Role of Nurr1 in Carcinogenesis and Tumor Immunology: A State of the Art Review
Simple Summary
Abstract
1. Introduction
2. Expression and Function of Nurr1 in Cancer
2.1. Breast Cancer
2.2. Bladder Cancer
2.3. Gastrointestinal Cancer
2.4. Lung Cancer
2.5. Cervical Cancer
2.6. Prostate Cancer
2.7. Pancreatic Cancer
2.8. Brain Cancer
2.9. Hematological Cancers
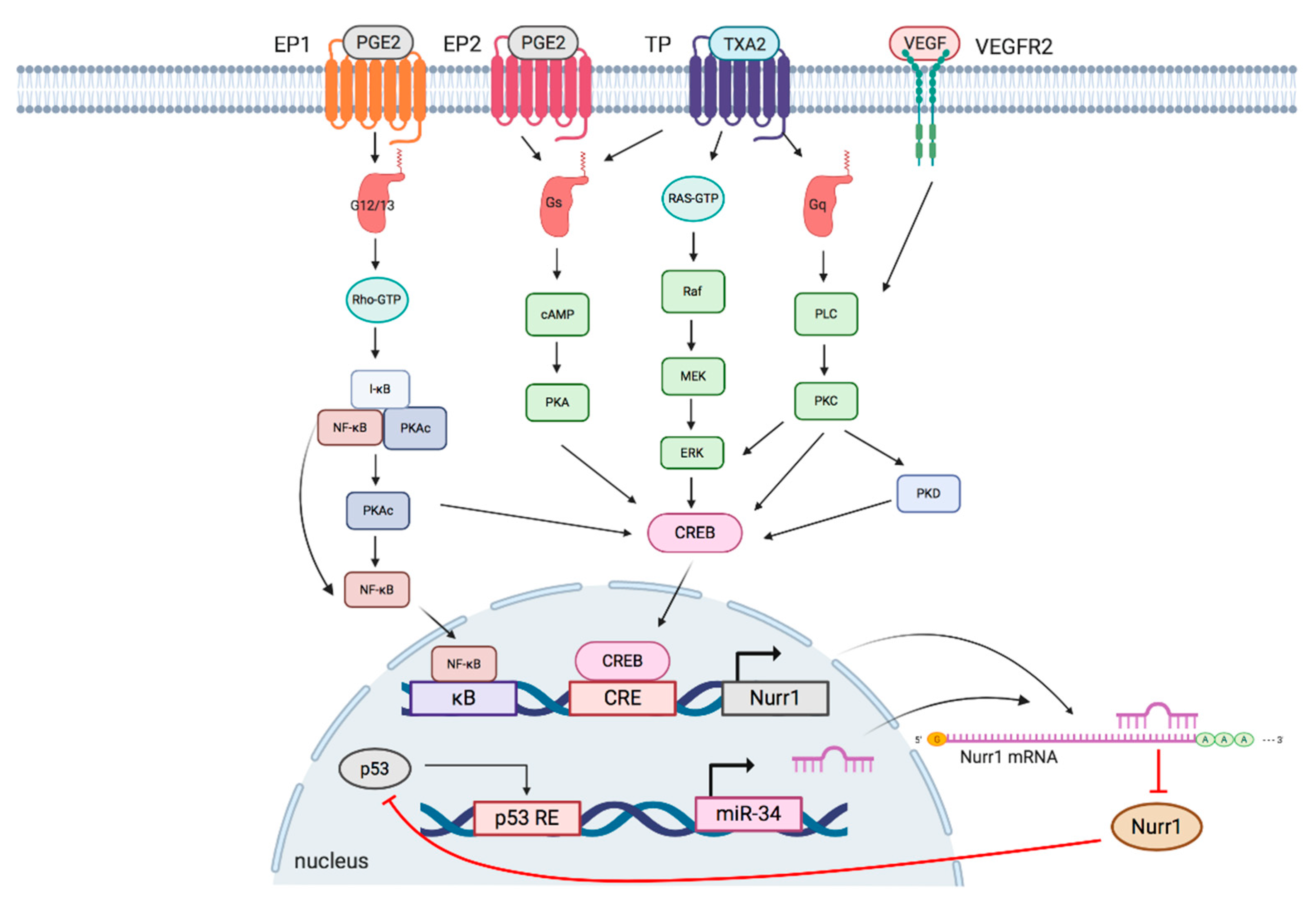
3. Signaling Pathways Regulating Nurr1 Expression
3.1. TXA2 Pathway
3.2. PGE2 Pathway
3.3. P53/miR-34/Nurr1 Loop
3.4. VEGF/Protein Kinase D (PKD) Pathway
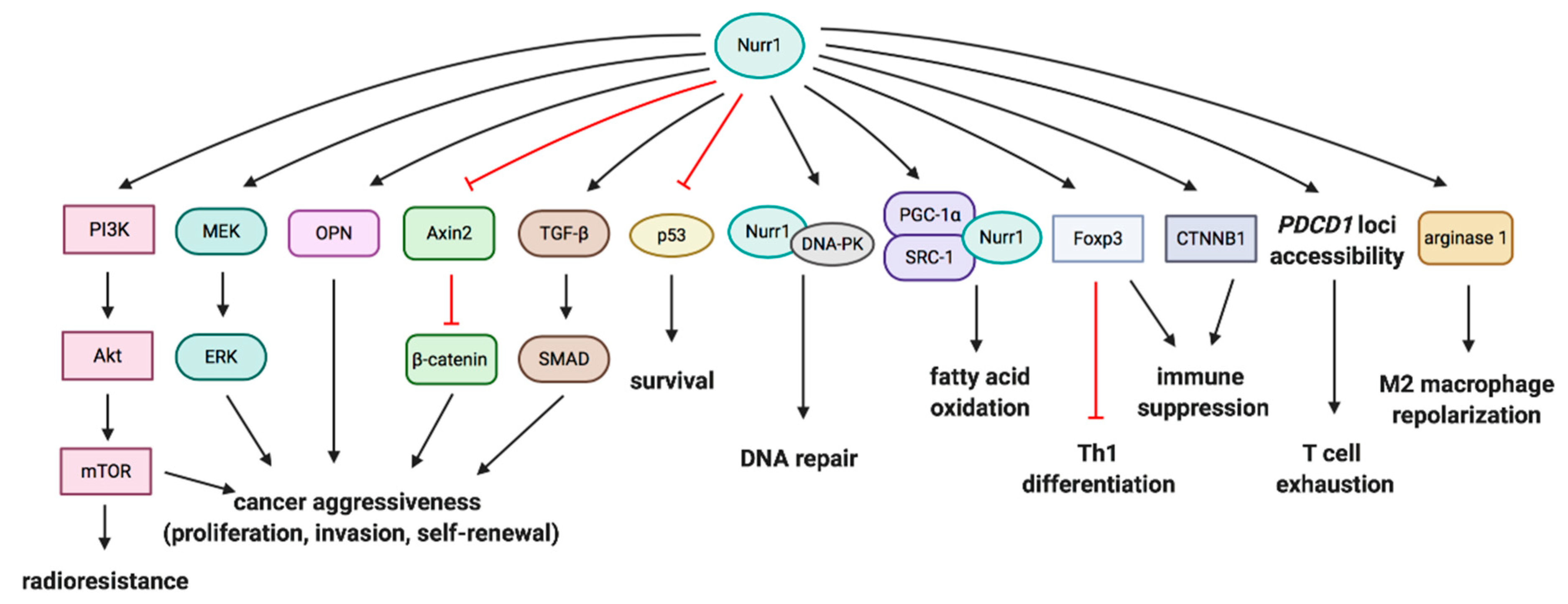
4. Crosstalk of Nurr1 with Pro-Tumorigenic and Tumor-Suppressive Molecules
4.1. PI3K/Akt/mTOR and MEK/ERK Pathways
4.2. Wnt/β-Catenin Signaling
4.3. P53
4.4. DNA-Dependent Protein Kinase (DNA-PK)
4.5. Fatty Acid Oxidation Pathway
4.6. Transforming Growth Factor-Beta (TGF-β) Pathway
5. Nurr1 and Inhibition of Antitumor Immunity
5.1. Treg-Mediated Immunosuppression
5.2. COX-2/Nurr1 Axis in Immunosuppression
5.3. Suppression of Anti-Leukemia Immunity
5.4. Treg Development
5.5. T Cell Exhaustion
5.6. M2 Macrophage Repolarization
5.7. Suppression of Immunotherapy
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giguere, V. Orphan nuclear receptors: From gene to function. Endocr. Rev. 1999, 20, 689–725. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, M.A.; Muscat, G.E. The NR4A subgroup: Immediate early response genes with pleiotropic physiological roles. Nucl. Recept. Signal. 2006, 4, e002. [Google Scholar] [CrossRef]
- Beard, J.A.; Tenga, A.; Chen, T. The interplay of NR4A receptors and the oncogene-tumor suppressor networks in cancer. Cell. Signal. 2015, 27, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sirin, O.; Lukov, G.L.; Mao, R.; Conneely, O.M.; Goodell, M.A. The orphan nuclear receptor Nurr1 restricts the proliferation of haematopoietic stem cells. Nat. Cell Biol. 2010, 12, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Cardenas, O.; Kardon, R.; Ediger, T.R.; Lydon, J.P.; Conneely, O.M. Cloning and structural organization of the gene encoding the murine nuclear receptor transcription factor, NURR1. Gene 1997, 187, 135–139. [Google Scholar] [CrossRef]
- Zhao, Y.; Bruemmer, D. NR4A Orphan Nuclear Receptors in Cardiovascular Biology. Drug Discov. Today Dis. Mech. 2009, 6, e43–e48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Benoit, G.; Liu, J.; Prasad, S.; Aarnisalo, P.; Liu, X.; Xu, H.; Walker, N.P.; Perlmann, T. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 2003, 423, 555–560. [Google Scholar] [CrossRef]
- Jakaria, M.; Haque, M.E.; Cho, D.-Y.; Azam, S.; Kim, I.-S.; Choi, D.-K. Molecular Insights into NR4A2(Nurr1): An Emerging Target for Neuroprotective Therapy Against Neuroinflammation and Neuronal Cell Death. Mol. Neurobiol. 2019, 56, 5799–5814. [Google Scholar] [CrossRef] [PubMed]
