffiths, J.R. Carbonic anhydrase IX is a pH-stat that sets an acidic tumour extracellular pH in vivo. Br. J. Cancer 2018, 119, 622–630. [Google Scholar] [CrossRef]
- Pilon-Thomas, S.; Kodumudi, K.N.; El-Kenawi, A.E.; Russell, S.; Weber, A.M.; Luddy, K.; Damaghi, M.; Wojtkowiak, J.W.; Mulé, J.J.; Ibrahim-Hashim, A.; et al. Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy. Cancer Res. 2015, 76, 1381–1390. [Google Scholar] [CrossRef]
- Kong, S.C.; Gianuzzo, A.; Enovak, I.; Pedersen, S.F. Acid-base transport in pancreatic cancer: Molecular mechanisms and clinical potential. Biochem. Cell Biol. 2014, 92, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Greco, M.R.; Zeeberg, K.; Zaccagnino, A.; Saccomano, M.; Bellizzi, A.; Bruns, P.; Menga, M.; Pilarsky, C.; Schwab, A.; et al. A Novel NHE1-Centered Signaling Cassette Drives Epidermal Growth Factor Receptor–Dependent Pancreatic Tumor Metastasis and Is a Target for Combination Therapy. Neoplasia 2015, 17, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Dolenšek, J.; Pohorec, V.; Rupnik, M.S.; Stožer, A. Pancreas Physiology. In Challenges in Pancreatic Pathology; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The Role of Carbonic Anhydrase 9 in Regulating Extracellular and Intracellular pH in Three-dimensional Tumor Cell Growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef]
- Nicholson, C. Dynamics of the brain cell microenvironment. Neurosci. Res. Progr. Bull. 1980, 18, 175–322. [Google Scholar]
- Welch, A.A.; Mulligan, A.; Bingham, S.A.; Khaw, K.-T. Urine pH is an indicator of dietary acid–base load, fruit and vegetables and meat intakes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br. J. Nutr. 2008, 99, 1335–1343. [Google Scholar] [CrossRef]
- Park, Y.-M.; Steck, S.E.; Fung, T.T.; Merchant, A.T.; Hodgson, M.E.; Keller, J.A.; Sandler, D.P. Higher diet-dependent acid load is associated with risk of breast cancer: Findings from the sister study. Int. J. Cancer 2018, 144, 1834–1843. [Google Scholar] [CrossRef]
- McDonald, P.C.; Chafe, S.C.; Brown, W.S.; Saberi, S.; Swayampakula, M.; Venkateswaran, G.; Nemirovsky, O.; Gillespie, J.A.; Karasinska, J.M.; Kalloger, S.E.; et al. Regulation of pH by Carbonic Anhydrase 9 Mediates Survival of Pancreatic Cancer Cells with Activated KRAS in Response to Hypoxia. Gastroenterology 2019, 157, 823–837. [Google Scholar] [CrossRef]
- Hamaguchi, R.; Narui, R.; Wada, H. Effects of Alkalization Therapy on Chemotherapy Outcomes in Metastatic or Recurrent Pancreatic Cancer. Anticancer Res. 2020, 40, 873–880. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. The Alkaline Diet: Is There Evidence That an Alkaline pH Diet Benefits Health? J. Environ. Public Health 2011, 2012, 1–7. [Google Scholar] [CrossRef]
- Welch, A.A.; MacGregor, A.J.; Skinner, J.; Spector, T.D.; Moayyeri, A.; Cassidy, A. A higher alkaline dietary load is associated with greater indexes of skeletal muscle mass in women. Osteoporos. Int. 2012, 24, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kankala, R.K.; Chen, B.; Long, R.; Cai, D.; Liu, Y.; Wang, S. Poly-allylamine hydrochloride and fucoidan-based self-assembled polyelectrolyte complex nanoparticles for cancer therapeutics. J. Biomed. Mater. Res. Part A 2018, 107, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Hashim, A.; Estrella, V. Acidosis and cancer: From mechanism to neutralization. Cancer Metastasis Rev. 2019, 38, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Cardone, R.A.; Reshkin, S.J. Synergy between Low Dose Metronomic Chemotherapy and the pH-centered Approach against Cancer. Int. J. Mol. Sci. 2019, 20, 5438. [Google Scholar] [CrossRef]
- Bolsinger, J.; Landstrom, M.; Pronczuk, A.; Auerbach, A.; Hayes, K. Low glycemic load diets protect against metabolic syndrome and Type 2 diabetes mellitus in the male Nile rat. J. Nutr. Biochem. 2017, 42, 134–148. [Google Scholar] [CrossRef]
- Thompson, H.J.; Neuhouser, M.L.; Lampe, J.W.; McGinley, J.N.; Neil, E.S.; Schwartz, Y.; McTiernan, A. Effect of low or high glycemic load diets on experimentally induced mammary carcinogenesis in rats. Mol. Nutr. Food Res. 2016, 60, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Sieri, S.; Agnoli, C.; Pala, V.; Grioni, S.; Brighenti, F.; Pellegrini, N.; Masala, G.; Palli, D.; Mattiello, A.; Panico, S.; et al. Dietary glycemic index, glycemic load, and cancer risk: Results from the EPIC-Italy study. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Sieri, S.; Krogh, V. Dietary glycemic index, glycemic load and cancer: An overview of the literature. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 18–31. [Google Scholar] [CrossRef]
- Choi, Y.; Giovannucci, E.; Lee, J.E. Glycaemic index and glycaemic load in relation to risk of diabetes-related cancers: A meta-analysis. Br. J. Nutr. 2012, 108, 1934–1947. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.M.; Vieira, A.R.; Rosenblatt, D.A.N.; Vieira, R.; Greenwood, D.C.; Cade, J.; Burley, V.; Norat, T. Dietary fructose, carbohydrates, glycemic indices and pancreatic cancer risk: A systematic review and meta-analysis of cohort studies. Ann. Oncol. 2012, 23, 2536–2546. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.-L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Odegaard, A.; Anderson, K.; Yuan, J.-M.; Gross, M.; Koh, W.-P.; Pereira, M.A. Soft drink and juice consumption and risk of pancreatic cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Åbacka, H.; Hansen, J.S.; Huang, P.; Venskutonytė, R.; Hyrenius-Wittsten, A.; Poli, G.; Tuccinardi, T.; Granchi, C.; Minutolo, F.; Hagström-Andersson, A.K.; et al. Targeting GLUT1 in acute myeloid leukemia to overcome cytarabine resistance. Haematologica 2020, 32554563. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; La Vecchia, C.; de Groh, M.; Negri, E.; Morrison, H.; Mery, L. Canadian Cancer Registries Epidemiology Research Group. Dietary cholesterol intake and cancer. Ann. Oncol. 2012, 23, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.-J.; Zhai, L.; Zhang, D. Association of cholesterol with risk of pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Haddy, N. IL-6, TNF-α and atherosclerosis risk indicators in a healthy family population: The STANISLAS cohort. Atherosclerosis 2003, 170, 277–283. [Google Scholar] [CrossRef]
- Baghurst, P.A.; McMichael, A.J.; Slavotinek, A.H.; Baghurst, K.I.; Boyle, P.; Walker, A.M. A Case-Control Study of Diet and Cancer of the Pancreas. Am. J. Epidemiol. 1991, 134, 167–179. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A. Blood pressure and risk of cancer progression—A possible connection with salt and voltage-gated sodium channel. Med. Hypotheses 2015, 85, 591–593. [Google Scholar] [CrossRef]
- Chen, W.C.-Y.; Boursi, B.; Mamtani, R.; Yang, Y.-X. Total Serum Cholesterol and Pancreatic Cancer: A Nested Case–Control Study. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 363–369. [Google Scholar] [CrossRef]
- Oni, T.E.; Biffi, G.; Baker, L.A.; Hao, Y.; Tonelli, C.; Somerville, T.D.; Deschênes, A.; Belleau, P.; Hwang, C.-I.; Sánchez-Rivera, F.J.; et al. SOAT1 promotes mevalonate pathway dependency in pancreatic cancer. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Matusewicz, L.; Czogalla, A.; Sikorski, A.F. Attempts to use statins in cancer therapy: An update. Tumour Biol. 2020, 42, 1010428320941760. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, D.; Crippa, S.; Partelli, S.; Archibugi, L.; Arcidiacono, P.G.; Falconi, M.; Capurso, G. Statin use improves survival in patients with pancreatic ductal adenocarcinoma: A meta-analysis. Dig. Liver Dis. 2020, 52, 392–399. [Google Scholar] [CrossRef]
- Walker, E.J.; Ko, A.H.; Holly, E.A.; Bracci, P.M. Statin use and risk of pancreatic cancer: Results from a large, clinic-based case-control study. Cancer 2015, 121, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, M.; Sun, C.; Qu, G.; Shi, T.; Min, M.; Wu, Y.; Sun, Y. Statin Use and Risk of Pancreatic Cancer. Pancreas 2019, 48, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Tian, J.; Zhang, J.-T.; Cheng, J.-X. Cholesterol esterification inhibition and gemcitabine synergistically suppress pancreatic ductal adenocarcinoma proliferation. PLoS ONE 2018, 13, e0193318. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Satija, A.; Pan, A.; Sotos-Prieto, M.; Rimm, E.; Willett, W.C.; Hu, F.B. Association of changes in red meat consumption with total and cause specific mortality among US women and men: Two prospective cohort studies. BMJ 2019, 365, l2110. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Red and processed meat consumption and risk of pancreatic cancer: Meta-analysis of prospective studies. Br. J. Cancer 2012, 106, 603–607. [Google Scholar] [CrossRef]
