Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments
Simple Summary
Abstract
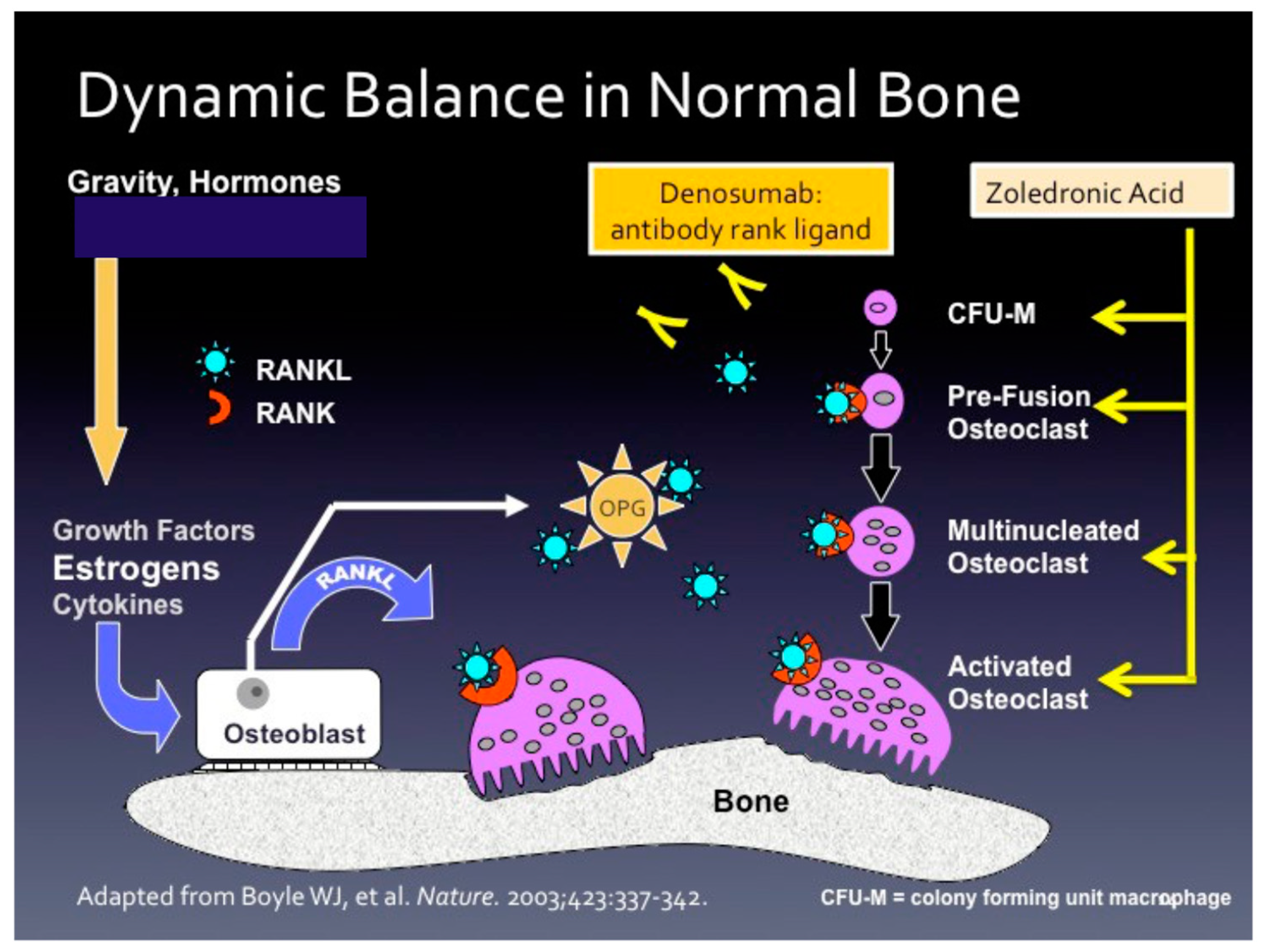
1. Healthy Bone Loss and the Osteoporosis Equation
2. Risk Factor Assessment and Screening for Osteoporosis
3. The Magnitude of Bone Loss Related to Breast Cancer Treatments
3.1. Aromatase Inhibitors
3.2. Selective Estrogen Receptor Modulators (SERM)
3.3. Oophorectomy, GnRH agonist +/− AI, and CIOF
4. Calcium and Vitamin D
5. Determining Fracture Risk
6. Assessing the Need for Anti-Osteoporosis Therapy
7. Oral and Intravenous Bisphosphonates and Rank Ligand Inhibitor
8. Anti-Osteoporosis Drugs and Their Anti-Cancer Activity
9. Conclusions
Funding
Conflicts of Interest
References
- The Basics of Bone in Health and Disease. In Bone Health and Osteoporisis; Office of the Surgeon General: Rockville, MD, USA, 2004.