- Bushue, N.; Wan, Y.J. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298. [Google Scholar] [CrossRef]
- Zagani, R.; Hamzaoui, N.; Cacheux, W.; de Reynies, A.; Terris, B.; Chaussade, S.; Romagnolo, B.; Perret, C.; Lamarque, D. Cyclooxygenase-2 inhibitors down-regulate osteopontin and Nr4A2-new therapeutic targets for colorectal cancers. Gastroenterology 2009, 137, 1358–1366. [Google Scholar] [CrossRef]
- Ke, N.; Claassen, G.; Yu, D.H.; Albers, A.; Fan, W.; Tan, P.; Grifman, M.; Hu, X.; Defife, K.; Nguy, V.; et al. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res. 2004, 64, 8208–8212. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Zou, Y.; Huang, G.L.; He, Z.W. Orphan nuclear receptor nurr1 as a potential novel marker for progression in human prostate cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Gong, C.; Ge, L.; Song, L.; Chen, F.; Jin, C.; Zhu, H.; Zhou, G. Orphan nuclear receptor Nurr1 as a potential novel marker for progression in human pancreatic ductal adenocarcinoma. Exp. Ther. Med. 2017, 13, 551–559. [Google Scholar] [CrossRef]
- Han, Y.; Cai, H.; Ma, L.; Ding, Y.; Tan, X.; Chang, W.; Guan, W.; Liu, Y.; Shen, Q.; Yu, Y.; et al. Expression of orphan nuclear receptor NR4A2 in gastric cancer cells confers chemoresistance and predicts an unfavorable postoperative survival of gastric cancer patients with chemotherapy. Cancer 2013, 119, 3436–3445. [Google Scholar] [CrossRef]
- Han, Y.; Cai, H.; Ma, L.; Ding, Y.; Tan, X.; Liu, Y.; Su, T.; Yu, Y.; Chang, W.; Zhang, H.; et al. Nuclear orphan receptor NR4A2 confers chemoresistance and predicts unfavorable prognosis of colorectal carcinoma patients who received postoperative chemotherapy. Eur J. Cancer 2013, 49, 3420–3430. [Google Scholar] [CrossRef] [PubMed]
- Kok-Ting Wan, P.; Ho-Yin Leung, T.; Kwan-Yee Siu, M.; Mo, X.; Wai-Man Tang, H.; Kar-Loen Chan, K.; Nga-Yin Cheung, A.; Yuen-Sheung Ngan, H. HPV-induced Nurr1 promotes cancer aggressiveness, self-renewal, and radioresistance via ERK and AKT signaling in cervical cancer. Cancer Lett. 2020. [Google Scholar] [CrossRef]
- Holla, V.R.; Mann, J.R.; Shi, Q.; DuBois, R.N. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J. Biol. Chem. 2006, 281, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tai, H.H. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis 2009, 30, 1606–1613. [Google Scholar] [CrossRef]
- Inamoto, T.; Czerniak, B.A.; Dinney, C.P.; Kamat, A.M. Cytoplasmic mislocalization of the orphan nuclear receptor Nurr1 is a prognostic factor in bladder cancer. Cancer 2010, 116, 340–346. [Google Scholar] [CrossRef]
- Misund, K.; Selvik, L.K.; Rao, S.; Norsett, K.; Bakke, I.; Sandvik, A.K.; Laegreid, A.; Bruland, T.; Prestvik, W.S.; Thommesen, L. NR4A2 is regulated by gastrin and influences cellular responses of gastric adenocarcinoma cells. PLoS ONE 2013, 8, e76234. [Google Scholar] [CrossRef]
