The Confounders of Cancer Immunotherapy: Roles of Lifestyle, Metabolic Disorders and Sociological Factors
Simple Summary
Abstract
1. Introduction
2. Roles of Sociological Factors and Sex/Gender in CPI Efficacy
2.1. Race
2.2. Ageing
2.3. Sex/Gender
3. Influence of Lifestyle on Outcome of CPI
3.1. Smoking
3.2. Alcohol Consumption
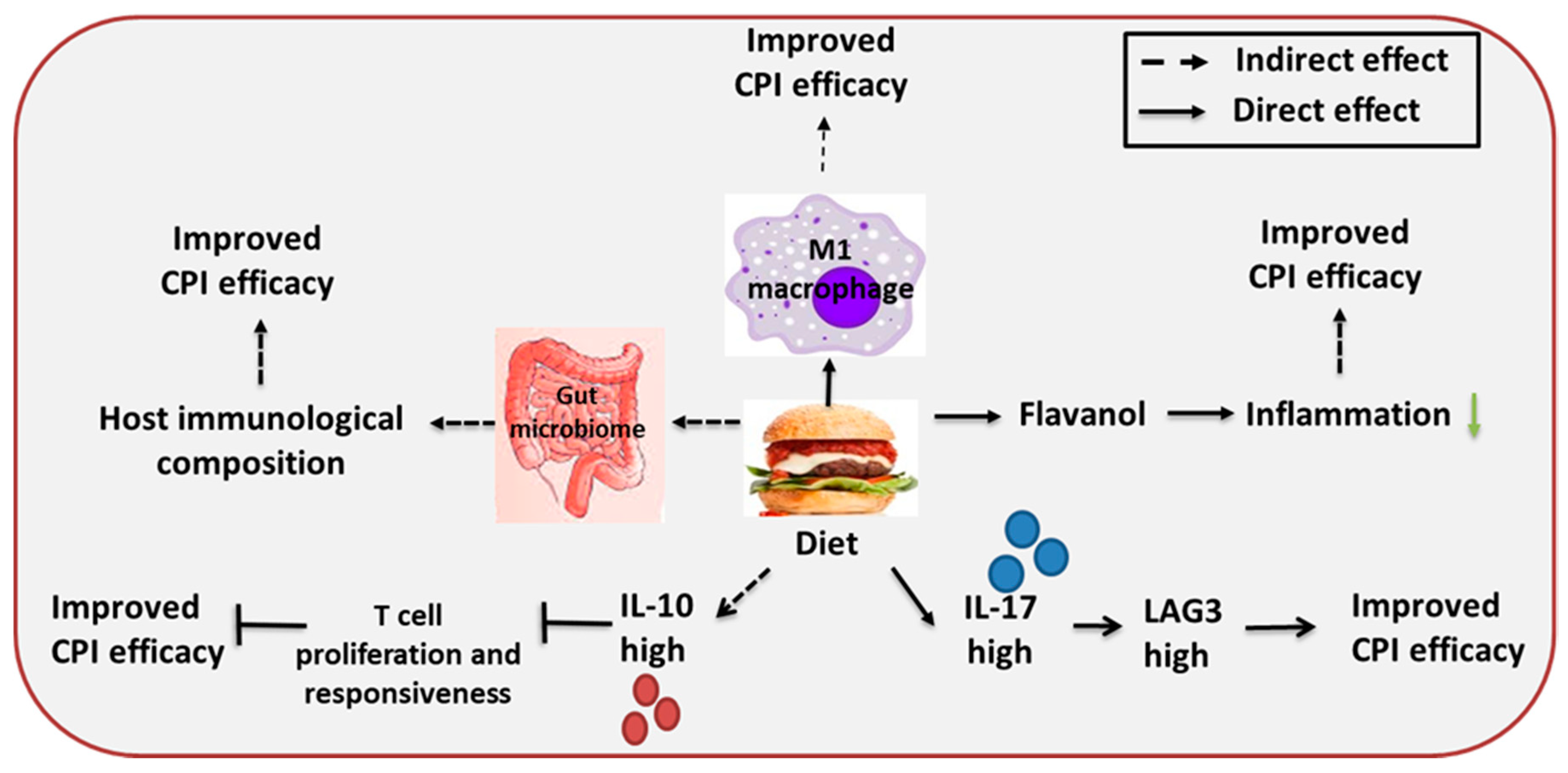
3.3. Diet
3.4. Exercise
3.5. Circadian Rhythms
3.6. Psycho-Emotional Disturbances
4. Effect of Metabolic Disorders on CPI Treatment
4.1. Obesity
4.2. Diabetes
4.3. Hypertension
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Sharma, P.K.; Krishnan, G.; Lockhart, A.C. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol. Rep. 2015, 3, 289–297. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharm. 2017, 8, 561. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.C.; Jin, L.; Zhang, X.D. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef]
- Jin, H.T.; Anderson, A.C.; Tan, W.G.; West, E.E.; Ha, S.J.; Araki, K.; Freeman, G.J.; Kuchroo, V.K.; Ahmed, R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 14733–14738. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Y.; Li, G.; Huang, H.; Zhang, G.; Wang, F.; Sun, J.; Yang, Q.; Zhang, X.; Lu, B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE 2012, 7, e30676. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A. Cutting edge: Molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, T.; Xuan, Q.; Zhao, H.; Qin, L.; Zhang, Q. Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Burton, B.R.; Britton, G.J.; Fang, H.; Verhagen, J.; Smithers, B.; Sabatos-Peyton, C.A.; Carney, L.J.; Gough, J.; Strobel, S.; Wraith, D.C. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat. Commun. 2014, 5, 4741. [Google Scholar] [CrossRef] [PubMed]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.F.; Joller, N.; Tan, D.J.; Teng, M.W.; Smyth, M.J.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009, 229, 12–26. [Google Scholar] [CrossRef]
- Hannani, D.; Vetizou, M.; Enot, D.; Rusakiewicz, S.; Chaput, N.; Klatzmann, D.; Desbois, M.; Jacquelot, N.; Vimond, N.; Chouaib, S.; et al. Anticancer immunotherapy by CTLA-4 blockade: Obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015, 25, 208–224. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Pesce, S.; Greppi, M.; Grossi, F.; Del Zotto, G.; Moretta, L.; Sivori, S.; Genova, C.; Marcenaro, E. PD/1-PD-Ls Checkpoint: Insight on the Potential Role of NK Cells. Front. Immunol. 2019, 10, 1242. [Google Scholar] [CrossRef]
- Hung, A.L.; Maxwell, R.; Theodros, D.; Belcaid, Z.; Mathios, D.; Luksik, A.S.; Kim, E.; Wu, A.; Xia, Y.; Garzon-Muvdi, T.; et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology 2018, 7, e1466769. [Google Scholar] [CrossRef]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Xiao, Z.; Guo, C.; Pu, Q.; Ma, L.; Liu, C.; Lin, F.; Liao, H.; You, Z.; Liu, L. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget 2016, 7, 34217–34228. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Ni, Z.; Liu, X.; Feng, S.; Dong, X.; Shi, X.; Zhai, J.; Mai, S.; Jiang, J.; Wang, Z.; et al. Beyond T Cells: Understanding the Role of PD-1/PD-L1 in Tumor-Associated Macrophages. J. Immunol. Res. 2019, 2019, 1919082. [Google Scholar] [CrossRef]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Arlauckas, S.P.; Garris, C.S.; Kohler, R.H.; Kitaoka, M.; Cuccarese, M.F.; Yang, K.S.; Miller, M.A.; Carlson, J.C.; Freeman, G.J.; Anthony, R.M.; et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Xing, F.; Liu, Y.; Wu, S.Y.; Wu, K.; Sharma, S.; Mo, Y.Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330. [Google Scholar] [CrossRef] [PubMed]
- Gershkovitz, M.; Yajuk, O.; Fainsod-Levi, T.; Granot, Z. The pd-l1/pd-1 axis blocks neutrophil cytotoxicity in cancer. bioRxiv 2020. [Google Scholar] [CrossRef]
- He, G.; Zhang, H.; Zhou, J.; Wang, B.; Chen, Y.; Kong, Y.; Xie, X.; Wang, X.; Fei, R.; Wei, L.; et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 141. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data from Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Soveral, I. The immune system and aging: A review. Gynecol. Endocrinol. 2014, 30, 16–22. [Google Scholar] [CrossRef]
- Dhillon, H.M.; Bell, M.L.; van der Ploeg, H.P.; Turner, J.D.; Kabourakis, M.; Spencer, L.; Lewis, C.; Hui, R.; Blinman, P.; Clarke, S.J.; et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann. Oncol. 2017, 28, 1889–1897. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Remon, J.; Besse, B.; Soria, J.C. Successes and failures: What did we learn from recent first-line treatment immunotherapy trials in non-small cell lung cancer? BMC Med. 2017, 15, 55. [Google Scholar] [CrossRef]
- Polk, A.; Svane, I.M.; Andersson, M.; Nielsen, D. Checkpoint inhibitors in breast cancer—Current status. Cancer Treat. Rev. 2018, 63, 122–134. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Faje, A.T.; Sullivan, R.; Lawrence, D.; Tritos, N.A.; Fadden, R.; Klibanski, A.; Nachtigall, L. Ipilimumab-induced hypophysitis: A detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J. Clin. Endocrinol. Metab. 2014, 99, 4078–4085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Solinas, C.; Gombos, A.; Latifyan, S.; Piccart-Gebhart, M.; Kok, M.; Buisseret, L. Targeting immune checkpoints in breast cancer: An update of early results. ESMO Open 2017, 2, e000255. [Google Scholar] [CrossRef]
- Klein, S.L.; Morgan, R. The impact of sex and gender on immunotherapy outcomes. Biol. Sex. Differ. 2020, 11, 24. [Google Scholar] [CrossRef]
- Parekh, A.; Fadiran, E.O.; Uhl, K.; Throckmorton, D.C. Adverse effects in women: Implications for drug development and regulatory policies. Expert Rev. Clin. Pharm. 2011, 4, 453–466. [Google Scholar] [CrossRef]
- Gloster, H.M.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Derm. 2006, 55, 741–760. [Google Scholar] [CrossRef]
- Morimoto, Y.; Conroy, S.M.; Ollberding, N.J.; Kim, Y.; Lim, U.; Cooney, R.V.; Franke, A.A.; Wilkens, L.R.; Hernandez, B.Y.; Goodman, M.T.; et al. Ethnic differences in serum adipokine and C-reactive protein levels: The multiethnic cohort. Int. J. Obes. (Lond.) 2014, 38, 1416–1422. [Google Scholar] [CrossRef]