- Bamia, C. Dietary patterns in association to cancer incidence and survival: Concept, current evidence, and suggestions for future research. Eur. J. Clin. Nutr. 2018, 72, 818–825. [Google Scholar] [CrossRef]
- Nöthlings, U.; Wilkens, L.R.; Murphy, S.P.; Hankin, J.H.; Henderson, B.E.; Kolonel, L.N. Meat and Fat Intake as Risk Factors for Pancreatic Cancer: The Multiethnic Cohort Study. J. Natl. Cancer Inst. 2005, 97, 1458–1465. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Zhao, Z.; Yin, Z.; Pu, Z.; Zhao, Q. Association between Consumption of Red and Processed Meat and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 486–493.e10. [Google Scholar] [CrossRef] [PubMed]
- Alomirah, H.F.; Al-Zenki, S.; Al Hooti, S.; Zaghloul, S.; Sawaya, W.; Ahmed, N.; Kannan, K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]
- Alaejos, M.S.; González, V.; Afonso, A.M. Exposure to heterocyclic aromatic amines from the consumption of cooked red meat and its effect on human cancer risk: A review. Food Addit. Contam. Part A 2007, 25, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Satija, A.; Blondin, S.A.; Janiszewski, M.; Emlen, E.; O’Connor, L.E.; Campbell, W.W.; Hu, F.B.; Willett, W.C.; Stampfer, M.J. Meta-Analysis of Randomized Controlled Trials of Red Meat Consumption in Comparison With Various Comparison Diets on Cardiovascular Risk Factors. Circulation 2019, 139, 1828–1845. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, W.; Li, T.; Liu, Y.; Simon, T.G.; Sui, J.; Wu, K.; Giovannucci, E.L.; Chan, A.T.; Zhang, X. Meat intake and risk of hepatocellular carcinoma in two large US prospective cohorts of women and men. Int. J. Epidemiol. 2019, 48, 1863–1871. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Kasi, P.D.; Loizzo, M.R.; Tundis, R. Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xun, P.; Brasky, T.M.; Gammon, M.D.; Stevens, J.; White, E. Types of Fish Consumed and Fish Preparation Methods in Relation to Pancreatic Cancer Incidence. Am. J. Epidemiol. 2012, 177, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-J.; Yu, J.; Xiao, J.; Cao, B.-W. The Consumption of Omega-3 Polyunsaturated Fatty Acids Improves Clinical Outcomes and Prognosis in Pancreatic Cancer Patients: A Systematic Evaluation. Nutr. Cancer 2014, 67, 112–118. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.G.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.A.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef]
- Werner, K.; De Gaudry, D.K.; Taylor, L.A.; Keck, T.; Unger, C.; Hopt, U.T.; Massing, U. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: Marine phospholipids versus fish oil—A randomized controlled double-blind trial. Lipids Health Dis. 2017, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Kang, K.S.; Okada, K.; Zhu, B.T. EPA, an omega-3 fatty acid, induces apoptosis in human pancreatic cancer cells: Role of ROS accumulation, caspase-8 activation, and autophagy induction. J. Cell. Biochem. 2012, 114, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Mullapudi, B.; Torres, C.; Mascariñas, E.; Mancinelli, G.; Diaz, A.M.; McKinney, R.; Barron, M.; Schultz, M.; Heiferman, M.; et al. Omega-3 Fatty Acids Prevent Early Pancreatic Carcinogenesis via Repression of the AKT Pathway. Nutrients 2018, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-S.; Jing, K.; Kim, J.-S.; Yun, E.-J.; Shin, S.; Seo, K.-S.; Park, J.-H.; Heo, J.Y.; Kang, J.X.; Suh, K.-S.; et al. Omega-3-Polyunsaturated Fatty Acids Suppress Pancreatic Cancer Cell Growth in vitro and in vivo via Downregulation of Wnt/β-Catenin Signaling. Pancreatology 2011, 11, 574–584. [Google Scholar] [CrossRef]
- Haqq, J.; Howells, L.M.; Garcea, G.; Dennison, A.R. Targeting pancreatic cancer using a combination of gemcitabine with the omega-3 polyunsaturated fatty acid emulsion, Lipidem™. Mol. Nutr. Food Res. 2015, 60, 1437–1447. [Google Scholar] [CrossRef]
- Arshad, A.; Isherwood, J.; Mann, C.; Cooke, J.; Pollard, C.; Runau, F.; Morgan, B.; Steward, W.; Metcalfe, M.; Dennison, A.R. Intravenous ω-3 Fatty Acids Plus Gemcitabine: Potential to Improve Response and Quality of Life in Advanced Pancreatic Cancer. J. Parenter. Enter. Nutr. 2016, 41, 398–403. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2019, 177, 1241–1257. [Google Scholar] [CrossRef]
- Schulpen, M.; Peeters, P.H.; Brandt, P.A.V.D. Mediterranean diet adherence and risk of pancreatic cancer: A pooled analysis of two Dutch cohorts. Int. J. Cancer 2018, 144, 1550–1560. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Sánchez, M.-J.; Buckland, G.; Bueno-De-Mesquita, H.B.; Weiderpass, E.; Amiano, P.; Wark, P.A.; Kühn, T.; Katzke, V.; Huerta, J.M.; et al. Mediterranean diet and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Br. J. Cancer 2017, 116, 811–820. [Google Scholar] [CrossRef]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.J.; Robinson, D.P.; Frank, R.D.; Anderson, K.E.; Bamlet, W.R.; Oberg, A.L.; Rabe, K.G.; Olson, J.E.; Sinha, R.; Petersen, G.M.; et al. Fatty acids found in dairy, protein and unsaturated fatty acids are associated with risk of pancreatic cancer in a case-control study. Int. J. Cancer 2013, 134, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Genkinger, J.M.; Wang, M.; Li, R.; Albanes, D.; Anderson, K.E.; Bernstein, L.; Brandt, P.A.V.D.; English, D.R.; Freudenheim, J.L.; Fuchs, C.S.; et al. Dairy products and pancreatic cancer risk: A pooled analysis of 14 cohort studies. Ann. Oncol. 2014, 25, 1106–1115. [Google Scholar] [CrossRef]
- Farvid, M.S.; Malekshah, A.F.; Pourshams, A.; Poustchi, H.; Sepanlou, S.G.; Sharafkhah, M.; Khoshnia, M.; Farvid, M.; Abnet, C.C.; Kamangar, F.; et al. Dairy Food Intake and All-Cause, Cardiovascular Disease, and Cancer Mortality. Am. J. Epidemiol. 2017, 185, 697–711. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Abou-Setta, A.M.; Grant, L.; Farshidfar, F.; Copstein, L.; Lys, J.; Gottschalk, T.; Desautels, D.; Czaykowski, P.; Pitz, M.; et al. Dairy product consumption and development of cancer: An overview of reviews. BMJ Open 2019, 9, e023625. [Google Scholar] [CrossRef]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef]
- Chan, J.M.; Wang, F.; Holly, E.A. Pancreatic cancer, animal protein and dietary fat in a population-based study, San Francisco Bay Area, California. Cancer Causes Control 2007, 18, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Thiébaut, A.C.M.; Jiao, L.; Silverman, D.T.; Cross, A.J.; Thompson, F.E.; Subar, A.F.; Hollenbeck, A.R.; Schatzkin, A.; Stolzenberg-Solomon, R.Z. Dietary Fatty Acids and Pancreatic Cancer in the NIH-AARP Diet and Health Study. J. Natl. Cancer Inst. 2009, 101, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Braun, H.; Brown, N.; Um, C.; Ehret, K.; Figueroa, J.; Barr, D.B. Production-related contaminants (pesticides, antibiotics and hormones) in organic and conventionally produced milk samples sold in the USA. Public Health Nutr. 2019, 22, 2972–2980. [Google Scholar] [CrossRef]
- Qin, L.; He, K.; Xu, J.-Y. Milk consumption and circulating insulin-like growth factor-I level: A systematic literature review. Int. J. Food Sci. Nutr. 2009, 60, 330–340. [Google Scholar] [CrossRef]
- Meyer, Z.; Höflich, C.; Wirthgen, E.; Olm, S.; Hammon, H.M.; Hoeflich, A. Analysis of the IGF-system in milk from farm animals—Occurrence, regulation, and biomarker potential. Growth Hormone IGF Res. 2017, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Knuppel, A.; Fensom, G.K.; Watts, E.L.; Gunter, M.J.; Murphy, N.; Papier, K.; Perez-Cornago, A.; Schmidt, J.A.; Byrne, K.S.; Travis, R.C.; et al. Circulating insulin-like growth factor-I (IGF-I) concentrations and incidence of 30 cancers: Prospective analyses in UK Biobank. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, B.; Gasiorowska, A.; Malecka-Panas, E. The Role of Insulin-like Growth Factor (IGF) Axis in Early Diagnosis of Pancreatic Adenocarcinoma (PDAC). J. Clin. Gastroenterol. 2018, 52, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Di Somma, C.; Macchia, P.E.; Falco, A.; Savanelli, M.C.; Orio, F.; Colao, A.; Savastano, S. Influence of nutrition on somatotropic axis: Milk consumption in adult individuals with moderate-severe obesity. Clin. Nutr. 2017, 36, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Porta, M.; Vioque, J.; López-Jiménez, T.; Mendez, M.A.; Pumarega, J.; Malats, N.; Crous-Bou, M.; Ngo, J.; Rifà, J.; et al. Food and nutrient intakes and K-ras mutations in exocrine pancreatic cancer. J. Epidemiol. Community Health 2007, 61, 641–649. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef]