- Maruyama, K.; Tsukada, T.; Bandoh, S.; Sasaki, K.; Ohkura, N.; Yamaguchi, K. Retinoic acids differentially regulate NOR-1 and its closely related orphan nuclear receptor genes in breast cancer cell line MCF-7. Biochem. Biophys. Res. Commun. 1997, 231, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Llopis, S.; Singleton, B.; Duplessis, T.; Carrier, L.; Rowan, B.; Williams, C. Dichotomous roles for the orphan nuclear receptor NURR1 in breast cancer. BMC Cancer 2013, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, T.; Papineni, S.; Chintharlapalli, S.; Cho, S.D.; Safe, S.; Kamat, A.M. 1,1-Bis(3′-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol. Cancer Ther. 2008, 7, 3825–3833. [Google Scholar] [CrossRef]
- Chang, W.; Ma, L.; Lin, L.; Gu, L.; Liu, X.; Cai, H.; Yu, Y.; Tan, X.; Zhai, Y.; Xu, X.; et al. Identification of novel hub genes associated with liver metastasis of gastric cancer. Int. J. Cancer 2009, 125, 2844–2853. [Google Scholar] [CrossRef]
- Sun, L.; Liu, M.; Sun, G.C.; Yang, X.; Qian, Q.; Feng, S.; Mackey, L.V.; Coy, D.H. Notch Signaling Activation in Cervical Cancer Cells Induces Cell Growth Arrest with the Involvement of the Nuclear Receptor NR4A2. J. Cancer 2016, 7, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Karki, K.; Li, X.; Jin, U.-H.; Mohankumar, K.; Zarei, M.; Michelhaugh, S.K.; Mittal, S.; Tjalkens, R.; Safe, S. Nuclear receptor 4A2 (NR4A2) is a druggable target for glioblastomas. J. Neuro Oncol. 2020, 146, 25–39. [Google Scholar] [CrossRef]
- Lin, B.; Kolluri, S.K.; Lin, F.; Liu, W.; Han, Y.H.; Cao, X.; Dawson, M.I.; Reed, J.C.; Zhang, X.K. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 2004, 116, 527–540. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. 2013, 19, 502–510. [Google Scholar] [CrossRef]
- Norregaard, R.; Kwon, T.H.; Frokiaer, J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res. Clin. Pract. 2015, 34, 194–200. [Google Scholar] [CrossRef]
- Likui, W.; Hong, W.; Shuwen, Z. Clinical significance of the upregulated osteopontin mRNA expression in human colorectal cancer. J. Gastrointest. Surg. 2010, 14, 74–81. [Google Scholar] [CrossRef]
- Agrawal, D.; Chen, T.; Irby, R.; Quackenbush, J.; Chambers, A.F.; Szabo, M.; Cantor, A.; Coppola, D.; Yeatman, T.J. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J. Natl. Cancer Inst. 2002, 94, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.F.; Cao, G.W. Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J. Gastroenterol. 2012, 18, 6865–6873. [Google Scholar] [CrossRef]
- Zhao, D.; Desai, S.; Zeng, H. VEGF stimulates PKD-mediated CREB-dependent orphan nuclear receptor Nurr1 expression: Role in VEGF-induced angiogenesis. Int. J. Cancer 2011, 128, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Chen, Y.-L.; Chiu, S.-C.; Yu, Y.-L.; Chen, S.-P.; Chien, M.-H.; Chen, K.-Y.; Chang, W.-L.; Lin, S.-Z.; Chiou, T.-W.; et al. Orphan nuclear receptor, Nurr-77 was a possible target gene of butylidenephthalide chemotherapy on glioblastoma multiform brain tumor. J. Neurochem. 2008, 106, 1017–1026. [Google Scholar] [CrossRef]