- Liu, P.; Morrison, C.; Wang, L.; Xiong, D.; Vedell, P.; Cui, P.; Hua, X.; Ding, F.; Lu, Y.; James, M.; et al. Identification of somatic mutations in non-small cell lung carcinomas using whole-exome sequencing. Carcinogenesis 2012, 33, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, E.; Togashi, Y.; Takeuchi, Y.; Shinya, S.; Tada, Y.; Kataoka, K.; Tane, K.; Sato, E.; Ishii, G.; Goto, K.; et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, S.; Jin, R.; Wang, X.; Wang, F.; Zang, R.; Xu, H.; Lu, Z.; Huang, J.; Lei, Y.; et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020, 470, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Tiu, A.C.; Potdar, R.; Djibo, D.A.; Masab, M.; Dourado, C. Clinical outcomes of African American patients with advanced or metastatic non-small cell lung cancer on Nivolumab in a single community-based cancer center. Med. Oncol. 2018, 35, 109. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Hida, T.; Atagi, S.; Sakai, H.; Nakagawa, K.; Takahashi, T.; Nogami, N.; Saka, H.; Takenoyama, M.; Maemondo, M.; et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. ESMO Open 2016, 1, e000108. [Google Scholar] [CrossRef]
- Hida, T.; Nishio, M.; Nogami, N.; Ohe, Y.; Nokihara, H.; Sakai, H.; Satouchi, M.; Nakagawa, K.; Takenoyama, M.; Isobe, H.; et al. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non-small cell lung cancer. Cancer Sci. 2017, 108, 1000–1006. [Google Scholar] [CrossRef]
- Nishino, M.; Dahlberg, S.E.; Adeni, A.E.; Lydon, C.A.; Hatabu, H.; Janne, P.A.; Hodi, F.S.; Awad, M.M. Tumor Response Dynamics of Advanced Non-small Cell Lung Cancer Patients Treated with PD-1 Inhibitors: Imaging Markers for Treatment Outcome. Clin. Cancer Res. 2017, 23, 5737–5744. [Google Scholar] [CrossRef]
- Peng, L.; Wu, Y.L. Immunotherapy in the Asiatic population: Any differences from Caucasian population? J. Thorac. Dis. 2018, 10, S1482–S1493. [Google Scholar] [CrossRef]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Sacca, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef]
- Joshi, S.S.; Maron, S.B.; Catenacci, D.V. Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma. Future Oncol. 2018, 14, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Betof, A.S.; Nipp, R.D.; Giobbie-Hurder, A.; Johnpulle, R.A.N.; Rubin, K.; Rubinstein, S.M.; Flaherty, K.T.; Lawrence, D.P.; Johnson, D.B.; Sullivan, R.J. Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma. Oncologist 2017, 22, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Horvat, T.Z.; Minehart, J.; Panageas, K.; Callahan, M.K.; Chapman, P.B.; Momtaz, P.; Postow, M.A.; Shoushtari, A.N.; Wolchok, J.D.; et al. Efficacy and safety of checkpoint blockade for treatment of advanced melanoma (mel) in patients (pts) age 80 and older (80+). J. Clin. Oncol. 2016, 34, 10009. [Google Scholar] [CrossRef]
- Muchnik, E.; Loh, K.P.; Strawderman, M.; Magnuson, A.; Mohile, S.G.; Estrah, V.; Maggiore, R.J. Immune Checkpoint Inhibitors in Real-World Treatment of Older Adults with Non-Small Cell Lung Cancer. J. Am. Geriatr. Soc. 2019, 67, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Sattar, J.; Kartolo, A.; Hopman, W.M.; Lakoff, J.M.; Baetz, T. The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population. J. Geriatr. Oncol. 2019, 10, 411–414. [Google Scholar] [CrossRef]