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar] [PubMed]
- Orwell, E.; Adler, R.A.; Amin, S.; Binkley, N.; Lewiecki, E.M.; Petak, S.M.; A Shapses, S.; Sinaki, M.; Watts, N.B.; Sibonga, J.D. Skeletal health in long-duration astronauts: Nature, assessment, and management recommendations from the NASA bone summit. J. Bone Miner. Res. 2013, 28, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.J.; Tom, C.; Guemes, M.; Polanco, G.; Mayorga, M.E.; Wend, K.; Miranda-Carboni, G.A.; Krum, S.A. ERalpha signaling regulates MMP3 expression to induce FasL cleavage and osteoclast apoptosis. J. Bone Miner. Res. 2013, 28, 283–290. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, M.; Zhang, H.Q.; Tang, M.X.; Guo, C.F.; Deng, A.; Chen, Y.; Xiao, L.G. Opposite Function of ERalpha and ERbeta in Controlling 17beta-Estradiol-mediated Osteogenesis in Osteoblasts. Arch. Med. Res. 2016, 47, 255–261. [Google Scholar] [CrossRef]
- Kameda, T.; Mano, H.; Yuasa, T.; Mori, Y.; Miyazawa, K.; Shiokawa, M.; Nakamaru, Y.; Hiroi, E.; Hiura, K.; Kameda, A.; et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 1997, 186, 489–495. [Google Scholar] [CrossRef]
- Tomkinson, A.; Reeve, J.; Shaw, R.W.; Noble, B.S. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J. Clin. Endocrinol. Metab. 1997, 82, 3128–3135. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. T cells: Unexpected players in the bone loss induced by estrogen deficiency and in basal bone homeostasis. Ann. N. Y. Acad. Sci. 2007, 1116, 360–375. [Google Scholar] [CrossRef]
- D’Amelio, P.; Grimaldi, A.; Di Bella, S.; Brianza, S.Z.; Cristofaro, M.A.; Tamone, C.; Giribaldi, G.; Ulliers, D.; Pescarmona, G.P.; Isaia, G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone 2008, 43, 92–100. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Shapiro, C.L. Osteopenia and osteoporosis in women with breast cancer. Semin. Oncol. 2003, 30, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Looker, A.C.; Tosteson, A.N.; Johansson, H.; Kanis, J.A.; Melton, L.J., 3rd. The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos. Int. 2010, 21, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Estrada, K.; Evangelou, E.; Ackert-Bicknell, C.; Akesson, K.; Beck, T.; Brown, S.J.; Capellini, T.; Carbone, L.; Cauley, J.; et al. Meta-Analysis of Genomewide Association Studies Reveals Genetic Variants for Hip Bone Geometry. J. Bone Miner. Res. 2019, 34, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019, 51, 258–266. [Google Scholar] [CrossRef]
- Liu, M.; Goss, P.E.; Ingle, J.N.; Kubo, M.; Furukawa, Y.; Batzler, A.; Jenkins, G.D.; Carlson, E.E.; Nakamura, Y.; Schaid, D.J.; et al. Aromatase inhibitor-associated bone fractures: A case-cohort GWAS and functional genomics. Mol. Endocrinol. 2014, 28, 1740–1751. [Google Scholar] [CrossRef]
- Artigalas, O.; Vanni, T.; Hutz, M.H.; Ashton-Prolla, P.; Schwartz, I.V. Influence of CYP19A1 polymorphisms on the treatment of breast cancer with aromatase inhibitors: A systematic review and meta-analysis. BMC Med. 2015, 13, 139. [Google Scholar] [CrossRef]
- Yang, S.; Leslie, W.D.; Yan, L.; Walld, R.; Roos, L.L.; Morin, S.N.; Majumdar, S.R.; Lix, L.M. Objectively Verified Parental Hip Fracture Is an Independent Risk Factor for Fracture: A Linkage Analysis of 478,792 Parents and 261,705 Offspring. J. Bone Miner. Res. 2016, 31, 1753–1759. [Google Scholar] [CrossRef]
- Lekamwaan, S.; Adachi, J.D.; Agnusdei, D. A framework for the development of guidelines for the managment of glucocorticoid-induced osteoporsis. Osteoporos. Int. 2012, 23, 2257–2276. [Google Scholar] [CrossRef]
- Jin, S.; Hsieh, E.; Peng, L.; Yu, C.; Wang, Y.; Wu, C.; Wang, Q.; Li, M.; Zeng, X. Incidence of fractures among patients with rheumatoid arthritis: A systematic review and meta-analysis. Osteoporos. Int. 2018, 29, 1263–1275. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z.; Yu, M.; Qu, X. Alcohol consumption and hip fracture risk. Osteoporos. Int. 2015, 26, 531–542. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; De Laet, C.; Johansson, H.; Oden, A.; Delmas, P.; Eisman, J.; Fujiwara, S.; Garnero, P.; Kroger, H.; et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004, 35, 375–382. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; De Laet, C.