- Mullican, S.E.; Zhang, S.; Konopleva, M.; Ruvolo, V.; Andreeff, M.; Milbrandt, J.; Conneely, O.M. Abrogation of nuclear receptors Nr4a3 andNr4a1 leads to development of acute myeloid leukemia. Nat. Med. 2007, 13, 730–735. [Google Scholar] [CrossRef]
- Ramirez-Herrick, A.M.; Mullican, S.E.; Sheehan, A.M.; Conneely, O.M. Reduced NR4A gene dosage leads to mixed myelodysplastic/myeloproliferative neoplasms in mice. Blood 2011, 117, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, K.; Troppan, K.; Neumeister, P.; Deutsch, A.J. The nuclear orphan receptor NR4A1 and NR4A3 as tumor suppressors in hematologic neoplasms. Curr. Drug Targets 2015, 16, 38–46. [Google Scholar] [CrossRef]
- Ariës, I.M.; van den Dungen, E.S.R.; Pieters, R.; den Boer, M.L. The NR4A orphan nuclear receptors do not confer prednisolone resistance in pediatric acute lymphoblastic leukemia. Leukemia 2014, 28, 422–425. [Google Scholar] [CrossRef]
- Ji, R.; Sanchez, C.M.; Chou, C.L.; Chen, X.B.; Woodward, D.F.; Regan, J.W. Prostanoid EP(1) receptors mediate up-regulation of the orphan nuclear receptor Nurr1 by cAMP-independent activation of protein kinase A, CREB and NF-kappaB. Br. J. Pharm. 2012, 166, 1033–1046. [Google Scholar] [CrossRef]
- Beard, J.A.; Tenga, A.; Hills, J.; Hoyer, J.D.; Cherian, M.T.; Wang, Y.D.; Chen, T. The orphan nuclear receptor NR4A2 is part of a p53-microRNA-34 network. Sci. Rep. 2016, 6, 25108. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujii, T.; Takahashi, Y.; Takahashi, Y.; Suzuki, T.; Ukai, M.; Tauchi, K.; Horikawa, N.; Tsukada, K.; Sakai, H. Up-regulation of Kv7.1 channels in thromboxane A2-induced colonic cancer cell proliferation. Pflug. Arch. 2014, 466, 541–548. [Google Scholar] [CrossRef]
- Young, M.R.; Young, M.E.; Lozano, Y.; Coogan, M.; Bagash, J.M. Regulation of protein kinase A activation and prostaglandin E2-stimulated migration of Lewis lung carcinoma clones. Int. J. Cancer 1991, 49, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Ushikubi, F.; Kakizuka, A.; Okuma, M.; Narumiya, S. Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J. Clin. Investig. 1996, 97, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Sobolesky, P.M.; Halushka, P.V.; Garrett-Mayer, E.; Smith, M.T.; Moussa, O. Regulation of the tumor suppressor FOXO3 by the thromboxane-A2 receptors in urothelial cancer. PLoS ONE 2014, 9, e107530. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.L.; Reid, H.M.; Eivers, S.B.; Mulvaney, E.P.; Kinsella, B.T. Transcriptional regulation of the human thromboxane A2 receptor gene by Wilms’ tumor (WT)1 and hypermethylated in cancer (HIC) 1 in prostate and breast cancers. Biochim. Biophys. Acta 2014, 1839, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Yang, Y.; Che, Q.; Jiang, F.; Wang, H.; Chen, Z.; Zhu, M.; Tong, H.; Zhang, H.; Yan, X.; et al. Prostaglandin E2 (PGE2) promotes proliferation and invasion by enhancing SUMO-1 activity via EP4 receptor in endometrial cancer. Tumor Biol. 2016, 37, 12203–12211. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Seo, S.Y.; Kim, S.L.; Zhu, S.M.; Chun, S.; Oh, J.M.; Lee, M.R.; Kim, S.H.; Kim, I.H.; Lee, S.O.; et al. MiRNA-206 suppresses PGE2-induced colorectal cancer cell proliferation, migration, and invasion by targetting TM4SF1. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]
- Wang, D.; Fu, L.; Sun, H.; Guo, L.; DuBois, R.N. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology 2015, 149, 1884–1895. [Google Scholar] [CrossRef]
- Juneja, J.; Casey, P.J. Role of G12 proteins in oncogenesis and metastasis. Br. J. Pharm. 2009, 158, 32–40. [Google Scholar] [CrossRef]
- Ji, R.; Chou, C.L.; Xu, W.; Chen, X.B.; Woodward, D.F.; Regan, J.W. EP1 prostanoid receptor coupling to G i/o up-regulates the expression of hypoxia-inducible factor-1 alpha through activation of a phosphoinositide-3 kinase signaling pathway. Mol. Pharmacol. 2010, 77, 1025–1036. [Google Scholar] [CrossRef]
- Hong, B.; van den Heuvel, A.P.; Prabhu, V.V.; Zhang, S.; El-Deiry, W.S. Targeting tumor suppressor p53 for cancer therapy: Strategies, challenges and opportunities. Curr. Drug Targets 2014, 15, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Synnott, N.C.; Crown, J. Mutant p53 as a target for cancer treatment. Eur. J. Cancer 2017, 83, 258–265. [Google Scholar] [CrossRef]
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell 2007, 26, 731–743. [Google Scholar] [CrossRef]
- Hermeking, H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, P.; Ren, H.; Fan, J.; Wang, G. NGFI-B nuclear orphan receptor Nurr1 interacts with p53 and suppresses its transcriptional activity. Mol. Cancer Res. 2009, 7, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivee, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Zeng, H.; Sanyal, S.; Mukhopadhyay, D. Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J. Biol. Chem. 2001, 276, 32714–32719. [Google Scholar] [CrossRef] [PubMed]
- Gille, H.; Kowalski, J.; Li, B.; LeCouter, J.; Moffat, B.; Zioncheck, T.F.; Pelletier, N.; Ferrara, N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J. Biol. Chem. 2001, 276, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kolluri, S.K.; Gu, J.; Dawson, M.I.; Cao, X.; Hobbs, P.D.; Lin, B.; Chen, G.; Lu, J.; Lin, F.; et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 2000, 289, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Lim, W.; Pearen, M.; Muscat, G.E.; Sturm, R.A. Regulation of NR4A nuclear receptor expression by oncogenic BRAF in melanoma cells. Pigment Cell Melanoma Res. 2011, 24, 551–563. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Shao, J.; Jung, C.; Liu, C.; Sheng, H. Prostaglandin E2 Stimulates the β-Catenin/T Cell Factor-dependent Transcription in Colon Cancer. J. Biol. Chem. 2005, 280, 26565–26572. [Google Scholar] [CrossRef]