- Waheed, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and cancer: A mechanistic review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef]
- Chow, P.; Kourghi, M.; Pei, J.V.; Nourmohammadi, S.; Yool, A.J. 5-Hydroxymethyl-Furfural and Structurally Related Compounds Block the Ion Conductance in Human Aquaporin-1 Channels and Slow Cancer Cell Migration and Invasion. Mol. Pharmacol. 2020, 98, 38–48. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Ansary, J.; Gil-Martín, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020, 325, 126881. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of Honey and Its Mechanisms of Action on the Development and Progression of Cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef]
- Badolato, M.; Carullo, G.; Cione, E.; Aiello, F.; Caroleo, M.C. From the hive: Honey, a novel weapon against cancer. Eur. J. Med. Chem. 2017, 142, 290–299. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Forbes-Hernández, T.Y.; Gasparrini, M.; Amici, A.; Cianciosi, D.; Quiles, J.L.; Battino, M. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free Radic. Biol. Med. 2018, 126, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Quiles, J.L.; Gil-Martín, E.; Bompadre, S.; Simal-Gandara, J.; Battino, M.; Giampieri, F. The Influence of In Vitro Gastrointestinal Digestion on the Anticancer Activity of Manuka Honey. Antioxidants 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, B.; Yen, S.; Trichopoulos, D.; Warren, K.; Nardi, G. Coffee and Cancer of the Pancreas. N. Engl. J. Med. 1981, 304, 630–633. [Google Scholar] [CrossRef]
- Guertin, M.J.; Freedman, N.D.; Loftfield, E.; Stolzenberg-Solomon, R.Z.; Graubard, B.I.; Sinha, R. A prospective study of coffee intake and pancreatic cancer: Results from the NIH-AARP Diet and Health Study. Br. J. Cancer 2015, 113, 1081–1085. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, C.D.; Kuan, A.S.; Reeves, G.K.; Green, J.; Floud, S.; Beral, V.; Yang, T.N.O.; Million Women Study Collaborators. Coffee and pancreatic cancer risk among never-smokers in the UK prospective Million Women Study. Int. J. Cancer 2018, 145, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zou, J.; Yu, X.-F. Coffee drinking and pancreatic cancer risk: A meta-analysis of cohort studies. World J. Gastroenterol. 2011, 17, 1204–1210. [Google Scholar] [CrossRef]
- Ran, H.-Q.; Wang, J.-Z.; Sun, C.-Q. Coffee Consumption and Pancreatic Cancer Risk: An Update Meta-analysis of Cohort Studies. Pak. J. Med. Sci. 2016, 32, 253–259. [Google Scholar] [CrossRef]
- Lv, L.; Cai, Q.; Jiang, Y.; Bai, K. Coffee consumption and risk of hepatocellular carcinoma: A meta-analysis of eleven epidemiological studies. OncoTargets Ther. 2016, 9, 4369–4375. [Google Scholar] [CrossRef]
- Kennedy, O.J.; Roderick, P.; Buchanan, R.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: A systematic review and dose–response meta-analysis. BMJ Open 2017, 7, e013739. [Google Scholar] [CrossRef]
- Bravi, F.; Tavani, A.; Bosetti, C.; Boffetta, P.; La Vecchia, C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: A systematic review and meta-analysis of prospective studies. Eur. J. Cancer Prev. 2017, 26, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.; Giese, N.; Hackert, T.; Strobel, O.; Schirmacher, P.; Felix, K.; Gaida, M.M. Overcoming chemoresistance in pancreatic cancer cells: Role of the bitter taste receptor T2R10. J. Cancer 2018, 9, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Loh, W.M.; Gopinath, S.C.; Bonam, S.R.; Fareez, I.M.; Mac Guad, R.; Sim, M.S.; Wu, Y.S. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm. Sin. B 2020, 10, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Davis-Yadley, A.H.; Malafa, M. Vitamins in Pancreatic Cancer: A Review of Underlying Mechanisms and Future Applications. Adv. Nutr. 2015, 6, 774–802. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Zhi, X.; Ta, N.; Jiang, H.; Zheng, J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 2016, 6, 38936. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Robinson, B.; Sarper, M.; Cortes, E.; Auernheimer, V.; Lachowski, D.; Attwood, S.; García, R.; Ghassemi, S.; Fabry, B.; et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat. Commun. 2016, 7, 12630. [Google Scholar] [CrossRef]
- Pettersson, F.; Colston, K.W.; Dalgleish, A.G. Retinoic Acid Enhances the Cytotoxic Effects of Gemcitabine and Cisplatin in Pancreatic Adenocarcinoma Cells. Pancreas 2001, 23, 273–279. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Er, T.K.; Bujanda, L. Retinoic Acid Reduces Stem Cell–Like Features in Pancreatic Cancer Cells. Pancreas 2015, 44, 918–924. [Google Scholar] [CrossRef]
- Wang, K.; Baldwin, G.S.; Nikfarjam, M.; He, H. Antitumor effects of all-trans retinoic acid and its synergism with gemcitabine are associated with downregulation of p21-activated kinases in pancreatic cancer. Am. J. Physiol. Liver Physiol. 2019, 316, G632–G640. [Google Scholar] [CrossRef]
- Kuroda, H.; Tachikawa, M.; Uchida, Y.; Inoue, K.; Ohtsuka, H.; Ohtsuki, S.; Unno, M.; Terasaki, T. All-trans retinoic acid enhances gemcitabine cytotoxicity in human pancreatic cancer cell line AsPC-1 by up-regulating protein expression of deoxycytidine kinase. Eur. J. Pharm. Sci. 2017, 103, 116–121. [Google Scholar] [CrossRef]
- Carapuça, E.F.; Gemenetzidis, E.; Feig, C.; Bapiro, T.E.; Williams, M.D.; Wilson, A.S.; DelVecchio, F.R.; Arumugam, P.; Grose, R.P.; Lemoine, N.R.; et al. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J. Pathol. 2016, 239, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Roa, F.J.; Peña, E.; Gatica, M.; Escobar-Acuña, K.; Saavedra, P.; Maldonado, M.; Cuevas, M.E.; Moraga-Cid, G.; Rivas, C.I.; Muñoz-Montesino, C. Therapeutic Use of Vitamin C in Cancer: Physiological Considerations. Front. Pharmacol. 2020, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, J.A.; Cullen, J.J. Treatment of Pancreatic Cancer with Pharmacological Ascorbate. Curr. Pharm. Biotechnol. 2015, 16, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Bradley, M.D.; Wagner, B.A.; Buettner, G.R.; Monga, V.V.; Milhem, M.; Spitz, D.R.; Allen, B.G. Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol. 2018, 14, 417–422. [Google Scholar] [CrossRef]
- Gómez-Tomás, Á.; Pumarega, J.; Alguacil, J.; Amaral, A.F.S.; Malats, N.; Pallarès, N.; Gasull, M.; Porta, M.; PANKRAS II Study Group. Concentrations of trace elements and KRAS mutations in pancreatic ductal adenocarcinoma. Environ. Mol. Mutagen. 2019, 60, 693–703. [Google Scholar] [CrossRef]
- Lener, M.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res. Treat. 2016, 48, 1056–1064. [Google Scholar] [CrossRef]
- Fan, H.; Kou, J.; Han, N.; Li, P.; Zhang, N.; Wu, Q.; He, Q. Association between vitamin C intake and the risk of pancreatic cancer: A meta-analysis of observational studies. Sci. Rep. 2015, 5, 13973. [Google Scholar] [CrossRef]
- Hua, Y.-F.; Wang, G.-Q.; Jiang, W.; Huang, J.; Chen, G.-C.; Lu, C.-D. Vitamin C Intake and Pancreatic Cancer Risk: A Meta-Analysis of Published Case-Control and Cohort Studies. PLoS ONE 2016, 11, e0148816. [Google Scholar] [CrossRef]
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.-H.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of Ascorbate-Induced Cytotoxicity in Pancreatic Cancer. Clin. Cancer Res. 2010, 16, 509–520. [Google Scholar] [CrossRef]
- Espey, M.G.; Chen, P.; Chalmers, B.; Drisko, J.; Sun, A.Y.; Levine, M.; Chen, Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic. Biol. Med. 2011, 50, 1610–1619. [Google Scholar] [CrossRef]
- Cullen, J.J.; Spitz, D.R.; Buettner, G.R. Comment on “Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer”, i.e., all we are saying is, give C a chance. Free Radic. Biol. Med. 2011, 50, 1726–1727. [Google Scholar] [CrossRef]