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; McCloskey, E.V.; Mellstrom, D.; et al. Smoking and fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Kanis, J.A.; Oden, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Khosla, S.; Malkasian, G.D.; Achenbach, S.J.; Oberg, A.L.; Riggs, B.L. Fracture risk after bilateral oophorectomy in elderly women. J. Bone Miner. Res. 2003, 18, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Arimidex, T.; Tamoxifen; Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008, 9, 45–53. [Google Scholar] [CrossRef]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Harbour, R.T.; Caldwell, L.M.; Creed, G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev. 2011, 7, CD000333. [Google Scholar] [CrossRef]
- Fornusek, C.P.; Kilbreath, S.L. Exercise for improving bone health in women treated for stages I-III breast cancer: A systematic review and meta-analyses. J. Cancer Surviv. 2017, 11, 525–541. [Google Scholar] [CrossRef]
- Cummings, S.R.; Bates, D.; Black, D.M. Clinical use of bone densitometry: Scientific review. JAMA 2002, 288, 1889–1897. [Google Scholar] [CrossRef]
- Kling, J.M.; Clarke, B.L.; Sandhu, N.P. Osteoporosis prevention, screening, and treatment: A review. J. Womens Health 2014, 23, 563–572. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Van Poznak, C.; Lacchetti, C.; Kirshner, J.; Eastell, R.; Gagel, R.; Smith, S.; Edwards, B.J.; Frank, E.; Lyman, G.H.; et al. Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2916–2946. [Google Scholar] [CrossRef]
- Love, R.R.; Barden, H.S.; Mazess, R.B.; Epstein, S.; Chappell, R.J. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch. Intern. Med. 1994, 154, 2585–2588. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.J.; Hickish, T.; Kanis, J.A.; Tidy, A.; Ashley, S. Effect of tamoxifen on bone mineral density measured by dual-energy X-ray absorptiometry in healthy premenopausal and postmenopausal women. J. Clin. Oncol. 1996, 14, 78–84. [Google Scholar] [CrossRef]
- Eastell, R.; Hannon, R.A.; Cuzick, J.; Dowsett, M.; Clack, G.; Adams, J.E. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J. Bone Miner. Res. 2006, 21, 1215–1223. [Google Scholar] [CrossRef]
- Fogelman, I.; Blake, G.M.; Blamey, R.; Palmer, M.; Sauerbrei, W.; Schumacher, M.; Serin, D.; Stewart, A.; Wilpshaar, W. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Osteoporos. Int. 2003, 14, 1001–1006. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Manola, J.; Leboff, M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J. Clin. Oncol. 2001, 19, 3306–3311. [Google Scholar] [CrossRef]
- Gnant, M.F.; Mlineritsch, B.; Luschin-Ebengreuth, G.; Grampp, S.; Kaessmann, H.; Schmid, M.; Menzel, C.; Piswanger-Soelkner, J.C.; Galid, A.; Mittlboeck, M.; et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: A report from the Austrian Breast and Colorectal Cancer Study Group. J. Clin. Oncol. 2007, 25, 820–828. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Baum, M.; Buzdar, A.; Howell, A.; Dowsett, M.; Forbes, J.F. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010, 11, 1135–1141. [Google Scholar] [CrossRef]
- Dowsett, M.; Forbes, J.F.; Bradley, R.; Ingle, J.; Aihara, T.; Bliss, J.; Boccardo, F.; Coates, A.; Coombes, R.C.; Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Pant, S.; Shapiro, C.L. Aromatase inhibitor-associated bone loss: Clinical considerations. Drugs 2008, 68, 2591–2600. [Google Scholar] [CrossRef]
- Milat, F.; Vincent, A.J. Management of bone disease in women after breast cancer. Climacteric 2015, 18 (Suppl. 2), 47–55. [Google Scholar] [CrossRef]
- Howell, A.; Cuzick, J.; Baum, M.; Buzdar, A.; Dowsett, M.; Forbes, J.F.; Hoctin-Boes, G.; Houghton, J.; Locker, G.Y.; Tobias, J.S.; et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005, 365, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Rabaglio, M.; Sun, Z.; Price, K.N.; Castiglione-Gertsch, M.; Hawle, H.; Thurlimann, B.; Mouridsen, H.; Campone, M.; Forbes, J.F.; Paridaens, R.J.; et al. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann. Oncol. 2009, 20, 1489–1498. [Google Scholar] [CrossRef]