- Rohde, F.; Rimkus, C.; Friederichs, J.; Rosenberg, R.; Marthen, C.; Doll, D.; Holzmann, B.; Siewert, J.R.; Janssen, K.P. Expression of osteopontin, a target gene of de-regulated Wnt signaling, predicts survival in colon cancer. Int. J. Cancer 2007, 121, 1717–1723. [Google Scholar] [CrossRef]
- Lammi, J.; Huppunen, J.; Aarnisalo, P. Regulation of the osteopontin gene by the orphan nuclear receptor NURR1 in osteoblasts. Mol. Endocrinol. 2004, 18, 1546–1557. [Google Scholar] [CrossRef]
- Kimelman, D.; Xu, W. β-Catenin destruction complex: Insights and questions from a structural perspective. Oncogene 2006, 25, 7482–7491. [Google Scholar] [CrossRef]
- Rajalin, A.M.; Aarnisalo, P. Cross-talk between NR4A orphan nuclear receptors and β-catenin signaling pathway in osteoblasts. Arch. Biochem. Biophys. 2011, 509, 44–51. [Google Scholar] [CrossRef]
- Goodwin, J.F.; Knudsen, K.E. Beyond DNA Repair: DNA-PK Function in Cancer. Cancer Discov. 2014, 4, 1126–1139. [Google Scholar] [CrossRef]
- Malewicz, M.; Kadkhodaei, B.; Kee, N.; Volakakis, N.; Hellman, U.; Viktorsson, K.; Leung, C.Y.; Chen, B.; Lewensohn, R.; van Gent, D.C.; et al. Essential role for DNA-PK-mediated phosphorylation of NR4A nuclear orphan receptors in DNA double-strand break repair. Genes Dev. 2011, 25, 2031–2040. [Google Scholar] [CrossRef]
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.-Y.; Fang, X. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Holla, V.R.; Wu, H.; Shi, Q.; Menter, D.G.; DuBois, R.N. Nuclear Orphan Receptor NR4A2 Modulates Fatty Acid Oxidation Pathways in Colorectal Cancer. J. Biol. Chem. 2011, 286, 30003–30009. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Drabsch, Y.; Dekker, T.J.A.; de Vinuesa, A.G.; Li, Y.; Hawinkels, L.J.A.C.; Sheppard, K.-A.; Goumans, M.-J.; Luwor, R.B.; de Vries, C.J.; et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-β signalling. Nat. Commun. 2014, 5, 3388. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Ono, M.; Setoguchi, R.; Yagi, H.; Hori, S.; Fehervari, Z.; Shimizu, J.; Takahashi, T.; Nomura, T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006, 212, 8–27. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nishikawa, H. Roles of regulatory T cells in cancer immunity. Int. Immunol. 2016, 28, 401–409. [Google Scholar] [CrossRef]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef]
- Sekiya, T.; Kashiwagi, I.; Inoue, N.; Morita, R.; Hori, S.; Waldmann, H.; Rudensky, A.Y.; Ichinose, H.; Metzger, D.; Chambon, P.; et al. The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat. Commun. 2011, 2, 269. [Google Scholar] [CrossRef]
- Sekiya, T.; Kashiwagi, I.; Yoshida, R.; Fukaya, T.; Morita, R.; Kimura, A.; Ichinose, H.; Metzger, D.; Chambon, P.; Yoshimura, A. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 2013, 14, 230–237. [Google Scholar] [CrossRef]