- Bigelsen, S. Evidence-based complementary treatment of pancreatic cancer: A review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin C, statins, metformin, curcumin, and aspirin. Cancer Manag. Res. 2018, 10, 2003–2018. [Google Scholar] [CrossRef]
- Ou, J.; Zhu, X.; Zhang, H.; Du, Y.; Chen, P.; Wang, J.; Peng, X.; Bao, S.; Zhang, X.; Zhang, T.; et al. A Retrospective Study of Gemcitabine and Carboplatin with or Without Intravenous Vitamin C on Patients with Advanced Triple-Negative Breast Cancer. Integr. Cancer Ther. 2020, 19. [Google Scholar] [CrossRef]
- Polireddy, K.; Dong, R.; Reed, G.; Yu, J.; Chen, P.; Williamson, S.; Violet, P.-C.; Pessetto, Z.; Godwin, A.K.; Fan, F.; et al. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Sci. Rep. 2017, 7, 17188. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, K.; Owzar, K.; Jiang, C.; Kindler, H.L.; Mulcahy, M.F.; Niedzwiecki, N.; O’Reilly, E.M.; Fuchs, C.; Innocenti, F.; Venook, A.P. 25-Hydroxyvitamin D Levels and Survival in Advanced Pancreatic Cancer: Findings From CALGB 80303 (Alliance). J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Layne, T.M.; Albanes, D. Vitamin D and Cancer Risk and Mortality: State of the Science, Gaps, and Challenges. Epidemiol. Rev. 2017, 39, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Ng, K.; Bao, Y.; Kraft, P.; Stampfer, M.J.; Michaud, D.S.; Ma, J.; Buring, J.E.; Sesso, H.D.; Lee, I.-M.; et al. Plasma 25-Hydroxyvitamin D and Risk of Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2011, 21, 82–91. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.-Z.; Chen, W.-J.; Wu, J.; Chen, Y.; Wu, C.-C.; Wang, Z.-N. Plasma 25-hydroxyvitamin D levels, vitamin D intake, and pancreatic cancer risk or mortality: A meta-analysis. Oncotarget 2017, 8, 64395–64406. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Sun, X.; Lu, S.; Liu, S. Vitamin intake and pancreatic cancer risk reduction. Medicine 2018, 97, e0114. [Google Scholar] [CrossRef]
- Ma, Y. Vitamin D in combination cancer treatment. J. Cancer 2010, 1, 101. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-D.; Ma, Y.; Flynn, G.; Muindi, J.R.; Kong, R.-X.; Trump, D.L.; Johnson, C.S. Calcitriol enhances gemcitabine antitumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle 2010, 9, 3094–3101. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, V.; Zhou, Y.; Yen, T.J. A synthetic lethal screen identifies the Vitamin D receptor as a novel gemcitabine sensitizer in pancreatic cancer cells. Cell Cycle 2014, 13, 3839–3856. [Google Scholar] [CrossRef] [PubMed]
- Gilzad-Kohan, H.; Sani, S.; Boroujerdi, M. Calcitriol Reverses Induced Expression of Efflux Proteins and Potentiates Cytotoxic Activity of Gemcitabine in Capan-2 Pancreatic Cancer Cells. J. Pharm. Pharm. Sci. 2017, 20, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef]
- Innocenti, F.; Owzar, K.; Jiang, C.; Etheridge, A.S.; Gordân, R.; Sibley, A.B.; Mulkey, F.; Niedzwiecki, N.; Glubb, D.; Neel, N.; et al. The vitamin D receptor gene as a determinant of survival in pancreatic cancer patients: Genomic analysis and experimental validation. PLoS ONE 2018, 13, e0202272. [Google Scholar] [CrossRef]
- Goyal, H.; Perisetti, A.; Rahman, M.R.; Levin, A.; Lippi, G. Vitamin D and Gastrointestinal Cancers: A Narrative Review. Dig. Dis. Sci. 2018, 64, 1098–1109. [Google Scholar] [CrossRef]
- Anbil, S.; Pigula, M.; Huang, H.-C.; Mallidi, S.; Broekgaarden, M.; Baglo, Y.; De Silva, P.; Simeone, D.M.; Mino-Kenudson, M.; Maytin, E.V.; et al. Vitamin D Receptor Activation and Photodynamic Priming Enables Durable Low-dose Chemotherapy. Mol. Cancer Ther. 2020, 19, 1308–1319. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Sliwinska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef]
- Kubesch, A.; Quenstedt, L.; Saleh, M.; Rüschenbaum, S.; Schwarzkopf, K.M.; Martinez, Y.; Welsch, C.; Zeuzem, S.; Welzel, T.M.; Lange, C.M. Vitamin D deficiency is associated with hepatic decompensation and inflammation in patients with liver cirrhosis: A prospective cohort study. PLoS ONE 2018, 13, e0207162. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.; Greenwood, D.; Manson, J.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Barone, E.; Corrado, A.; Gemignani, F.; Landi, S. Environmental risk factors for pancreatic cancer: An update. Arch. Toxicol. 2016, 90, 2617–2642. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, C.; Javle, M.; Tan, W.; Ma, Y.; Fetterly, G.; Iyer, R.V.; Johnson, C. A phase 1, open-label, dose escalation study of intravenous paricalcitol in combination with gemcitabine in patients with advanced malignancies. Cancer 2018, 124, 3890–3899. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, L.; Liu, X.; Lu, Q.; Tang, T. Vitamin E Intake and Pancreatic Cancer Risk: A Meta-Analysis of Observational Studies. Med. Sci. Monit. 2015, 21, 1249–1255. [Google Scholar] [CrossRef]
- Abu-Fayyad, A.; Nazzal, S. Gemcitabine-vitamin E conjugates: Synthesis, characterization, entrapment into nanoemulsions, and in-vitro deamination and antitumor activity. Int. J. Pharm. 2017, 528, 463–470. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sung, B.; Ravindran, J.; Diagaradjane, P.; Deorukhkar, A.A.; Dey, S.; Koca, C.; Yadav, V.R.; Tong, Z.; Gelovani, J.G.; et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010, 70, 8695–8705. [Google Scholar] [CrossRef]
- Husain, K.; Centeno, B.A.; Coppola, D.; Trevino, J.; Sebti, S.M.; Malafa, M. δ-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget 2017, 8, 31554–31567. [Google Scholar] [CrossRef]
- Mène-Saffrané, L. Vitamin E Biosynthesis and Its Regulation in Plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef]
- Kanai, M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J. Gastroenterol. 2014, 20, 9384–9391. [Google Scholar]
- Bimonte, S.; Barbieri, A.; Leongito, M.; Piccirillo, M.; Giudice, A.; Pivonello, C.; De Angelis, C.; Granata, V.; Palaia, R.; Izzo, F. Curcumin AntiCancer Studies in Pancreatic Cancer. Nutrients 2016, 8, 433. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, C.; Xie, F.; Chen, L.; Liu, L.; Liang, X.; Wu, X.; Wang, P.; Meng, Z. Curcumin suppresses epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells by inhibiting cancer-associated fibroblasts. Am. J. Cancer Res. 2017, 7, 125–133. [Google Scholar] [PubMed]
- Yoshida, K.; Toden, S.; Ravindranathan, P.; Han, H.; Goel, A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 2017, 38, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xi, H.; Gao, Y.; Xu, D.; Li, C. Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway. Mol. Med. Rep. 2015, 12, 5415–5422. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Zhao, D. Curcumin induces apoptotic cell death in human pancreatic cancer cells via the miR-340/XIAP signaling pathway. Oncol. Lett. 2017, 14, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.D.S.; Monteiro, G. Gemcitabine: Metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur. J. Pharmacol. 2014, 741, 8–16. [Google Scholar] [CrossRef]
- Pignanelli, C.; Ma, D.; Noel, M.; Ropat, J.; Mansour, F.; Curran, C.; Pupulin, S.; Larocque, K.; Wu, J.; Liang, G.; et al. Selective Targeting of Cancer Cells by Oxidative Vulnerabilities with Novel Curcumin Analogs. Sci. Rep. 2017, 7, 1105. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin Potentiates Antitumor Activity of Gemcitabine in an Orthotopic Model of Pancreatic Cancer through Suppression of Proliferation, Angiogenesis, and Inhibition of Nuclear Factor-κB–Regulated Gene Products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef]
- Khan, S.; Setua, S.; Kumari, S.; Dan, N.; Massey, A.; Bin Hafeez, B.; Yallapu, M.M.; Stiles, Z.E.; Alabkaa, A.; Yue, J.; et al. Superparamagnetic iron oxide nanoparticles of curcumin enhance gemcitabine therapeutic response in pancreatic cancer. Biomaterials 2019, 208, 83–97. [Google Scholar] [CrossRef]
- Pastorelli, D.; Fabricio, A.S.C.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G.; et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Vyas, A.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F. Perspectives on New Synthetic Curcumin Analogs and their Potential Anticancer Properties. Curr. Pharm. Des. 2013, 19, 2047–2069. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin®) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Hassanian, S.M.; Mohammadzadeh, E.; Shahidsales, S.; Maftouh, M.; Fayazbakhsh, H.; Khazaei, M.; Ghayour-Mobarhan, M.; Mohamamdazade, E. Therapeutic Potential of Curcumin in Treatment of Pancreatic Cancer: Current Status and Future Perspectives. J. Cell. Biochem. 2017, 118, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Cheng, L.; Mei, C.; Ma, J.; Shi, Y.; Zeng, F.; Wang, Z.; Wang, Z. Genistein inhibits cell growth and invasion through regulation of miR-27a in pancreatic cancer cells. Curr. Pharm. Des. 2014, 20, 5348–5353. [Google Scholar] [CrossRef]