- van de Velde, C.J.; Rea, D.; Seynaeve, C.; Putter, H.; Hasenburg, A.; Vannetzel, J.M.; Paridaens, R.; Markopoulos, C.; Hozumi, Y.; Hille, E.T.; et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): A randomised phase 3 trial. Lancet 2011, 377, 321–331. [Google Scholar] [CrossRef]
- Jakesz, R.; Jonat, W.; Gnant, M.; Mittlboeck, M.; Greil, R.; Tausch, C.; Hilfrich, J.; Kwasny, W.; Menzel, C.; Samonigg, H.; et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005, 366, 455–462. [Google Scholar] [CrossRef]
- Goss, P.E.; Ingle, J.N.; Martino, S.; Robert, N.J.; Muss, H.B.; Piccart, M.J.; Castiglione, M.; Tu, D.; Shepherd, L.E.; Pritchard, K.I.; et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: Updated findings from NCIC CTG MA.17. J. Natl. Cancer Inst. 2005, 97, 1262–1271. [Google Scholar] [CrossRef]
- Kristensen, B.; Ejlertsen, B.; Mouridsen, H.T.; Andersen, K.W.; Lauritzen, J.B. Femoral fractures in postmenopausal breast cancer patients treated with adjuvant tamoxifen. Breast Cancer Res. Treat. 1996, 39, 321–326. [Google Scholar] [CrossRef]
- Parker, W.H.; Jacoby, V.; Shoupe, D.; Rocca, W. Effect of bilateral oophorectomy on women’s long-term health. Womens Health 2009, 5, 565–576. [Google Scholar] [CrossRef]
- Stearns, V.; Schneider, B.; Henry, N.L.; Hayes, D.F.; Flockhart, D.A. Breast cancer treatment and ovarian failure: Risk factors and emerging genetic determinants. Nat. Rev. Cancer 2006, 6, 886–893. [Google Scholar] [CrossRef]
- Gracia, C.R.; Sammel, M.D.; Freeman, E.; Prewitt, M.; Carlson, C.; Ray, A.; Vance, A.; Ginsberg, J.P. Impact of cancer therapies on ovarian reserve. Fertil. Steril. 2012, 97, 134–140. [Google Scholar] [CrossRef]
- Col, N.F.; Ochs, L.; Springmann, V.; Aragaki, A.K.; Chlebowski, R.T. Metformin and breast cancer risk: A meta-analysis and critical literature review. Breast Cancer Res. Treat. 2012, 135, 639–646. [Google Scholar] [CrossRef]
- Partridge, A.; Gelber, S.; Gelber, R.D.; Castiglione-Gertsch, M.; Goldhirsch, A.; Winer, E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: Long-term results from International Breast Cancer Study Group Trials V and VI. Eur. J. Cancer 2007, 43, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Avenell, A.; Mak, J.C.; O’Connell, D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014, 2014, CD000227. [Google Scholar] [CrossRef]
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium intake and risk of fracture: Systematic review. BMJ 2015, 351, h4580. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pang, Q. The effect of vitamin D and calcium supplementation on falls in older adults: A systematic review and meta-analysis. Orthopade 2017, 46, 729–736. [Google Scholar] [CrossRef]
- Dhaliwal, R.; Aloia, J.F. Effect of Vitamin D on Falls and Physical Performance. Endocrinol. Metab. Clin. N. Am. 2017, 46, 919–933. [Google Scholar] [CrossRef]
- Datta, M.; Schwartz, G.G. Calcium and vitamin D supplementation and loss of bone mineral density in women undergoing breast cancer therapy. Crit. Rev. Oncol. Hematol. 2013, 88, 613–624. [Google Scholar] [CrossRef]
- Hadji, P.; Aapro, M.S.; Body, J.J.; Gnant, M.; Brandi, M.L.; Reginster, J.Y.; Zillikens, M.C.; Gluer, C.C.; de Villiers, T.; Baber, R.; et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J. Bone Oncol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Tremollieres, F.A.; Ceausu, I.; Depypere, H.; Lambrinoudaki, I.; Mueck, A.; Perez-Lopez, F.R.; van der Schouw, Y.T.; Senturk, L.M.; Simoncini, T.; Stevenson, J.C.; et al. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas 2017, 95, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Suskin, J.; Shapiro, C.L. Osteoporosis and musculoskeletal complications related to therapy of breast cancer. Gland. Surg. 2018, 7, 411–423. [Google Scholar] [CrossRef]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Friedman, C.F.; DeMichele, A.; Su, H.I.; Feng, R.; Kapoor, S.; Desai, K.; Mao, J.J. Vitamin d deficiency in postmenopausal breast cancer survivors. J. Womens Health 2012, 21, 456–462. [Google Scholar] [CrossRef]
- Nogues, X.; Servitja, S.; Pena, M.J.; Prieto-Alhambra, D.; Nadal, R.; Mellibovsky, L.; Albanell, J.; Diez-Perez, A.; Tusquets, I. Vitamin D deficiency and bone mineral density in postmenopausal women receiving aromatase inhibitors for early breast cancer. Maturitas 2010, 66, 291–297. [Google Scholar] [CrossRef]