- Hibino, S.; Chikuma, S.; Kondo, T.; Ito, M.; Nakatsukasa, H.; Omata-Mise, S.; Yoshimura, A. Inhibition of Nr4a Receptors Enhances Antitumor Immunity by Breaking Treg-Mediated Immune Tolerance. Cancer Res. 2018, 78, 3027–3040. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, A.J.; Subbaramaiah, K. Targeting cyclooxygenase-2 in human neoplasia: Rationale and promise. Cancer Cell 2003, 4, 431–436. [Google Scholar] [CrossRef]
- Zelenay, S.; van der Veen, A.G.; Bottcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A.; et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015, 162, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Amarachintha, S.; Xu, J.; Oley, F., Jr.; Du, W. Mesenchymal COX2-PG secretome engages NR4A-WNT signalling axis in haematopoietic progenitors to suppress anti-leukaemia immunity. Br. J. Haematol. 2018, 183, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.R.; Kaminski, J.; Barnitz, R.A.; Kurachi, M.; Gerdemann, U.; Yates, K.B.; Tsao, H.W.; Godec, J.; LaFleur, M.W.; Brown, F.D.; et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–1169. [Google Scholar] [CrossRef]
- Wherry, E.J.; Ha, S.J.; Kaech, S.M.; Haining, W.N.; Sarkar, S.; Kalia, V.; Subramaniam, S.; Blattman, J.N.; Barber, D.L.; Ahmed, R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007, 27, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lopez-Moyado, I.F.; Seo, H.; Lio, C.J.; Hempleman, L.J.; Sekiya, T.; Yoshimura, A.; Scott-Browne, J.P.; Rao, A. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019, 567, 530–534. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Mognol, G.P.; Spreafico, R.; Wong, V.; Scott-Browne, J.P.; Togher, S.; Hoffmann, A.; Hogan, P.G.; Rao, A.; Trifari, S. Exhaustion-associated regulatory regions in CD8(+) tumor-infiltrating T cells. Proc. Natl. Acad. Sci. USA 2017, 114, E2776–E2785. [Google Scholar] [CrossRef] [PubMed]
- Scott-Browne, J.P.; Lopez-Moyado, I.F.; Trifari, S.; Wong, V.; Chavez, L.; Rao, A.; Pereira, R.M. Dynamic Changes in Chromatin Accessibility Occur in CD8(+) T Cells Responding to Viral Infection. Immunity 2016, 45, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.J.; Pereira, R.M.; Aijo, T.; Kim, E.Y.; Marangoni, F.; Pipkin, M.E.; Togher, S.; Heissmeyer, V.; Zhang, Y.C.; Crotty, S.; et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity 2015, 42, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.-S.; Yang, J.; Beltran, C.; Cho, S. Cell Surface CD36 Protein in Monocyte/Macrophage Contributes to Phagocytosis during the Resolution Phase of Ischemic Stroke in Mice. J. Biol. Chem. 2016, 291, 23654–23661. [Google Scholar] [CrossRef]
- Mahajan, S.; Saini, A.; Chandra, V.; Nanduri, R.; Kalra, R.; Bhagyaraj, E.; Khatri, N.; Gupta, P. Nuclear Receptor Nr4a2 Promotes Alternative Polarization of Macrophages and Confers Protection in Sepsis. J. Biol. Chem. 2015, 290, 18304–18314. [Google Scholar] [CrossRef]