- Bi, Y.L.; Min, M.; Shen, W.; Liu, Y. Genistein induced anticancer effects on pancreatic cancer cell lines nvolves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine 2018, 39, 10–16. [Google Scholar] [CrossRef]
- Li, L.; Leung, P.S. Use of herbal medicines and natural products: An alternative approach to overcoming the apoptotic resistance of pancreatic cancer. Int. J. Biochem. Cell Biol. 2014, 53, 224–236. [Google Scholar] [CrossRef]
- Subramaniam, D.; Kaushik, G.; Dandawate, P.; Anant, S. Targeting Cancer Stem Cells for Chemoprevention of Pancreatic Cancer. Curr. Med. Chem. 2018, 25, 2585–2594. [Google Scholar] [CrossRef]
- Suzuki, R.; Kang, Y.; Li, X.; Roife, D.; Zhang, R.; Fleming, J.B. Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014, 34, 4685–4692. [Google Scholar]
- Ma, J.; Zeng, F.; Ma, C.; Pang, H.; Fang, B.; Lian, C.; Yin, B.; Zhang, X.; Wang, Z.P.; Xia, J. Synergistic reversal effect of epithelial-to-mesenchymal transition by miR-223 inhibitor and genistein in gemcitabine-resistant pancreatic cancer cells. Am. J. Cancer Res. 2016, 6, 1384–1395. [Google Scholar]
- Mesmar, F.; Dai, B.; Ibrahim, A.; Hases, L.; Jafferali, M.H.; Augustine, J.J.; DiLorenzo, S.; Kang, Y.; Zhao, Y.; Wang, J.; et al. Clinical candidate and genistein analogue AXP107-11 has chemoenhancing functions in pancreatic adenocarcinoma through G protein-coupled estrogen receptor signaling. Cancer Med. 2019, 8, 7705–7719. [Google Scholar] [CrossRef]
- Wang, X.; McKernan, R.; Kim, K.H.; Alvero, A.B.; Whiting, A.; Thompson, J.A.; Mor, G.; Saif, M.W.; Husband, A.J.; Brown, D.M.; et al. Triphendiol (NV-196), development of a novel therapy for pancreatic cancer. Anti Cancer Drugs 2011, 22, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Go, V.L.W.; Sarkar, F.H. The Role of Nutraceuticals in Pancreatic Cancer Prevention and Therapy. Pancreas 2015, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.-M.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Xiaokaiti, Y.; Fan, S.; Pan, Y.; Li, X. Direct interaction between caffeic acid phenethyl ester and human neutrophil elastase inhibits the growth and migration of PANC-1 cells. Oncol. Rep. 2017, 37, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Shih, S.-C.; Wang, H.-Y.; Lin, C.-C.; Liu, C.-Y.; Wang, T.-E.; Chu, C.-H.; Chen, Y.-J. Caffeic Acid Phenethyl Ester Inhibits Epithelial-Mesenchymal Transition of Human Pancreatic Cancer Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Fraser, S.P.; Hemsley, F.; Djamgoz, M.B.A. Caffeic acid phenethyl ester: Inhibition of metastatic cell behaviours via voltage-gated sodium channel in human breast cancer in vitro. Int. J. Biochem. Cell Biol. 2016, 71, 111–118. [Google Scholar] [CrossRef]
- Oršolic, N.; Car, N.; Lisičić, D.; Benković, V.; Knežević, A.H.; Domagoj, D.; Petrik, J. Synergism Between Propolis and Hyperthermal Intraperitoneal Chemotherapy with Cisplatin on Ehrlich Ascites Tumor in Mice. J. Pharm. Sci. 2013, 102, 4395–4405. [Google Scholar] [CrossRef]
- Salvador-Barbero, B.; Álvarez-Fernández, M.; Zapatero-Solana, E.; el Bakkali, A.; Menéndez, M.D.C.; López-Casas, P.P.; di Domenico, T.; Xie, T.; VanArsdale, T.; Shields, D.J.; et al. CDK4/6 inhibitors impair recovery from cytotoxic chemotherapy in pancreatic adenocarcinoma. Cancer Cell 2020, 37, 340–353.e6. [Google Scholar] [CrossRef]
- Kim, S.T.; Kim, S.Y.; Lee, J.; Kim, K.-H.; Park, S.H.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Park, J.O. Triptolide as a novel agent in pancreatic cancer: The validation using patient derived pancreatic tumor cell line. BMC Cancer 2018, 18, 1103. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.-J.; Li, L.; Lu, C.; Lu, A. Effect of the Natural Product Triptolide on Pancreatic Cancer: A Systematic Review of Preclinical Studies. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Dai, H.; Jiang, Y.; Luo, Y.; Bie, P.; Chen, Z. Triptolide enhances TRAIL sensitivity of pancreatic cancer cells by activating autophagy via downregulation of PUM1. Phytomedicine 2019, 62, 152953. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; He, M.; He, M.; Li, W.; Wang, X.; Wang, Y.; Kuai, Q.; Li, C.; Ren, S.; Yu, Q. Synergistic antitumor activity of gemcitabine combined with triptolide in pancreatic cancer cells. Oncol. Lett. 2016, 11, 3527–3533. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.X.; Sun, Y.L.; Yu, Y.; Zhang, J.; Wu, H.Y.; Yu, X.F. Triptolide enhances the sensitivity of pancreatic cancer PANC-1 cells to gemcitabine by inhibiting TLR4/NF-κB signaling. Am. J. Transl. Res. 2019, 11, 3750–3760. [Google Scholar]
- Wang, C.; Liu, B.; Xu, X.; Zhuang, B.; Li, H.; Yin, J.; Cong, M.; Xu, W.; Lu, A. Toward targeted therapy in chemotherapy-resistant pancreatic cancer with a smart triptolide nanomedicine. Oncotarget 2016, 7, 8360–8372. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wahid, A.; Wang, Z.; Xie, C.; Thakkar, A.; Prabhu, S.; Wang, J. Triptolide and celastrol loaded silk fibroin nanoparticles show synergistic effect against human pancreatic cancer cells. Nanoscale 2017, 9, 11739–11753. [Google Scholar] [CrossRef]
- Modi, S.; Kir, D.; Giri, B.; Majumder, K.; Arora, N.; Dudeja, V.; Banerjee, S.; Saluja, A.K. Minnelide Overcomes Oxaliplatin Resistance by Downregulating the DNA Repair Pathway in Pancreatic Cancer. J. Gastrointest. Surg. 2015, 20, 13–24. [Google Scholar] [CrossRef]
- Chugh, R.; Sangwan, V.; Patil, S.P.; Dudeja, V.; Dawra, R.K.; Banerjee, S.; Schumacher, R.J.; Blazar, B.R.; Georg, G.I.; Vickers, S.M.; et al. A Preclinical Evaluation of Minnelide as a Therapeutic Agent Against Pancreatic Cancer. Sci. Transl. Med. 2012, 4, 156ra139. [Google Scholar] [CrossRef]
- Banerjee, S.; Saluja, A.K. Minnelide, a novel drug for pancreatic and liver cancer. Pancreatology 2015, 15, S39–S43. [Google Scholar] [CrossRef]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, and Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Guzmán, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef]
- Amin, R.; Ali, D. Pharmacology of Medical Cannabis. Adv. Exp. Med. Biol. 2019, 1162, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, G.; He, H.; Nikfarjam, M. Potential Use of Cannabinoids for the Treatment of Pancreatic Cancer. J. Pancreat. Cancer 2019, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Donadelli, M.; Dando, I.; Zaniboni, T.; Costanzo, C.; Pozza, E.D.; Scupoli, M.T.; Scarpa, A.; Zappavigna, S.; Marra, M.; Abbruzzese, A.; et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011, 2, e152. [Google Scholar] [CrossRef] [PubMed]
- Ferro, R.; Adamska, A.; Lattanzio, R.; Mavrommati, I.; Edling, C.E.; Arifin, S.A.; Fyffe, C.A.; Sala, G.; Sacchetto, L.; Chiorino, G.; et al. GPR55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene 2018, 37, 6368–6382. [Google Scholar] [CrossRef]
- Moreau, M.; Ibeh, U.; Decosmo, K.; Bih, N.; Yasmin-Karim, S.; Toyang, N.; Lowe, H.; Ngwa, W. Flavonoid Derivative of Cannabis Demonstrates Therapeutic Potential in Preclinical Models of Metastatic Pancreatic Cancer. Front. Oncol. 2019, 9, 660. [Google Scholar] [CrossRef]
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef]
- Pai, M.; Spalding, D. Pancreatic cancer. Medicine 2015, 43, 329–333. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Andreotti, G.; Silverman, D.T. Occupational risk factors and pancreatic cancer: A review of recent findings. Mol. Carcinog. 2011, 51, 98–108. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Nissim, S.; Leshchiner, I.; Mancias, J.D.; Greenblatt, M.B.; Maertens, O.; Cassa, C.A.; Rosenfeld, J.A.; Cox, A.G.; Hedgepeth, J.; Wucherpfennig, J.I.; et al. Mutations in RABL3 alter KRAS prenylation and are associated with hereditary pancreatic cancer. Nat. Genet. 2019, 51, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Naudin, S.; Viallon, V.; Hashim, D.; Freisling, H.; Jenab, M.; Weiderpass, E.; Perrier, F.; McKenzie, F.; Bueno-De-Mesquita, H.B.; Olsen, A.; et al. Healthy lifestyle and the risk of pancreatic cancer in the EPIC study. Eur. J. Epidemiol. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bracci, P.M. Obesity and pancreatic cancer: Overview of epidemiologic evidence and biologic mechanisms. Mol. Carcinog. 2011, 51, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Xu, M.; Jung, X.; Hines, O.J.; Eibl, G.; Chen, Y. Obesity and Pancreatic Cancer. Pancreas 2018, 47, 158–162. [Google Scholar] [CrossRef]