- Aspray, T.J. Fragility fracture: Recent developments in risk assessment. Ther. Adv. Musculoskelet. Dis. 2015, 7, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Reddy, S.; Berkman, N.; Cullen, K.; Middleton, J.C.; Nicholson, W.K.; Kahwati, L.C. Screening to Prevent Osteoporotic Fractures: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 2532–2551. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Giralt, N.; Pineda-Moncusi, M.; Overjero, D.; Ovejero, I.; Soldada-Folgado, J.; Campodarve, J. Risk factors for Incident fracture in patients with breast cancer treated with aromatase inhibitors: B-ABLE cohort. Rev. Osteoporos. Metab. Miner. 2020, 12, 1–7. [Google Scholar]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Strom, O.; Borgstrom, F. Development and use of FRAX in osteoporosis. Osteoporos. Int. 2010, 21 (Suppl. 2), S407–S413. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Morin, S.N.; Lix, L.M.; Niraula, S.; McCloskey, E.V.; Johansson, H.; Harvey, N.C.; Kanis, J.A. Performance of FRAX in Women with Breast Cancer Initiating Aromatase Inhibitor Therapy: A Registry-Based Cohort Study. J. Bone Miner. Res. 2019, 34, 1428–1435. [Google Scholar] [CrossRef]
- Leslie, W.D.; Morin, S.N.; Lix, L.M.; Niraula, S.; McCloskey, E.V.; Johansson, H.; Harvey, N.C.; Kanis, J.A. Fracture Risk in Women with Breast Cancer Initiating Aromatase Inhibitor Therapy: A Registry-Based Cohort Study. Oncologist 2019, 24, 1432–1438. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef]
- Hadji, P. Cancer Treatment-Induced Bone Loss in women with breast cancer. Bonekey Rep. 2015, 4, 692. [Google Scholar] [CrossRef]
- Gralow, J.R.; Biermann, J.S.; Farooki, A.; Fornier, M.N.; Gagel, R.F.; Kumar, R.N.; Shapiro, C.L.; Shields, A.; Smith, M.R.; Srinivas, S.; et al. NCCN Task Force Report: Bone Health in Cancer Care. J. Natl. Compr. Cancer Netw. 2009, 7 (Suppl. 3), S1–S32. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Cosman, F.; Lewiecki, E.M.; Schousboe, J.T.; Bauer, D.C.; Black, D.M.; Brown, T.D.; Cheung, A.M.; Cody, K.; Cooper, C.; et al. Goal-Directed Treatment for Osteoporosis: A Progress Report From the ASBMR-NOF Working Group on Goal-Directed Treatment for Osteoporosis. J. Bone Miner. Res. 2017, 32, 3–10. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; Johansson, H.; Liu, E.; Vandenput, L.; Lorentzon, M.; Leslie, W.D.; McCloskey, E.V. A decade of FRAX: How has it changed the management of osteoporosis? Aging Clin. Exp. Res. 2020, 32, 187–196. [Google Scholar] [CrossRef]
- Clezardin, P.; Ebetino, F.H.; Fournier, P.G. Bisphosphonates and cancer-induced bone disease: Beyond their antiresorptive activity. Cancer Res. 2005, 65, 4971–4974. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, M.R.; Mansinho, A.; Abreu, C.; Cavaco-Silva, J.; Casimiro, S.; Costa, L. Clinical and translational pharmacology of drugs for the prevention and treatment of bone metastases and cancer-induced bone loss. Br. J. Clin. Pharmacol. 2019, 85, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Fleisch, H.A. Bisphosphonates: Mechanisms of action. J. Clin. Invest. 1996, 97, 2692–2696. [Google Scholar] [CrossRef]
- Deeks, E.D. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging 2018, 35, 163–173. [Google Scholar] [CrossRef]
- Heeke, A.; Nunes, M.R.; Lynce, F. Bone-Modifying Agents in Early-Stage and Advanced Breast Cancer. Curr. Breast Cancer Rep. 2018, 10, 241–250. [Google Scholar] [CrossRef]
- Cremers, S.; Ebetino, F.H.; Phipps, R. On the pharmacological evaluation of bisphosphonates in humans. Bone 2020, 139, 115501. [Google Scholar] [CrossRef]
- Dahiya, N.; Khadka, A.; Sharma, A.K.; Gupta, A.K.; Singh, N.; Brashier, D.B. Denosumab: A bone antiresorptive drug. Med. J. Armed Forces India 2015, 71, 71–75. [Google Scholar] [CrossRef]
- de Groot, A.F.; Appelman-Dijkstra, N.M.; van der Burg, S.H.; Kroep, J.R. The anti-tumor effect of RANKL inhibition in malignant solid tumors—A systematic review. Cancer Treat. Rev. 2018, 62, 18–28. [Google Scholar] [CrossRef]