- Visekruna, A.; Volkov, A.; Steinhoff, U. A key role for NF-kappaB transcription factor c-Rel in T-lymphocyte-differentiation and effector functions. Clin. Dev. Immunol. 2012, 2012, 239368. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.M.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef]
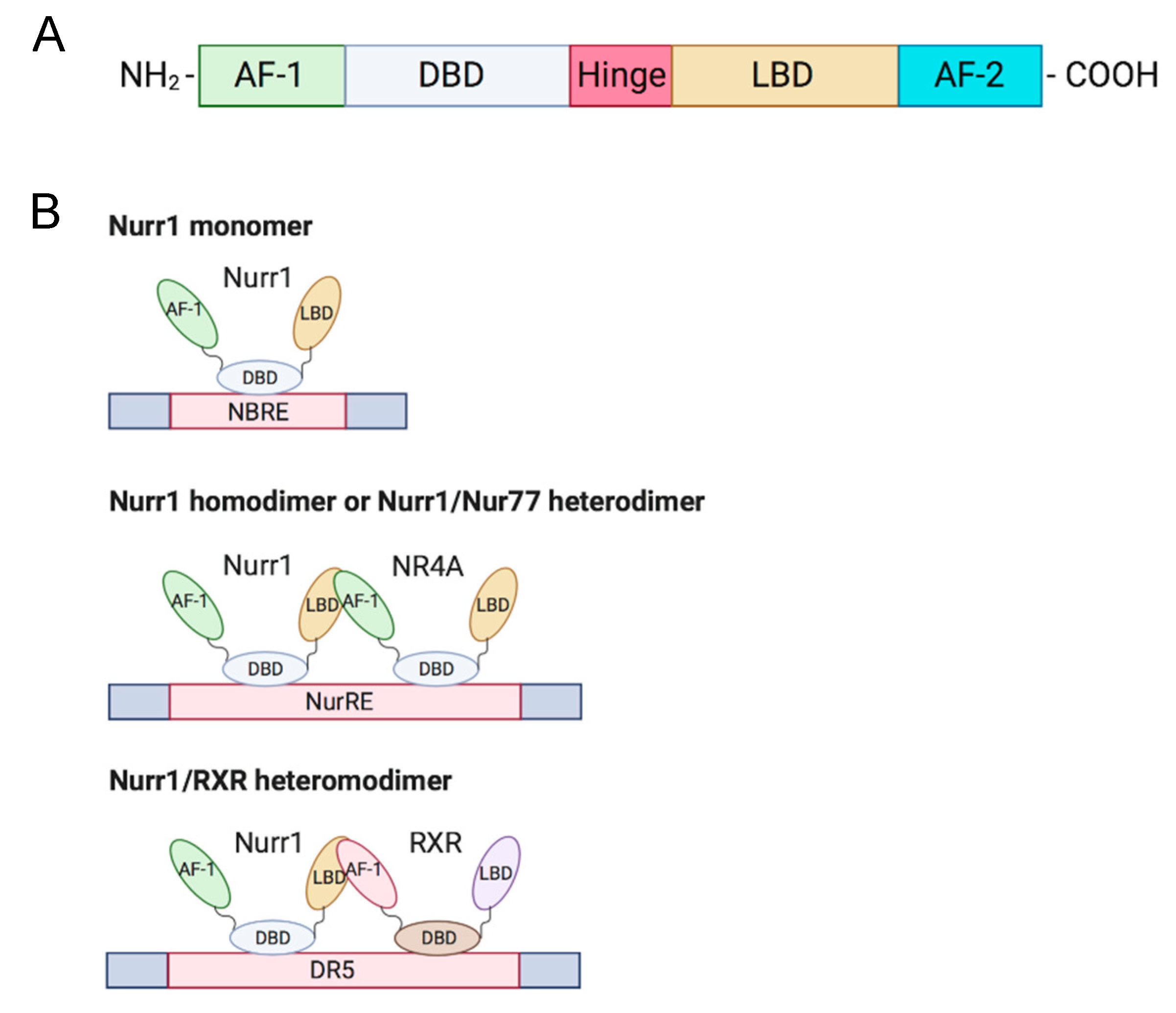
| Cancer | Nurr1 Expression | Functions | Stage Correlation | Survival Correlation | Ref. |
|---|---|---|---|---|---|
| Breast | Low | Inhibited tumor growth and metastasis in vivo * | No ^ | Yes ^ | [22] |
| Bladder | High | Promoted migration and tumor growth in vivo | Yes ^^^ | Yes ^^^ | [19,23] |
| Colon | High | Promoted cell proliferation, migration, and chemoresistance to 5-fluorouracil | No ^^ | Yes ^^ | [10,15] |
| Gastric | Low | Promoted apoptosis and inhibited gastrin-induced migration and invasion | NA | NA | [20,24] |
| High | Promoted tumor growth in vivo and chemoresistance to 5-fluorouracil | No ^^ | Yes ^^ | [14] | |
| Cervical | High | Promoted anchorage-independent growth, anoikis | NA | NA | [11,25] |
| Prostate | High | Promoted cell proliferation, migration, invasion, and resistance to apoptosis | Yes ^ | NA | [12] |
| Pancreatic | High | Promoted cell proliferation and resistance to apoptosis | Yes ^ | Yes ^ | [13] |
| Brain | High | Promoted cell proliferation, migration, invasion and survival | NA | Yes # | [26] |
| Pathway | Cancer | Mode of Regulation |
|---|---|---|
| TXA2 pathway | Lung cancer [18] | Transcriptional regulation |
| PGE2 pathway | Neuroblastoma [39]; Colorectal cancer [17]; Lung cancer [41] | Transcriptional regulation |
| p53/miR-34/Nurr1 loop | Colorectal cancer [40] | Post-transcriptional regulation |
| VEGF/PKD pathway | Endothelia angiogenesis [33] | Transcriptional regulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, P.K.-T.; Siu, M.K.-Y.; Leung, T.H.-Y.; Mo, X.-T.; Chan, K.K.-L.; Ngan, H.Y.-S. Role of Nurr1 in Carcinogenesis and Tumor Immunology: A State of the Art Review. Cancers 2020, 12, 3044. https://doi.org/10.3390/cancers12103044
Wan PK-T, Siu MK-Y, Leung TH-Y, Mo X-T, Chan KK-L, Ngan HY-S. Role of Nurr1 in Carcinogenesis and Tumor Immunology: A State of the Art Review. Cancers. 2020; 12(10):3044. https://doi.org/10.3390/cancers12103044
Chicago/Turabian StyleWan, Peter Kok-Ting, Michelle Kwan-Yee Siu, Thomas Ho-Yin Leung, Xue-Tang Mo, Karen Kar-Loen Chan, and Hextan Yuen-Sheung Ngan. 2020. "Role of Nurr1 in Carcinogenesis and Tumor Immunology: A State of the Art Review" Cancers 12, no. 10: 3044. https://doi.org/10.3390/cancers12103044
APA StyleWan, P. K.-T., Siu, M. K.-Y., Leung, T. H.-Y., Mo, X.-T., Chan, K. K.-L., & Ngan, H. Y.-S. (2020). Role of Nurr1 in Carcinogenesis and Tumor Immunology: A State of the Art Review. Cancers, 12(10), 3044. https://doi.org/10.3390/cancers12103044