- Preziosi, G.; Oben, J.A.; Fusai, G. Obesity and pancreatic cancer. Surg. Oncol. 2014, 23, 61–71. [Google Scholar] [CrossRef]
- Carreras-Torres, R.; Johansson, M.; Gaborieau, V.; Haycock, P.C.; Wade, K.H.; Relton, C.L.; Martin, R.M.; Smith, G.D.; Brennan, P. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J. Natl. Cancer Inst. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, D.; Liu, H.; Du, L.-T.; Wang, Y.-S.; Xu, J.-W.; Qiu, F.-B.; Hu, S.-Y.; Zhan, H. Obesity and pancreatic cancer: An update of epidemiological evidence and molecular mechanisms. Pancreatology 2019, 19, 941–950. [Google Scholar] [CrossRef]
- Arslan, A.A.; Helzlsouer, K.J.; Kooperberg, C.; Shu, X.-O.; Steplowski, E.; Bueno-De-Mesquita, H.B.; Fuchs, C.S.; Gross, M.D.; Jacobs, E.J.; Lacroix, A.Z.; et al. Anthropometric Measures, Body Mass Index, and Pancreatic Cancer. Arch. Intern. Med. 2010, 170, 791–802. [Google Scholar] [CrossRef]
- Cascetta, P.; Cavaliere, A.; Epiro, G.; Torroni, L.; Santoro, R.; Tortora, G.; Emelisi, D.; Ecarbone, C. Pancreatic Cancer and Obesity: Molecular Mechanisms of Cell Transformation and Chemoresistance. Int. J. Mol. Sci. 2018, 19, 3331. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S.; Crowley, M.J.; Dar, M.S.; Coffman, C.J.; Jeffreys, A.S.; Maciejewski, M.L.; Voils, C.I.; Bradley, A.B.; Edelman, D. Comparison of Group Medical Visits Combined with Intensive Weight Management vs Group Medical Visits Alone for Glycemia in Patients With Type 2 Diabetes. JAMA Intern. Med. 2020, 180, 70. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.M.R.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; Lucas, A.S.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Sah, R.P.; Nagpal, S.J.S.; Mukhopadhyay, D.; Chari, S.T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 423–433. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef]
- Song, S.; Wang, B.; Zhang, X.; Hao, L.; Hu, X.; Li, Z.; Sun, S. Long-Term Diabetes Mellitus Is Associated with an Increased Risk of Pancreatic Cancer: A Meta-Analysis. PLoS ONE 2015, 10, e0134321. [Google Scholar] [CrossRef]
- Risch, H.A. Diabetes and Pancreatic Cancer: Both Cause and Effect. J. Natl. Cancer Inst. 2018, 111, 1–2. [Google Scholar] [CrossRef]
- Eijgenraam, P.; Heinen, M.; Verhage, B.A.J.; Keulemans, Y.C.; Schouten, L.J.; Brandt, P.A.V.D. Diabetes type II, other medical conditions and pancreatic cancer risk: A prospective study in The Netherlands. Br. J. Cancer 2013, 109, 2924–2932. [Google Scholar] [CrossRef]
- Liao, W.-C.; Tu, Y.-K.; Wu, M.-S.; Lin, J.-T.; Wang, H.-P.; Chien, K.-L. Blood glucose concentration and risk of pancreatic cancer: Systematic review and dose-response meta-analysis. BMJ 2015, 349, g7371. [Google Scholar] [CrossRef]
- Rahn, S.; Zimmermann, V.; Viol, F.; Knaack, H.; Stemmer, K.; Peters, L.; Lenk, L.; Ungefroren, H.; Saur, D.; Schäfer, H.; et al. Diabetes as risk factor for pancreatic cancer: Hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett. 2018, 415, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Pauli, C.; Du, X.; Wang, D.G.; Li, X.; Wu, D.; Amadiume, S.C.; Goncalves, M.D.; Hodakoski, C.; Lundquist, M.R.; et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nat. Cell Biol. 2018, 560, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Kakehi, E.; Kotani, K.; Nakamura, T.; Takeshima, T.; Kajii, E. Non-diabetic Glucose levels and Cancer Mortality: A Literature Review. Curr. Diabetes Rev. 2017, 13, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.E.; Lashinger, L.M.; Hays, D.; Harrison, L.M.; Lewis, K.; Fischer, S.M.; Hursting, S.D. Calorie Restriction Decreases Murine and Human Pancreatic Tumor Cell Growth, Nuclear Factor-κB Activation, and Inflammation-Related Gene Expression in an Insulin-like Growth Factor-1−Dependent Manner. PLoS ONE 2014, 9, e94151. [Google Scholar] [CrossRef] [PubMed]
- De Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.; Ehsan, I.; Vreeswijk, M.P.; Smit, V.T.; De Graaf, H.; Heijns, J.B.; Dutch Breast Cancer Research Group (BOOG); et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. 2012, 23, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.; Peveri, G.; Bosetti, C.; Bagnardi, V.; Crippa, A.; Orsini, N.; Rota, M.; Gallus, S. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: A comprehensive review and meta-analysis. Eur. J. Cancer 2018, 104, 117–126. [Google Scholar] [CrossRef]
- Zou, L.; Zhong, R.; Shen, N.; Chen, W.; Zhu, B.; Ke, J.; Lu, X.; Zhang, T.; Lou, J.; Wang, Z.; et al. Non-linear dose–response relationship between cigarette smoking and pancreatic cancer risk: Evidence from a meta-analysis of 42 observational studies. Eur. J. Cancer 2014, 50, 193–203. [Google Scholar] [CrossRef]
- Barreto, S.G. How does cigarette smoking cause acute pancreatitis? Pancreatology 2016, 16, 157–163. [Google Scholar] [CrossRef]
- Maddatu, J.; Anderson-Baucum, E.; Evans-Molina, C. Smoking and the risk of type 2 diabetes. Transl. Res. 2017, 184, 101–107. [Google Scholar] [CrossRef]
- Lynch, S.M.; Vrieling, A.; Lubin, J.H.; Kraft, P.; Mendelsohn, J.B.; Hartge, P.; Canzian, F.; Steplowski, E.; Arslan, A.A.; Gross, M.; et al. Cigarette Smoking and Pancreatic Cancer: A Pooled Analysis from the Pancreatic Cancer Cohort Consortium. Am. J. Epidemiol. 2009, 170, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Tibensky, M.; Horvathova, L.; Babal, P. E-Cigarettes and Cancer Risk. Cancer Prev. Res. 2019, 13, 137–144. [Google Scholar] [CrossRef]
- Iodice, S.; Gandini, S.; Maisonneuve, P.; Lowenfels, A.B. Tobacco and the risk of pancreatic cancer: A review and meta-analysis. Langenbecks Arch. Surg. 2008, 393, 535–545. [Google Scholar] [CrossRef]
- Pericleous, M.; Rossi, R.E.; Mandair, D.; Whyand, T.; Caplin, M. Nutrition and pancreatic cancer. Anticancer Res. 2014, 34, 9–21. [Google Scholar] [PubMed]
- Tong, X.; Chaudhry, Z.; Lee, C.-C.; Bone, R.N.; Kanojia, S.; Maddatu, J.; Sohn, P.; Weaver, S.A.; Robertson, M.A.; Petrache, I.; et al. Cigarette smoke exposure impairs β-cell function through activation of oxidative stress and ceramide accumulation. Mol. Metab. 2020, 37, 100975. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, T.; Horikoshi, Y.; Nakaso, K.; Nakasone, M.; Kitagawa, Y.; Amisaki, M.; Arai, Y.; Tokuyasu, N.; Sakamoto, T.; Honjo, S.; et al. Nicotine enhances the malignant potential of human pancreatic cancer cells via activation of atypical protein kinase C. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 2404–2415. [Google Scholar] [CrossRef]
- Al-Wadei, M.H.; Banerjee, J.; Al-Wadei, H.A.; Schuller, H.M. Nicotine induces self-renewal of pancreatic cancer stem cells via neurotransmitter-driven activation of sonic hedgehog signalling. Eur. J. Cancer 2016, 52, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Nimmakayala, R.K.; Seshacharyulu, P.; Lakshmanan, I.; Rachagani, S.; Chugh, S.; Karmakar, S.; Rauth, S.; Vengoji, R.; Atri, P.; Talmon, G.A.; et al. Cigarette Smoke Induces Stem Cell Features of Pancreatic Cancer Cells via PAF1. Gastroenterology 2018, 155, 892–908.e6. [Google Scholar] [CrossRef]
- Korc, M.; Jeon, C.Y.; Edderkaoui, M.; Pandol, S.J.; Petrov, M.S. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 529–536. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Chang, J.S. Environmental Risk Factors of Pancreatic Cancer. J. Clin. Med. 2019, 8, 1427. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, H.; English, D.R.; Hodge, A.M.; Room, R.; Hopper, J.L.; Milne, R.L.; Giles, G.G.; MacInnis, R.J. Lifetime alcohol intake and pancreatic cancer incidence and survival: Findings from the Melbourne Collaborative Cohort Study. Cancer Causes Control 2019, 30, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Holmes, M.V.; Chen, Z.; Kartsonaki, C. A review of lifestyle, metabolic risk factors, and blood-based biomarkers for early diagnosis of pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 2019, 34, 330–345. [Google Scholar] [CrossRef]