- Nakai, Y.; Okamoto, K.; Terashima, A.; Ehata, S.; Nishida, J.; Imamura, T.; Ono, T.; Takayanagi, H. Efficacy of an orally active small-molecule inhibitor of RANKL in bone metastasis. Bone Res. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Z.K.; Yu, Y.; Zhuo, Z.; Zhang, G.; Zhang, B.T. Pros and Cons of Denosumab Treatment for Osteoporosis and Implication for RANKL Aptamer Therapy. Front. Cell Dev. Biol. 2020, 8, 325. [Google Scholar] [CrossRef]
- Yarom, N.; Shapiro, C.L.; Peterson, D.E.; Van Poznak, C.H.; Bohlke, K.; Ruggiero, S.L.; Migliorati, C.A.; Khan, A.; Morrison, A.; Anderson, H.; et al. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2270–2290. [Google Scholar] [CrossRef] [PubMed]
- Sestak, I.; Singh, S.; Cuzick, J.; Blake, G.M.; Patel, R.; Gossiel, F.; Coleman, R.; Dowsett, M.; Forbes, J.F.; Howell, A.; et al. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: An international, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2014, 15, 1460–1468. [Google Scholar] [CrossRef]
- Rossini, M.; Orsolini, G.; Adami, S.; Kunnathully, V.; Gatti, D. Osteoporosis treatment: Why ibandronic acid? Expert Opin. Pharmacother. 2013, 14, 1371–1381. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- Cummings, S.R.; Ferrari, S.; Eastell, R.; Gilchrist, N.; Jensen, J.B.; McClung, M.; Roux, C.; Torring, O.; Valter, I.; Wang, A.T.; et al. Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J. Bone Miner. Res. 2018, 33, 190–198. [Google Scholar] [CrossRef]
- Freemantle, N.; Cooper, C.; Diez-Perez, A.; Gitlin, M.; Radcliffe, H.; Shepherd, S.; Roux, C. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: A meta-analysis. Osteoporos. Int. 2013, 24, 209–217. [Google Scholar] [CrossRef]
- Levis, S.; Theodore, G. Summary of AHRQ’s comparative effectiveness review of treatment to prevent fractures in men and women with low bone density or osteoporosis: Update of the 2007 report. J. Manag. Care Pharm. 2012, 18, S1–S15. [Google Scholar] [CrossRef]
- Wilson, C.; Bell, R.; Hinsley, S.; Marshall, H.; Brown, J.; Cameron, D.; Dodwell, D.; Coleman, R. Adjuvant zoledronic acid reduces fractures in breast cancer patients; an AZURE (BIG 01/04) study. Eur. J. Cancer 2018, 94, 70–78. [Google Scholar] [CrossRef]
- Coleman, R.; de Boer, R.; Eidtmann, H.; Llombart, A.; Davidson, N.; Neven, P.; von Minckwitz, G.; Sleeboom, H.P.; Forbes, J.; Barrios, C.; et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): Final 60-month results. Ann. Oncol. 2013, 24, 398–405. [Google Scholar] [CrossRef]
- Hershman, D.L.; McMahon, D.J.; Crew, K.D.; Cremers, S.; Irani, D.; Cucchiara, G.; Brafman, L.; Shane, E. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J. Clin. Oncol. 2008, 26, 4739–4745. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Halabi, S.; Hars, V.; Archer, L.; Weckstein, D.; Kirshner, J.; Sikov, W.; Winer, E.; Burstein, H.J.; Hudis, C.; et al. Zoledronic acid preserves bone mineral density in premenopausal women who develop ovarian failure due to adjuvant chemotherapy: Final results from CALGB trial 79809. Eur. J. Cancer 2011, 47, 683–689. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Luschin-Ebengreuth, G.; Kainberger, F.; Kassmann, H.; Piswanger-Solkner, J.C.; Seifert, M.; Ploner, F.; Menzel, C.; Dubsky, P.; et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008, 9, 840–849. [Google Scholar] [CrossRef]
- Brufsky, A.; Harker, W.G.; Beck, J.T.; Carroll, R.; Tan-Chiu, E.; Seidler, C.; Hohneker, J.; Lacerna, L.; Petrone, S.; Perez, E.A. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J. Clin. Oncol. 2007, 25, 829–836. [Google Scholar] [CrossRef]
- Ellis, G.K.; Bone, H.G.; Chlebowski, R.; Paul, D.; Spadafora, S.; Smith, J.; Fan, M.; Jun, S. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J. Clin. Oncol. 2008, 26, 4875–4882. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Van Poznak, C.; Hannon, R.A.; Mackey, J.R.; Campone, M.; Apffelstaedt, J.P.; Clack, G.; Barlow, D.; Makris, A.; Eastell, R. Prevention of aromatase inhibitor-induced bone loss using risedronate: The SABRE trial. J. Clin. Oncol. 2010, 28, 967–975. [Google Scholar] [CrossRef]