- Vanella, G.; Archibugi, L.; Stigliano, S.; Capurso, G. Alcohol and gastrointestinal cancers. Curr. Opin. Gastroenterol. 2019, 35, 107–113. [Google Scholar] [CrossRef]
- Yu, W.; Ma, Y.; Shankar, S.; Srivastava, R.K. Chronic ethanol exposure of human pancreatic normal ductal epithelial cells induces cancer stem cell phenotype through SATB2. J. Cell. Mol. Med. 2018, 22, 3920–3928. [Google Scholar] [CrossRef]
- Yallew, W.; Bamlet, W.R.; Oberg, A.L.; Anderson, K.E.; Olson, J.E.; Sinha, R.; Petersen, G.M.; Stolzenberg-Solomon, R.Z.; Jansen, R.J. Association between Alcohol Consumption, Folate Intake, and Risk of Pancreatic Cancer: A Case-Control Study. Nutrients 2017, 9, 448. [Google Scholar] [CrossRef]
- Pezzilli, R.; Caputo, F.; Testino, G.; Patussi, V.; Greco, G.; Macciò, L.; Rossin, M.R.; Mioni, D.; Balbinot, P.; Gandin, C.; et al. Alcohol-related chronic exocrine pancreatic insufficiency: Diagnosis and therapeutic management. A proposal for treatment by the Italian Association for the Study of the Pancreas (AISP) and the Italian Society of Alcohology (SIA). Minerva Med. 2019, 110, 425–438. [Google Scholar] [CrossRef]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef]
- Rahman, F.; Cotterchio, M.; Cleary, S.P.; Gallinger, S. Association between Alcohol Consumption and Pancreatic Cancer Risk: A Case-Control Study. PLoS ONE 2015, 10, e0124489. [Google Scholar] [CrossRef]
- Di Rocco, G.; Baldari, S.; Pani, G.; Toietta, G. Stem cells under the influence of alcohol: Effects of ethanol consumption on stem/progenitor cells. Cell. Mol. Life Sci. 2018, 76, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Gou, Y.-W.; Jin, W.-W.; Xiao, M.; Fang, H.-Y. Association between alcohol intake and the risk of pancreatic cancer: A dose-response meta-analysis of cohort studies. BMC Cancer 2016, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Schadler, K.L.; Thomas, N.J.; Galie, P.A.; Bhang, D.H.; Roby, K.C.; Addai, P.; Till, J.E.; Sturgeon, K.; Zaslavsky, A.; Chen, C.S.; et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 2016, 7, 65429–65440. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Ba, K.S.A.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Astell-Burt, T.; Feng, X. Association of Urban Green Space with Mental Health and General Health Among Adults in Australia. JAMA Netw. Open 2019, 2, e198209. [Google Scholar] [CrossRef] [PubMed]
- Stan, S.D.; Singh, S.V.; Brand, R.E. Chemoprevention strategies for pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 347–356. [Google Scholar] [CrossRef]
- Efferth, T. Editorial: Chemoprevention of cancer by natural products. Cancer Lett. 2019, 459, 13–14. [Google Scholar] [CrossRef]
- Moffat, G.T.; Epstein, A.S.; O’Reilly, E.M. Pancreatic cancer—A disease in need: Optimizing and integrating supportive care. Cancer 2019, 125, 3927–3935. [Google Scholar] [CrossRef]
- Fung, S.; Forte, T.; Rahal, R.; Niu, J.; Bryant, H. Provincial rates and time trends in pancreatic cancer outcomes. Curr. Oncol. 2013, 20, 279–281. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Initiative, A.P.C.G.; Łuksza, M.; Zhao, J.N.; Makarov, V.; Moral, J.A.; Remark, R.; Herbst, B.; Askan, G.; Bhanot, U.; et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nat. Cell Biol. 2017, 551, 512–516. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Fenclova, M.; Novakova, A.; Viktorova, J.; Jonatova, P.; Dzuman, Z.; Ruml, T.; Kren, V.; Hajslova, J.; Vítek, L.; Stranska-Zachariasova, M. Poor chemical and microbiological quality of the commercial milk thistle-based dietary supplements may account for their reported unsatisfactory and non-reproducible clinical outcomes. Sci. Rep. 2019, 9, 11118. [Google Scholar] [CrossRef]
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant Nutrition for Human Nutrition: Hints from Rice Research and Future Perspectives. Mol. Plant 2020, 13, 825–835. [Google Scholar] [CrossRef]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural Product Target Network Reveals Potential for Cancer Combination Therapies. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Pandit, A.P.; Joshi, S.R.; Dalal, P.S.; Patole, V.C. Curcumin as a permeability enhancer enhanced the antihyperlipidemic activity of dietary green tea extract. BMC Complement. Altern. Med. 2019, 19, 129. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Hood, S.; Drummond, P.D. Multiple antidepressant potential modes of action of curcumin: A review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J. Psychopharmacol. 2012, 26, 1512–1524. [Google Scholar] [CrossRef]
- O’Neil, N.J.; Bailey, M.L.; Hieter, P. Synthetic lethality and cancer. Nat. Rev. Genet. 2017, 18, 613–623. [Google Scholar] [CrossRef]
- Regel, I.; Mayerle, J.; Mukund, M.U.; Mahajan, U. Current Strategies and Future Perspectives for Precision Medicine in Pancreatic Cancer. Cancers 2020, 12, 1024. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.; Initiative, A.P.C.G.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nat. Cell Biol. 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Balaji, U.; Eslinger, C.; McMillan, E.; Conway, W.; Posner, B.; Mills, G.B.; O’Reilly, E.M.; Knudsen, E.S. Integrated Patient-Derived Models Delineate Individualized Therapeutic Vulnerabilities of Pancreatic Cancer. Cell Rep. 2016, 16, 2017–2031. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, E.B.; Claghorn, K.; Dixon, S.W.; Hill, E.B.; Braun, A.; Lipinski, E.; Platek, M.E.; Vergo, M.T.; Spees, C.K. Inadequate Nutrition Coverage in Outpatient Cancer Centers: Results of a National Survey. J. Oncol. 2019, 2019. [Google Scholar] [CrossRef]
- Fokas, E.; Neill, E.O.; Gordon-Weeks, A.; Mukherjee, S.; McKenna, W.G.; Muschel, R.J. Pancreatic ductal adenocarcinoma: From genetics to biology to radiobiology to oncoimmunology and all the way back to the clinic. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1855, 61–82. [Google Scholar] [CrossRef]
- Nishikawa, G.; Luo, J.; Prasad, V. A comprehensive review of exceptional responders to anticancer drugs in the biomedical literature. Eur. J. Cancer 2018, 101, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Garcia-Perez, I.; Posma, J.M.; Chambers, E.S.; Mathers, J.C.; Draper, J.; Beckmann, M.; Nicholson, J.K.; Holmes, E.; Frost, G.S. Dietary metabotype modelling predicts individual responses to dietary interventions. Nat. Food 2020, 1, 355–364. [Google Scholar] [CrossRef]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-Integrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; Silva, A.N.D.L.E.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef]
- Lee, L.O.; James, P.; Zevon, E.S.; Kim, E.S.; Trudel-Fitzgerald, C.; Spiro, A.; Grodstein, F.; Kubzansky, L.D. Optimism Is Associated with Exceptional Longevity in 2 Epidemiologic Cohorts of Men and Women. Proc. Natl. Acad. Sci. USA 2019, 116, 18357–18362. [Google Scholar] [CrossRef]
- Boilly, B.; Faulkner, S.; Jobling, P.; Hondermarck, H. Nerve Dependence: From Regeneration to Cancer. Cancer Cell 2017, 31, 342–354. [Google Scholar] [CrossRef]
- Arese, M.; Bussolino, F.; Pergolizzi, M.; Bizzozero, L.; Pascal, D. Tumor progression: The neuronal input. Ann. Transl. Med. 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, C.; Phillips, J.A.; Djamgoz, M.B.A. Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 188411. [Google Scholar] [CrossRef]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaanan, T.L.; Schiller, M.; Azulay-Debby, H.; Korin, B.; Boshnak, N.; Koren, T.; Krot, M.; Shakya, J.; Rahat, M.A.; Hakim, F.; et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.A.; Coombes, R.C.; Schwab, A. Ion transport and cancer: From initiation to metastasis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130092. [Google Scholar] [CrossRef]
- Jiang, S.-H.; Hu, L.-P.; Wang, X.; Li, J.; Zhang, Z.-G. Neurotransmitters: Emerging targets in cancer. Oncogene 2019, 39, 503–515. [Google Scholar] [CrossRef]
- Jung, E.; Alfonso, J.; Monyer, H.; Wick, W.; Winkler, F. Neuronal signatures in cancer. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Onkal, R. Persistent Current Blockers of Voltage-Gated Sodium Channels: A Clinical Opportunity for Controlling Metastatic Disease. Recent Patents Anti Cancer Drug Discov. 2012, 8, 66–84. [Google Scholar] [CrossRef]
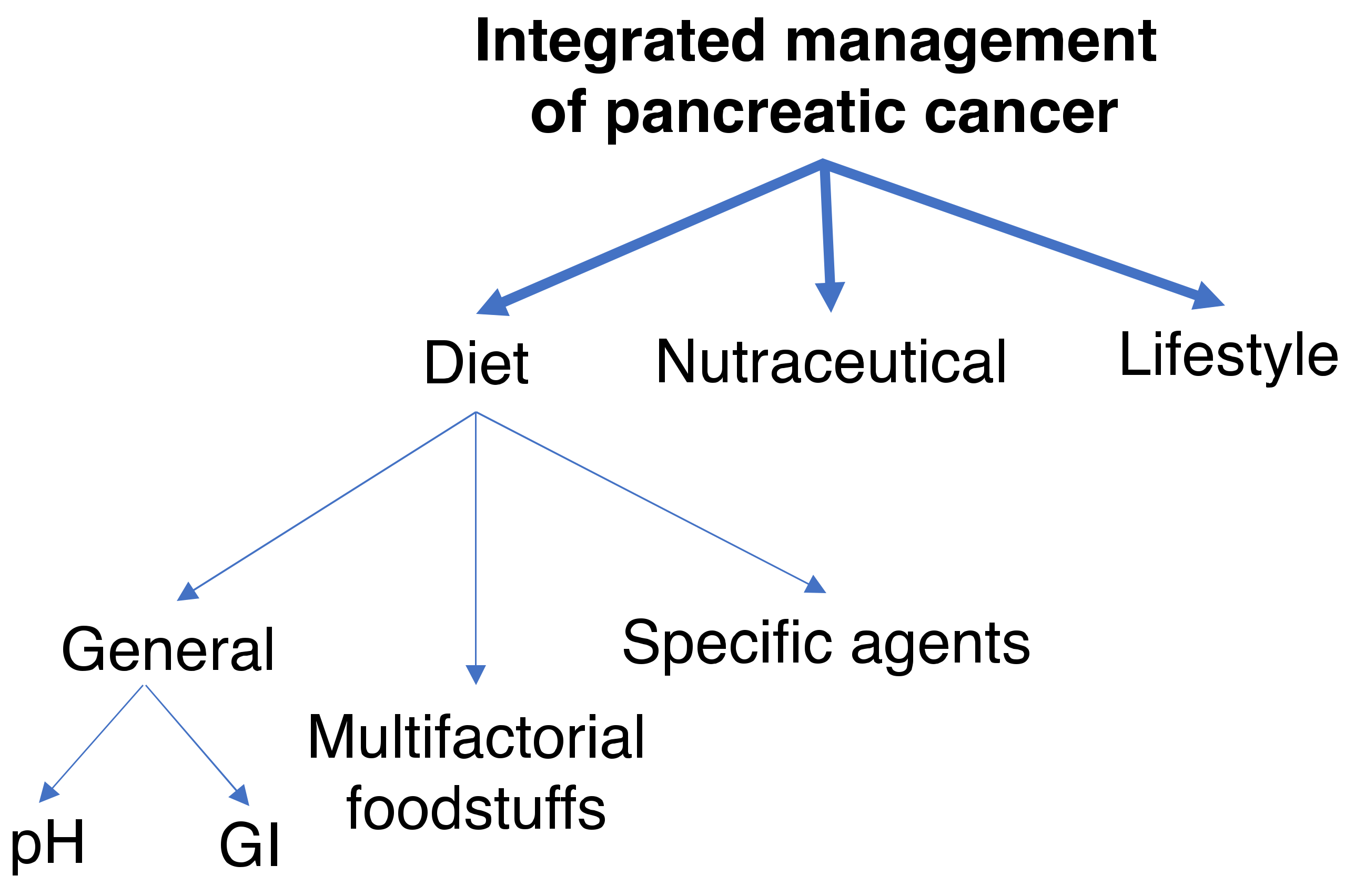
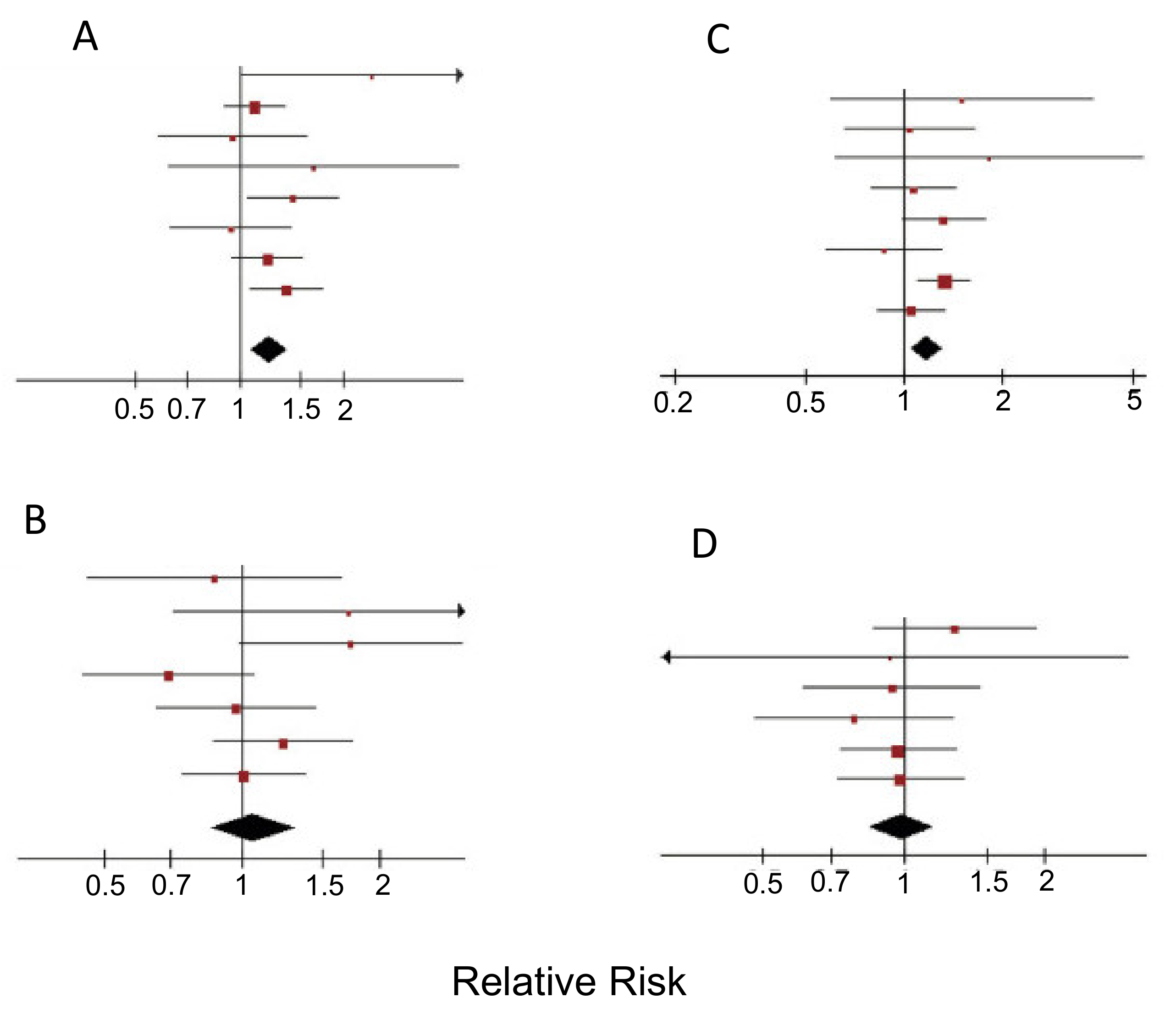
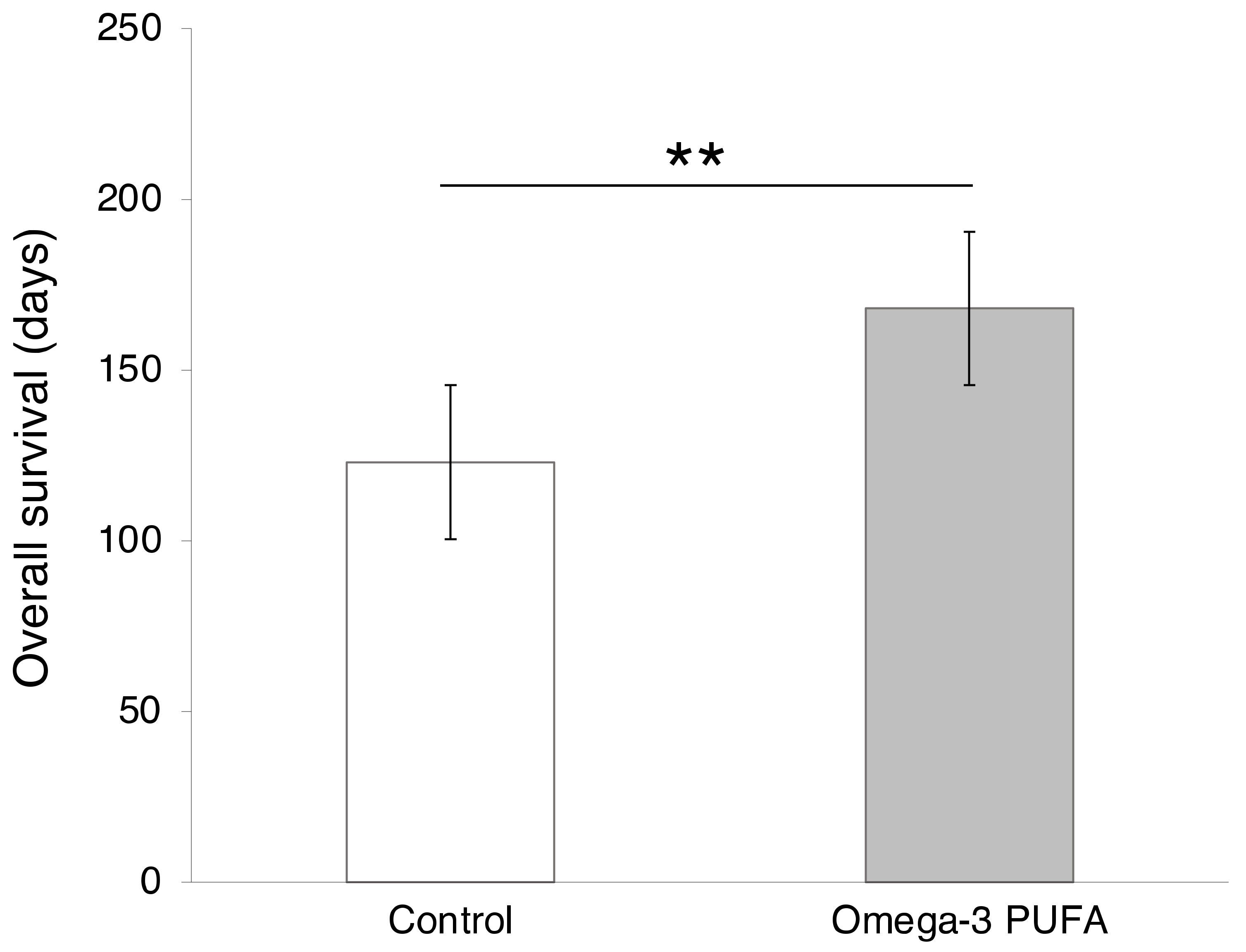

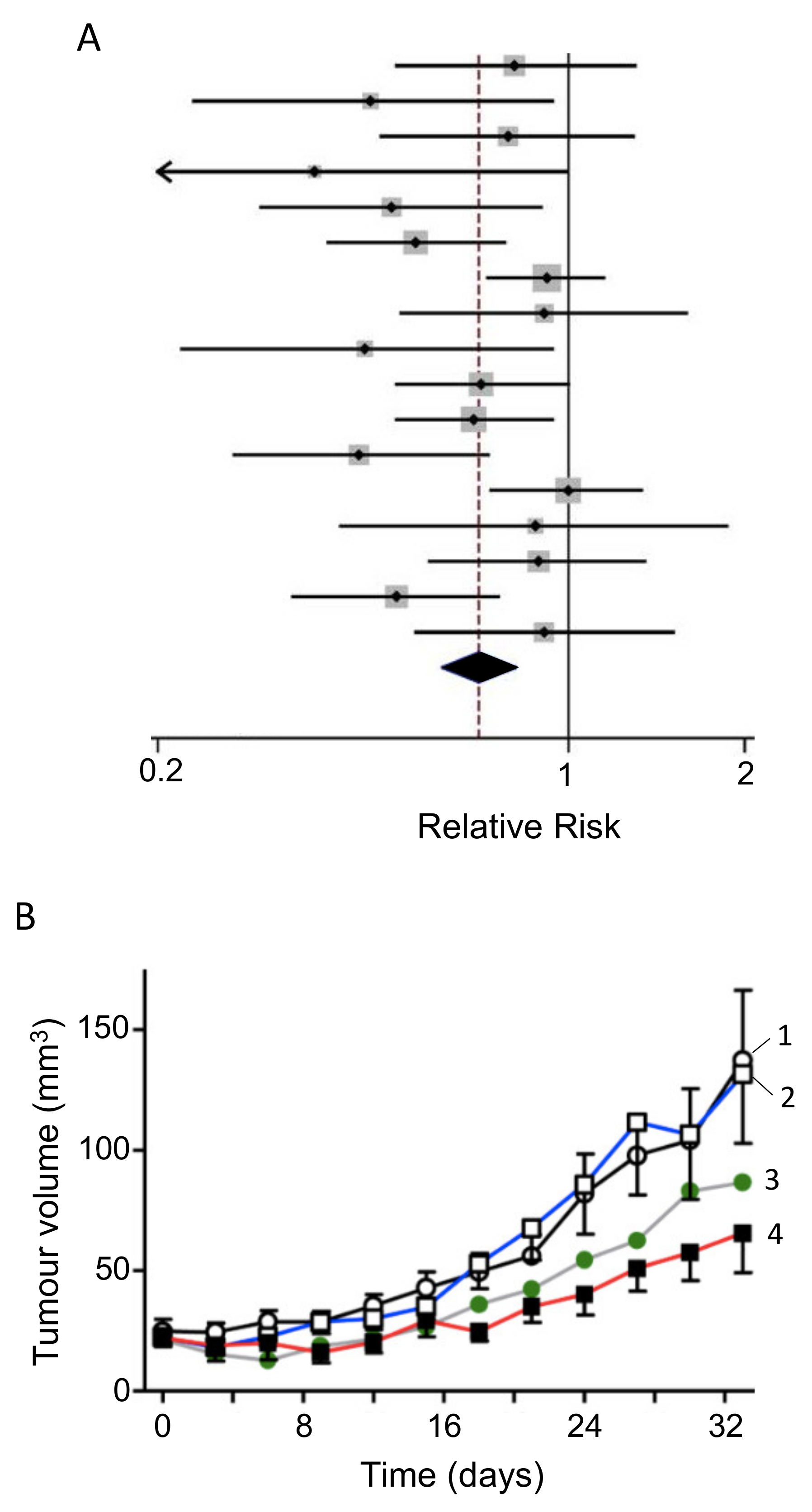