- Gralow, J.R.; Barlow, W.E.; Paterson, A.H.G.; M’Iao, J.L.; Lew, D.L.; Stopeck, A.T.; Hayes, D.F.; Hershman, D.L.; Schubert, M.M. Phase III Randomized Trial of Bisphosphonates as Adjuvant Therapy in Breast Cancer: S0307. J. Natl. Cancer Inst. 2020, 112, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Hiligsmann, M.; Bours, S.P.; Boonen, A. A Review of Patient Preferences for Osteoporosis Drug Treatment. Curr. Rheumatol. Rep. 2015, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Morizio, P.; Burkhart, J.I.; Ozawa, S. Denosumab: A Unique Perspective on Adherence and Cost-effectiveness Compared With Oral Bisphosphonates in Osteoporosis Patients. Ann. Pharmacother. 2018, 52, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Zhang, Y.; Wu, D.B.; Liu, J.; Qian, X.; Luo, N.; Mori, T. Cost-Effectiveness of Zoledronic Acid Versus Oral Alendronate for Postmenopausal Osteoporotic Women in China. Front. Pharmacol. 2020, 11, 456. [Google Scholar] [CrossRef]
- Canon, J.R.; Roudier, M.; Bryant, R.; Morony, S.; Stolina, M.; Kostenuik, P.J.; Dougall, W.C. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin. Exp. Metastasis 2008, 25, 119–129. [Google Scholar] [CrossRef]
- Beuzeboc, P.; Scholl, S. Prevention of Bone Metastases in Breast Cancer Patients. Therapeutic Perspectives. J. Clin. Med. 2014, 3, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Eber, M.R.; Berry, J.E.; Taichman, R.S. Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. Bonekey Rep. 2015, 4, 689. [Google Scholar] [CrossRef]
- Domschke, C.; Diel, I.J.; Englert, S.; Kalteisen, S.; Mayer, L.; Rom, J.; Heil, J.; Sohn, C.; Schuetz, F. Prognostic value of disseminated tumor cells in the bone marrow of patients with operable primary breast cancer: A long-term follow-up study. Ann. Surg. Oncol. 2013, 20, 1865–1871. [Google Scholar] [CrossRef]
- Aft, R.; Naughton, M.; Trinkaus, K.; Watson, M.; Ylagan, L.; Chavez-MacGregor, M.; Zhai, J.; Kuo, S.; Shannon, W.; Diemer, K.; et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: An open label, randomised, phase 2 trial. Lancet Oncol. 2010, 11, 421–428. [Google Scholar] [CrossRef]
- Banys, M.; Solomayer, E.F.; Gebauer, G.; Janni, W.; Krawczyk, N.; Lueck, H.J.; Becker, S.; Huober, J.; Kraemer, B.; Wackwitz, B.; et al. Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: Results of a prospective clinical trial. BMC Cancer 2013, 13, 480. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Knauer, M.; Moik, M.; Jakesz, R.; Seifert, M.; Taucher, S.; Bjelic-Radisic, V.; et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: Final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 2015, 26, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Marshall, H.; Cameron, D.; Dodwell, D.; Burkinshaw, R.; Keane, M.; Gil, M.; Houston, S.J.; Grieve, R.J.; Barrett-Lee, P.J.; et al. Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 2011, 365, 1396–1405. [Google Scholar] [CrossRef]
- Paterson, A.H.; Anderson, S.J.; Lembersky, B.C.; Fehrenbacher, L.; Falkson, C.I.; King, K.M.; Weir, L.M.; Brufsky, A.M.; Dakhil, S.; Lad, T.; et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): A multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012, 13, 734–742. [Google Scholar] [CrossRef]
- Coleman, R.; Cameron, D.; Dodwell, D.; Bell, R.; Wilson, C.; Rathbone, E.; Keane, M.; Gil, M.; Burkinshaw, R.; Grieve, R.; et al. Adjuvant zoledronic acid in patients with early breast cancer: Final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014, 15, 997–1006. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative, G.; Coleman, R.; Powles, T.; Paterson, A.; Gnant, M.; Anderson, S.; Diel, I.; Gralow, J.; von Minckwitz, G.; Moebus, V.; et al. Adjuvant bisphosphonate treatment in early breast cancer: Meta-analyses of individual patient data from randomised trials. Lancet 2015, 386, 1353–1361. [Google Scholar] [CrossRef]
- O’Carrigan, B.; Wong, M.H.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017, 10, CD003474. [Google Scholar] [CrossRef]
- Dhesy-Thind, S.; Fletcher, G.G.; Blanchette, P.S.; Clemons, M.J.; Dillmon, M.S.; Frank, E.S.; Gandhi, S.; Gupta, R.; Mates, M.; Moy, B.; et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 2062–2081. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Balic, M.; Thomssen, C.; Wurstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Perianez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant denosumab in early breast cancer (D-CARE): An international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 60–72. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Steger, G.G.; Egle, D.; Greil, R.; Fitzal, F.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger-Zeinitzer, E.; et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): Disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 339–351. [Google Scholar] [CrossRef]