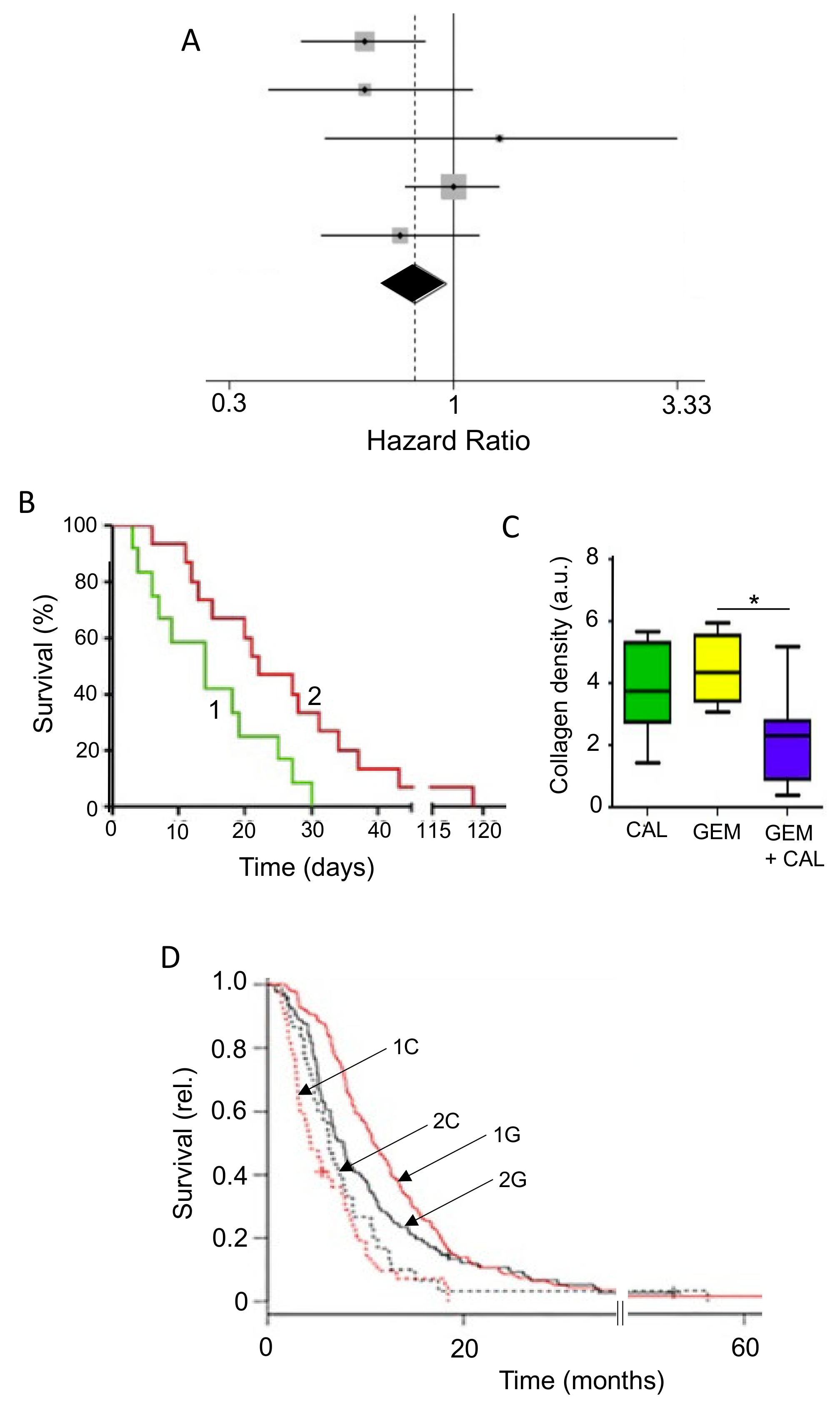
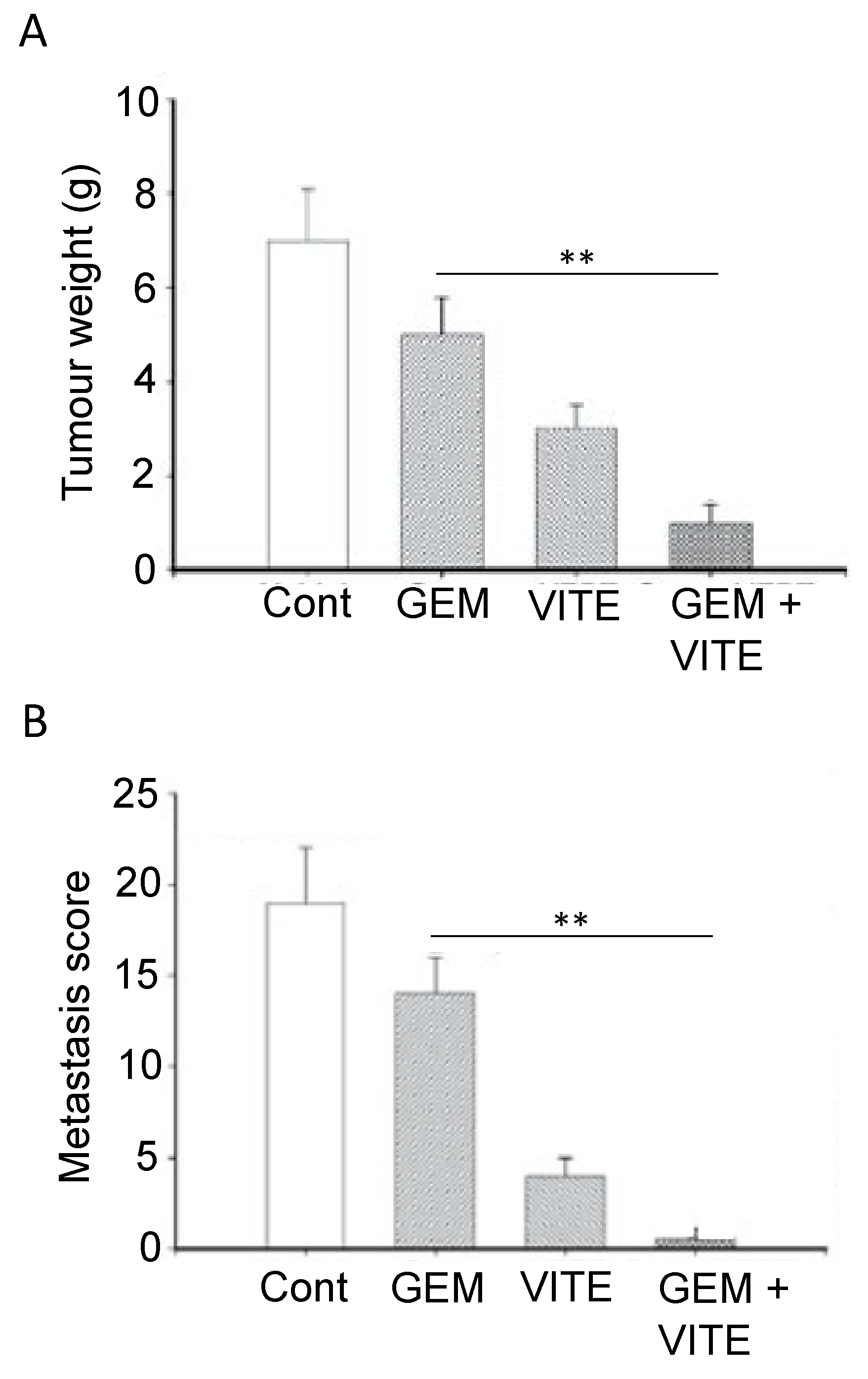
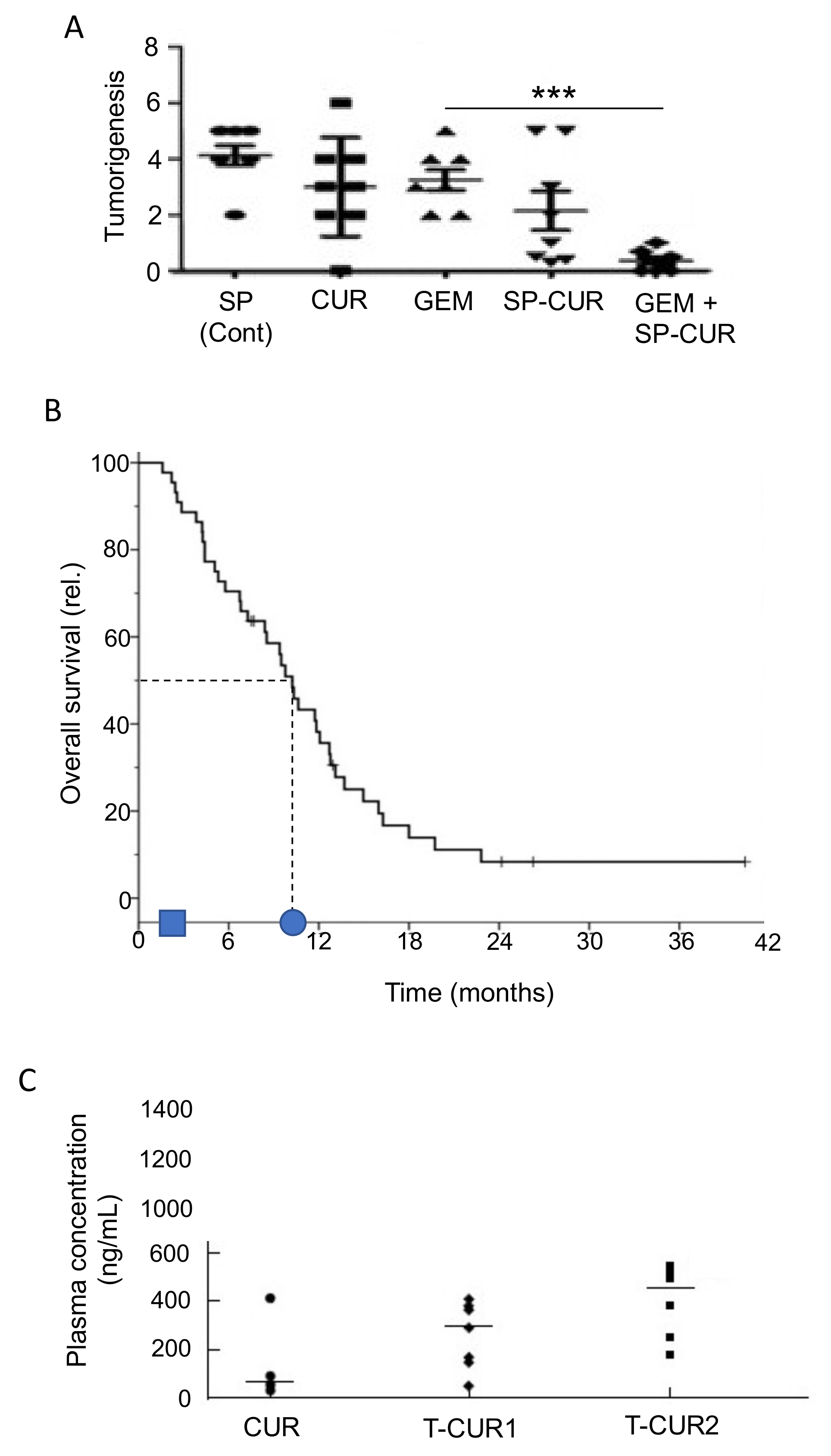
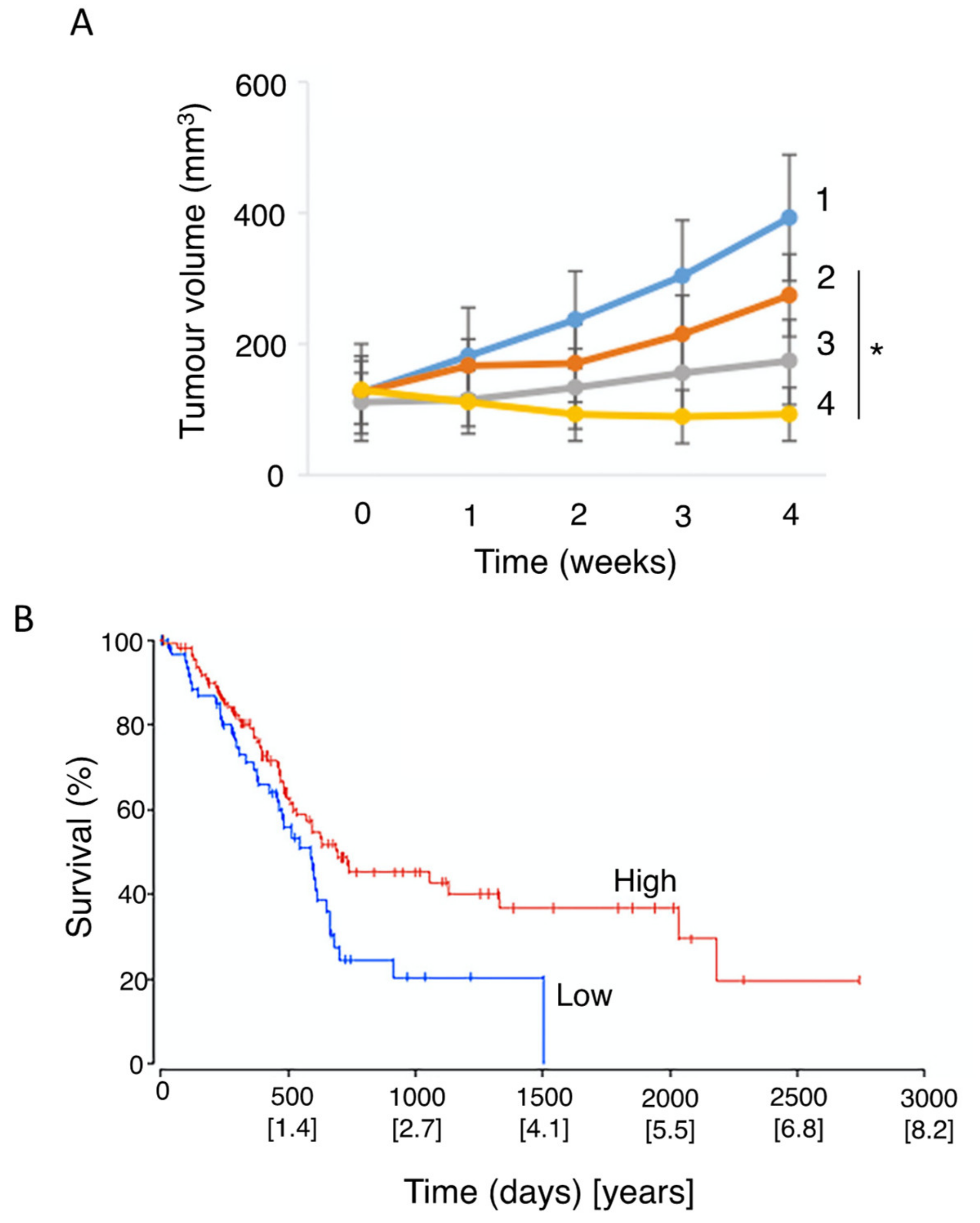
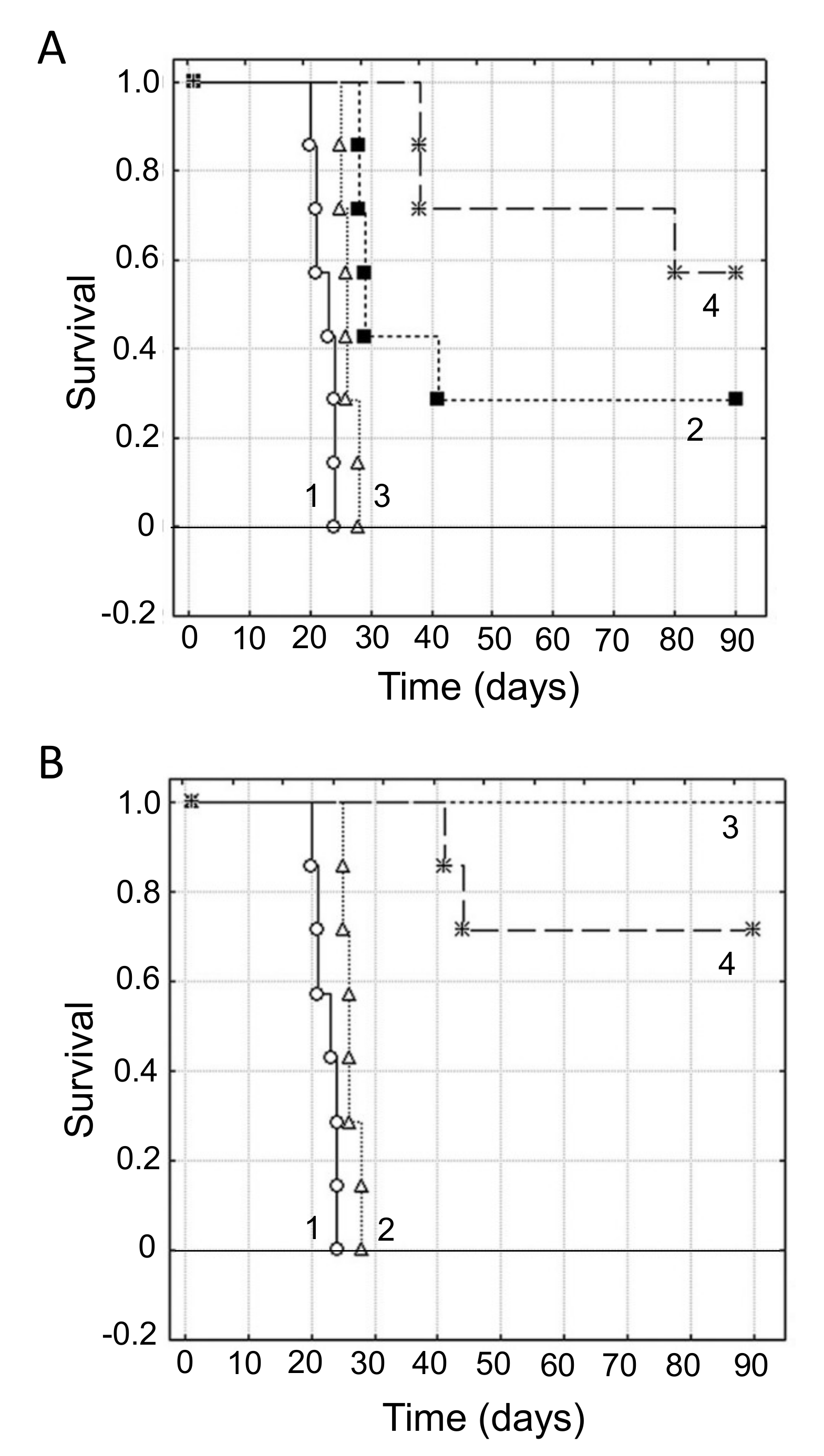
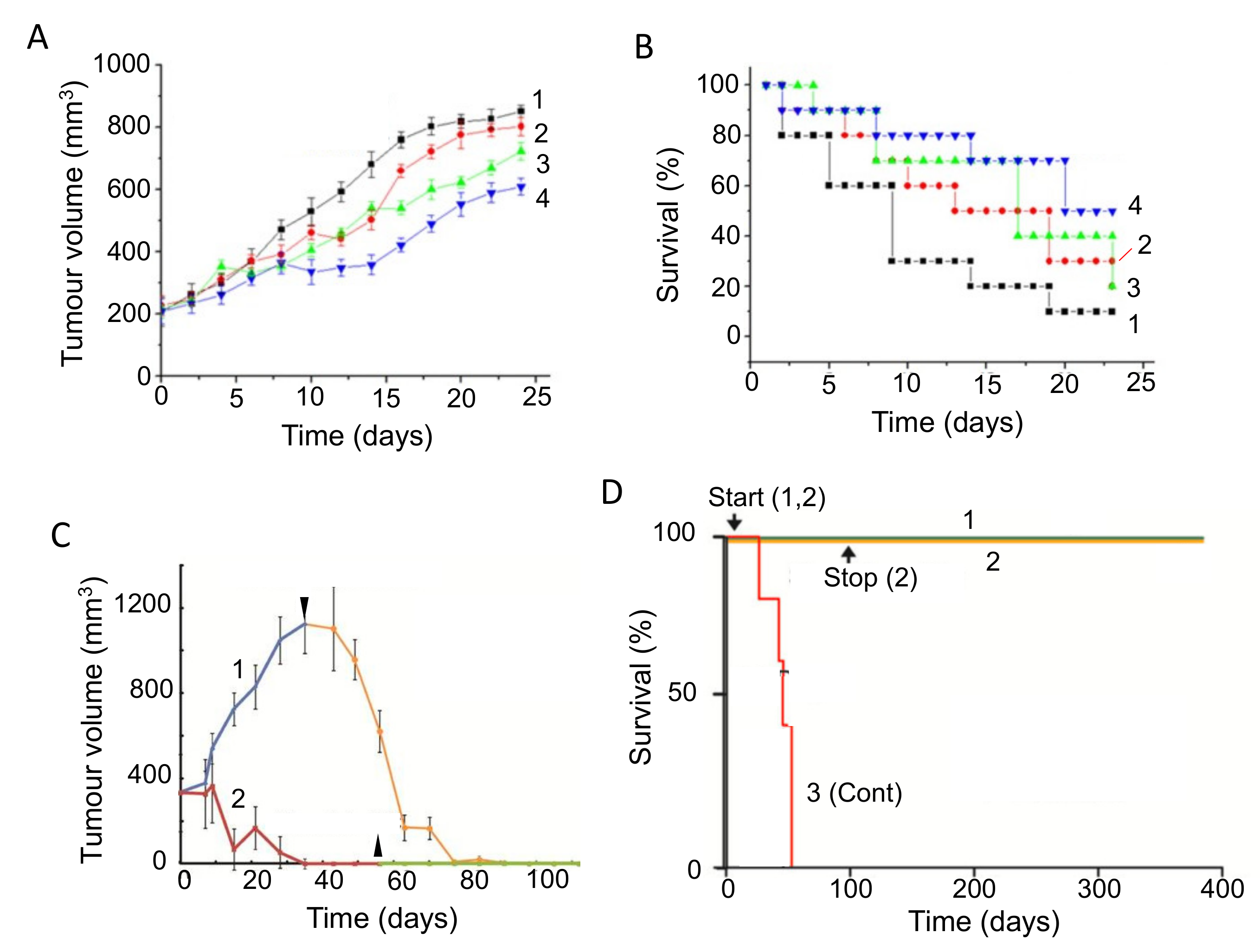
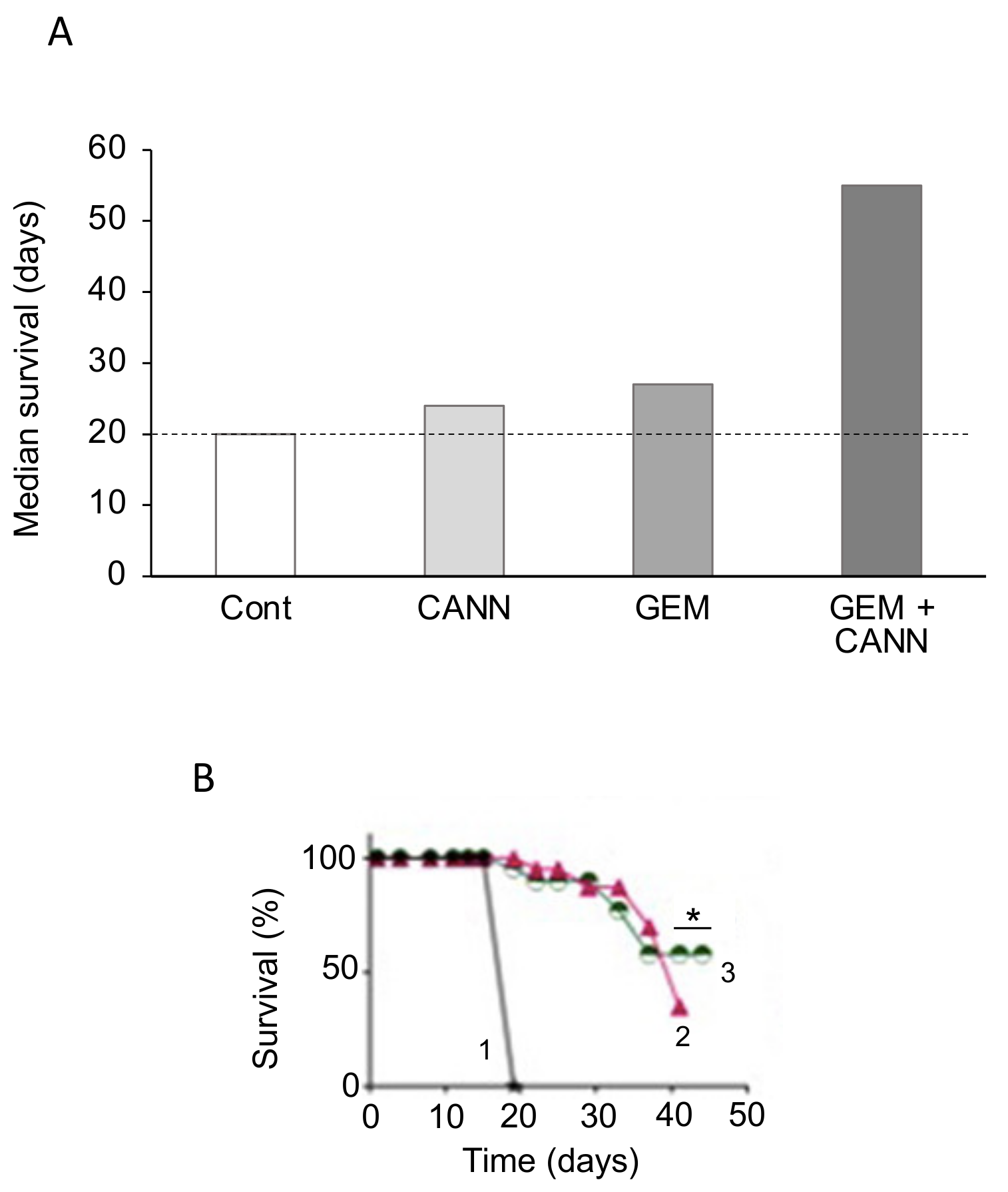
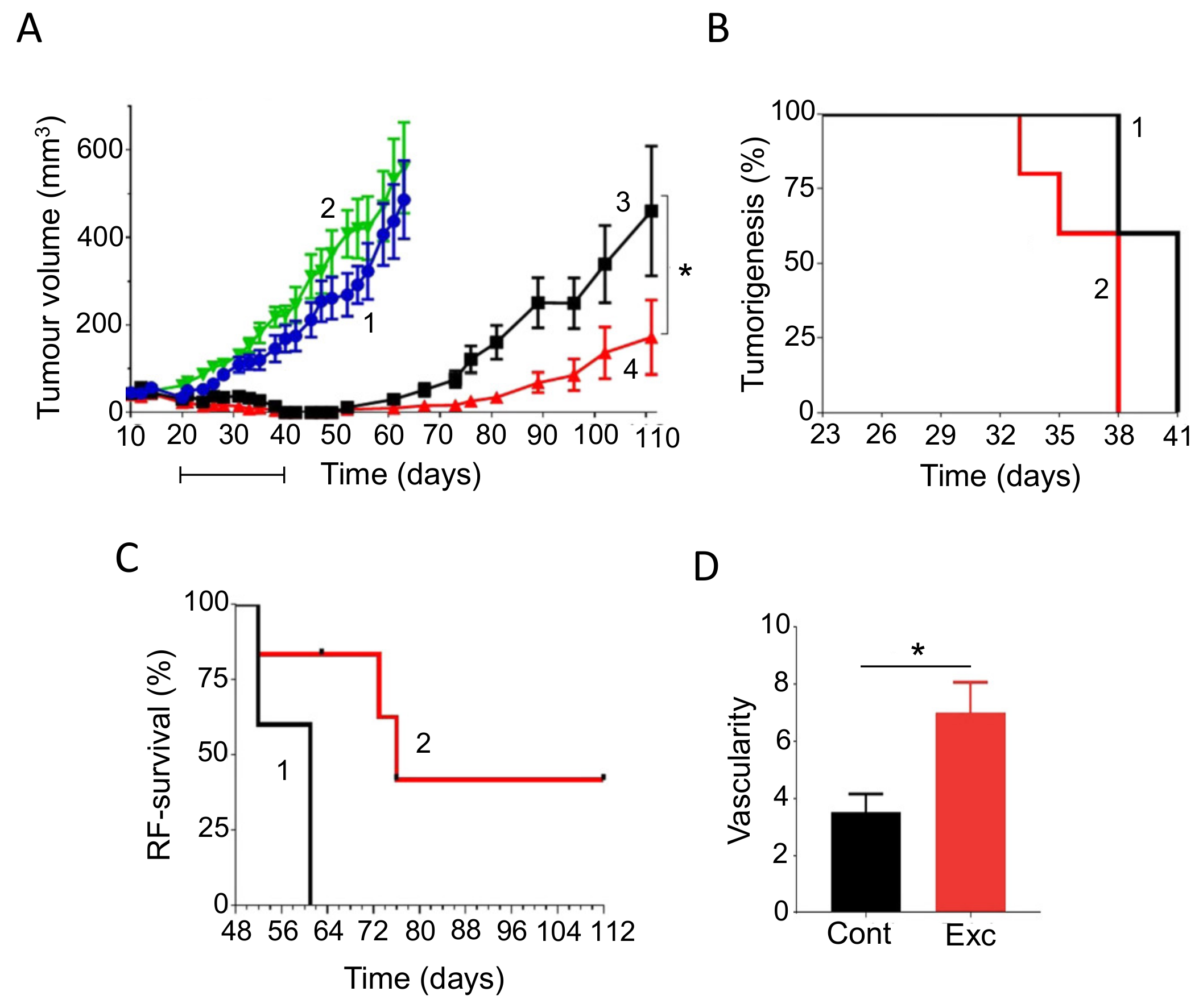
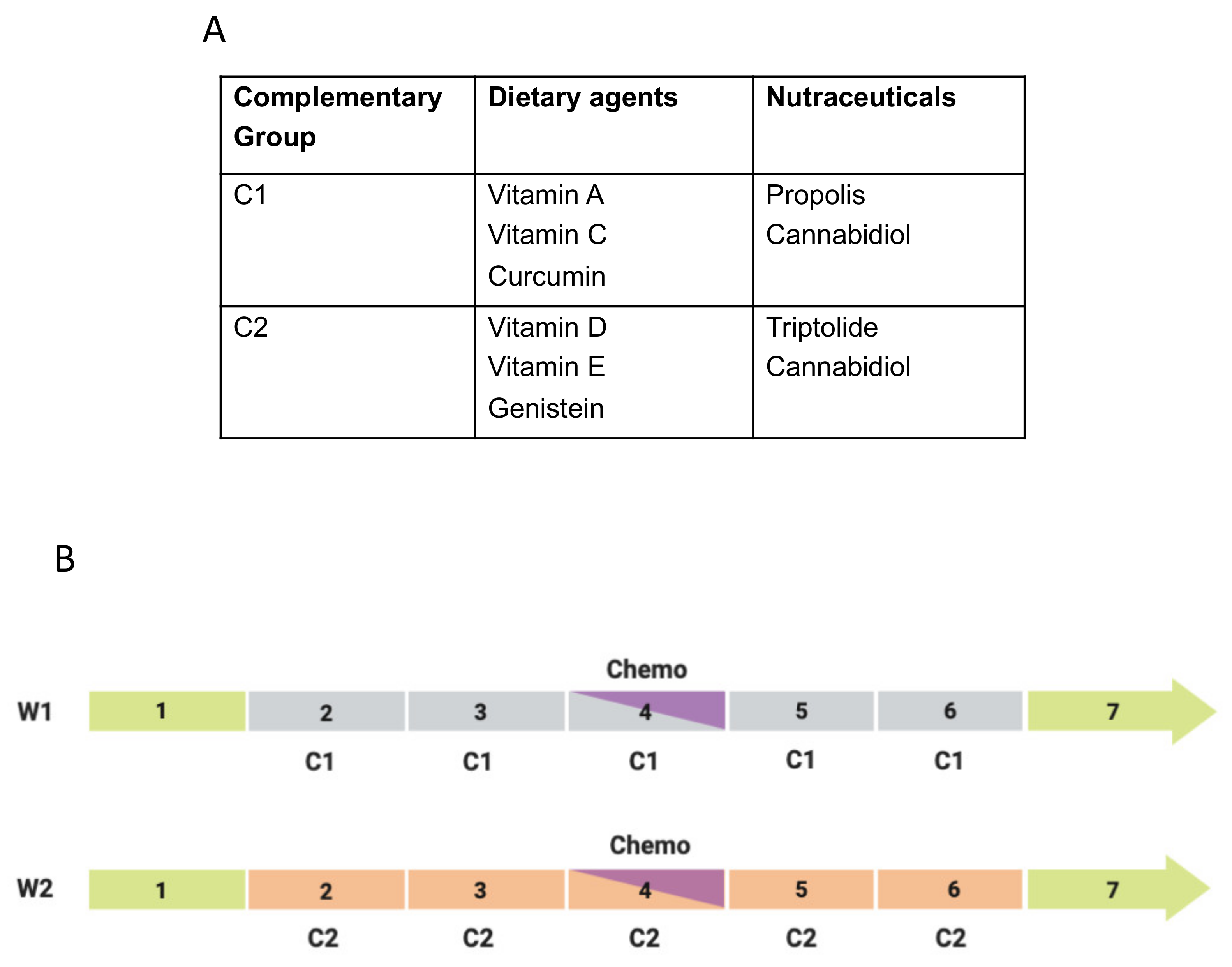
| Serial Letter | National Clinical Trial (NCT) Number | Title | Primary Investigator | Site(s) | No. of Patients | Completion Date | Reference(s)/Comments |
|---|---|---|---|---|---|---|---|
| A | 01019382 | Phase II trial of the effect of gemcitabine with intravenous omega-3 fish oil infusion in patients with unresectable pancreatic adenocarcinoma | Ashley Dennison | Leicester University Hospitals (UK) | 50 | June 2014 | [33] |
| B | 03307148 | A Phase 1b study repurposing ATRA as stromal targeting agent along with gemcitabine and nab-paclitaxel for pancreatic cancer (STAR-PAC) | David Propper | Barts and The London NHS Trust (UK) | 34 | March 2018 | No results posted (NRP) |
| C | 01852890 | Gemcitabine, ascorbate, radiation therapy for pancreatic cancer, Phase I | Joseph J Cullen | The University of Iowa Hospitals & Clinics (USA) | 16 | January 2019 | [34] |
| D | 02905578 | A Phase II trial of pharmacological ascorbate, gemcitabine, and nab-paclitaxel for metastatic pancreatic cancer (PACMAN 2.1) | Joseph J Cullen | University of Iowa (USA) | 65 | December 2025 | - |
| E | 03410030 | Phase Ib/II trial of high dose ascorbic acid (AA) + nanoparticle paclitaxel protein bound + cisplatin + gemcitabine (AA NABPLAGEM) in patients who have no prior therapy for their metastatic pancreatic cancer | Gayle S Jameson | HonorHealth Research Institute (USA) | 36 | July 2020 | - |
| F | 02896907 | A pilot study of intravenous ascorbic acid and FOLFIRINOX in the treatment of advanced pancreatic cancer | James Posey | Sidney Kimmel Cancer Center at Thomas Jefferson University (USA) | 8 | October 2019 | Some adverse effects reported, reason(s) and any benefit not clear. |
| G | 03520790 | Vitamin D receptor agonist paricalcitol plus gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer | Kimberly Perez | Dana-Farber Cancer Institute (USA) | 112 | November 2025 | - |
| H | 03519308 | A pilot study of perioperative nivolumab and paricalcitol to target the microenvironment in resectable pancreatic cancer | Peter O’Dwyer | Abramson Cancer Center of the University of Pennsylvania (USA) | 20 | July 2022 | - |
| I | 03415854 | A Phase II pilot trial of paclitaxel protein bound plus cisplatin plus gemcitabine and the addition of paricalcitol upon disease progression in patients with previously untreated metastatic pancreatic ductal adenocarcinoma (NABPLAGEMD) | Erkut Borazanci | HonorHealth Research Institute (USA) | 14 | December 2020 | - |
| J | 03300921 | A Phase Ib pharmacodynamic study of neoadjuvant paricalcitol in resectable pancreatic cancer | Peter O’Dwyer | Abramson Cancer Center of the University of Pennsylvania (USA) | 20 | April 2020 | Suspended/NRP |
| K | 03331562 | A SU2C Catalyst ® randomized Phase II trial of the PD1 inhibitor pembrolizumab with or without a vitamin D receptor agonist paricalcitol in patients with stage IV pancreatic cancer who have been placed in best possible response | Daniel Von Hoff | Translational Genomics Research Institute (USA) | 24 | June 2021 | - |
| L | 00985777 | A Phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of vitamin E δ-tocotrienol administered to subjects with resectable pancreatic exocrine neoplasia | Gregory Springett | H. Lee Moffitt Cancer Center and Research Institute (USA) | 26 | June 2015 | [35] |
| M | - | A Phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer | Masashi Kanai | Kyoto University Hospital (Japan) | 21 | - | [36] |
| N | 01182246 | Safety, pharmacokinetics and efficacy of AXP107-11 in combination with standard gemcitabine (Gemzar®) treatment in patients with locally advanced or metastatic, unresectable, adenocarcinoma of the pancreas, stage III-IV: a prospective, open label, multi-center, sequential Phase Ib/IIa study | Mattias Löhr | Karolinska Institute (Sweden) | 44 | March 2016 | [37] |
| O | 01375088 | Phase I assessing the preventing and therapeutic effect of propolis in radiotherapy induced mucositis of head and neck cancers | Arghavan Tonkaboni | Mashhad University of Medical Sciences (Iran) | 20 | March 2011 | NRP |
| P | 03416127 | Effect of propolis or metformin administration on glycemic control in patients with type 2 diabetes mellitus without pharmacological treatment | Manuel González Ortiz | Intstituto de Terapeútica Experimental y Clínica, Universidad de Guadalajara (Mexico) | 36 | October 2020 | - |
| Q | 03117920 | MinPAC: A Phase II, international open label trial of Minnelide™ in patients with refractory pancreatic cancer | David Propper | Barts & The London NHS Trust (UK) | 35 | May 2020 | NRP |
| R | 03129139 | A Phase I, multi-center, open-label, dose-escalation, safety, pharmacokinetic, and pharmacodynamic study of Minnelide™ capsules given alone or in combination with protein-bound paclitaxel in patients with advanced solid tumors | Cameron Wright | Minneamrita Therapeutics LLC (USA) | 54 | December 2021 | - |
| S | 03245658 | The effect of medical cannabis inpatients with palliative pancreatic cancer | Jens Rikardt Andersen | University of Copenhagen (Denmark) | 32 | October 2018 | NRP |
| T | 03984214 | Efficacy and safety of dronabinol in the improvement of chemotherapy-induced and tumor-related symptoms in advanced pancreatic cancer | Felix Keil | Arbeitsgemeinschaft Medikamentoese Tumortherapie (Austria) | 140 | June 2022 | - |
| U | 02295956 | Preoperative rehabilitation during neoadjuvant therapy for pancreatic cancer: A pilot study | Matthew H. Katz | Anderson Cancer Center (USA) | 75 | June 2019 | [38] |
| Agent | Formula | Mechanisms of Action/Cellular Effects | Food Sources |
|---|---|---|---|
| Vitamin A (Retinol) | C20H30O | Multiple—metabolic substrate, transcriptional regulator of growth and differentiation (epithelial, bone and immune cells) | Carrots, eggs, leafy green vegetables, carrots, dark leafy green vegetables, dried apricots, cantaloupe, bell peppers, fish, liver, tropical fruits |
| Vitamin C | C6H8O6 | Regulator of oxidation-reduction processes, mainly as an antioxidant and immune regulation | Citrus fruits, broccoli, cantaloupe, cauliflower, kale, kiwi, papaya, sweet peppers, tomatoes |
| Vitamin D: D2 (Ergocalciferol) D3 (Cholecalciferol) | C28H44O C27H44O | Multiple—wide-ranging genomic and non-genomic regulator, including of calcium metabolism and immune response | Oily fish, eggs, mushrooms |
| Vitamin E | C29H50O2 | Mainly an antioxidant that inhibits production of reactive oxygen species | Nuts, seeds, sunflower oil, soybeans, avocado, green leafy vegetables, spinach |
| Curcumin | C21H20O6 | Pleiotropic—several modes of action especially antioxidant and anti-inflammatory | Turmeric |
| Genistein | C15H10O5 | Multiple—modulator of growth factor signaling, pro-apoptotic, inhibitor of cancer cell survival, proliferation, angiogenesis | Soybeans and soy products |
| Agent | Formula | Mechanisms of Action/Cellular Effects | Source |
|---|---|---|---|
| Propolis | Variable | Multiple—anti-inflammatory, antioxidant | Honey (comb) |
| Triptolide | C20H24O6 | Complex—anti-inflammatory, antioxidant, immune-modulator | Thunder god vine |
| Cannabidiol | C21H30O2 | Multi-modal—immune-modulator, neuromodulator (analgesic, anti-convulsant) | Cannabis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jentzsch, V.; Davis, J.; Djamgoz, M.B.A. Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management. Cancers 2020, 12, 3096. https://doi.org/10.3390/cancers12113096
Jentzsch V, Davis J, Djamgoz MBA. Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management. Cancers. 2020; 12(11):3096. https://doi.org/10.3390/cancers12113096
Chicago/Turabian StyleJentzsch, Valerie, James Davis, and Mustafa B. A. Djamgoz. 2020. "Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management" Cancers 12, no. 11: 3096. https://doi.org/10.3390/cancers12113096
APA StyleJentzsch, V., Davis, J., & Djamgoz, M. B. A. (2020). Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management. Cancers, 12(11), 3096. https://doi.org/10.3390/cancers12113096