- Tremblay, D.; Patel, V.; Fifer, K.M.; Caro, J.; Kolodka, O.; Mandelli, J.; Shapiro, C.L. Management of bone health in postmenopausal women on aromatase inhibitors (AIs): A single health care system experience. Support. Care Cancer 2018, 26, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; Lix, L.M.; Azimaee, M.; Metge, C.; Caetano, P.; Leslie, W.D. Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos. Int. 2011, 22, 2439–2448. [Google Scholar] [CrossRef]



| Risk Factor in General Population | With BMD | Ref | |
|---|---|---|---|
| RR | 95% CI | ||
| Parental History of Non-Traumatic Fracture | 2.11 | 1.41–3.14 | [18] |
| Ever Use of Steroids α | 2.25 | 1.60–3.15 | [19] |
| Rheumatoid Arthritis α | 1.73 | 0.94–2.30 | [20] |
| Alcohol Intake of More than 2–3 Drinks/Day | 1.70 | 1.20–2.42 | [21] |
| Prior Non-Traumatic Fracture after Age 50 years α | 1.62 | 1.30–2.01 | [22] |
| Current Smoking | 1.60 | 1.27–2.02 | [23] |
| Low Body Mass Index α | 1.42 | 1.23–1.65 | [24] |
| Risk factors for Fractures in Women with Early Stage Breast Cancer | |||
| Hypogonadism (CIOF or GnRH agonist +/− AI) | NA | ||
| Oophorectomy | 1.54 δ | 1.29–1.82 | [25] |
| AI | 1.55 ∗,β | 1.31–1.83 | [26] |
| Trial | n | Follow-Up (mo.) | Treatment | Fractures (%) | p-Value | Ref |
|---|---|---|---|---|---|---|
| AI vs. TAM | ||||||
| ATAC | 9336 | 100 | ANA vs. TAM | 11 vs. 7.7 | <0.001 | [42] |
| BIG 1-98 | 4922 | 60 | LET vs. TAM | 9.3 vs. 6.5 | 0.002 | [43] |
| AI after 2–3 years. of TAM | ||||||
| TEAM | 9779 | 61 | EXE vs. TAM | 5.0 vs. 3.0 | 0.0001 | [44] |
| ABCSG8/ARNO | 3224 | 28 | ANA vs. TAM | 2.0 vs. 1.0 | 0.015 | [45] |
| AI after 5 years. of TAM | ||||||
| MA-17 | 5187 | 63 | LET vs. TAM | 5.2 vs. 3.1 | 0.02 | [46] |
| Factor | ZA (iv) | DEN (sc) |
|---|---|---|
| Dose | 4 or 5 mg † | 60 mg |
| Mechanism | Osteoclast inhibitor | RANKL monoclonal antibody |
| Metabolism | Not Metabolized | Not Metabolized |
| Half-life | 2.5 h ¶, 188 days ¥ | 28 days |
| Clearance | Renal | RES |
| Common side effects | Fever, chills; muscle, bone or joint pain; nausea; fatigue; headaches | Joint, muscle pains; hypocalcemia |
| Rare side effects | Osteonecrosis; renal insufficiency §; atypical femur fractures [89] | Osteonecrosis; rebound vertebral fractures [90] |
| Dose modifications | Renal insufficiency (creatinine clearance < 30 mL/min) | --- |
| Costs & (US dollars) | 252.00 | 1906.00 |
| Trial | Treatments | n | Results (L/S BMD) † | p Value | Ref |
|---|---|---|---|---|---|
| CIOF | |||||
| Hershman | ZA 4 mg q3 mo for 1 yr. vs. placebo | 101 | 0 vs. −3.0 | <0.001 | [95] |
| Shapiro | ZA 4 mg q3 mo for 1 yr vs. control | 441 | 1.2 vs. −6.7 | <0.001 | [96] |
| Gnant | ZA 4 mg q6 mo for 3 yrs vs. control | 401 | 4.0 vs. −6.7 | 0.02 | [97] |
| AI | |||||
| Brufsky | ZA 4 mg iv q6 mo for 1 yr vs. delayed | 502 | 2.0 vs. −2.5 | <0.001 | [98] |
| Coleman | ZA 4 mg iv q6 mo for 5 yrs vs. delayed | 1065 | 4.3 vs. −5.4 | <0.0001 | [94] |
| Ellis | DEN 60 mg sc q6 mo for 2 years vs. placebo | 262 | 6.0 vs. −1.6 | <0.0001 | [99] |
| Gnant | DEN 60 mg sc q6 mo for 5 years vs. placebo | 3425 | HR fractures = 0.50 95% CI 0.39–0.65 | <0.0001 | [100] |
| Van Poznak | Risedronate oral 35 mg/week for 2 years vs. placebo | 111 | 2.2 vs. −1.85 | <0.0001 | [101] |
| Sestak | Risedronate oral 35 mg/week for 3 years vs. placebo | 150 | 1.1 vs. −2.6 | <0.0001 | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapiro, C.L. Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers 2020, 12, 3094. https://doi.org/10.3390/cancers12113094
Shapiro CL. Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers. 2020; 12(11):3094. https://doi.org/10.3390/cancers12113094
Chicago/Turabian StyleShapiro, Charles L. 2020. "Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments" Cancers 12, no. 11: 3094. https://doi.org/10.3390/cancers12113094
APA StyleShapiro, C. L. (2020). Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers, 12(11), 3094. https://doi.org/10.3390/cancers12113094