- Freeman, M.; Weber, J. Subset analysis of the safety and efficacy of nivolumab in elderly patients with metastatic melanoma. J. Immunother. Cancer 2015, 3, P133. [Google Scholar] [CrossRef]
- O’Connor, J.M.; Fessele, K.L.; Steiner, J.; Seidl-Rathkopf, K.; Carson, K.R.; Nussbaum, N.C.; Yin, E.S.; Adelson, K.B.; Presley, C.J.; Chiang, A.C.; et al. Speed of Adoption of Immune Checkpoint Inhibitors of Programmed Cell Death 1 Protein and Comparison of Patient Ages in Clinical Practice vs Pivotal Clinical Trials. JAMA Oncol. 2018, 4, e180798. [Google Scholar] [CrossRef]
- Rosato, E.; Salsano, F. Immunity, autoimmunity and autoimmune diseases in older people. J. Biol. Regul. Homeost. Agents 2008, 22, 217–224. [Google Scholar]
- Pang, H.H.; Wang, X.; Stinchcombe, T.E.; Wong, M.L.; Cheng, P.; Ganti, A.K.; Sargent, D.J.; Zhang, Y.; Hu, C.; Mandrekar, S.J.; et al. Enrollment Trends and Disparity Among Patients with Lung Cancer in National Clinical Trials, 1990 to 2012. J. Clin. Oncol. 2016, 34, 3992–3999. [Google Scholar] [CrossRef]
- Freedman, R.A.; Foster, J.C.; Seisler, D.K.; Lafky, J.M.; Muss, H.B.; Cohen, H.J.; Mandelblatt, J.; Winer, E.P.; Hudis, C.A.; Partridge, A.H.; et al. Accrual of Older Patients with Breast Cancer to Alliance Systemic Therapy Trials over Time: Protocol A151527. J. Clin. Oncol. 2017, 35, 421–431. [Google Scholar] [CrossRef]
- Singh, H.; Beaver, J.A.; Kim, G.; Pazdur, R. Enrollment of older adults on oncology trials: An FDA perspective. J. Geriatr. Oncol. 2017, 8, 149–150. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Ponnappan, S.; Ponnappan, U. Aging and immune function: Molecular mechanisms to interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Derhovanessian, E.; Larbi, A. Immunosenescence and cancer. Crit. Rev. Oncol. Hematol. 2010, 75, 165–172. [Google Scholar] [CrossRef]
- Tomihara, K.; Curiel, T.J.; Zhang, B. Optimization of immunotherapy in elderly cancer patients. Crit. Rev. Oncog. 2013, 18, 573–583. [Google Scholar] [CrossRef]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Moschos, S.J. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treat. Rev. 2016, 45, 30–37. [Google Scholar] [CrossRef]
- Ferris, R.L.; Lu, B.; Kane, L.P. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J. Immunol. 2014, 193, 1525–1530. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Fagnoni, F.F.; Vescovini, R.; Passeri, G.; Bologna, G.; Pedrazzoni, M.; Lavagetto, G.; Casti, A.; Franceschi, C.; Passeri, M.; Sansoni, P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood 2000, 95, 2860–2868. [Google Scholar] [CrossRef]
- Saavedra, D.; Garcia, B.; Lage, A. T Cell Subpopulations in Healthy Elderly and Lung Cancer Patients: Insights from Cuban Studies. Front. Immunol. 2017, 8, 146. [Google Scholar] [CrossRef]
- Jeske, S.S.; Schuler, P.J.; Doescher, J.; Theodoraki, M.N.; Laban, S.; Brunner, C.; Hoffmann, T.K.; Wigand, M.C. Age-related changes in T lymphocytes of patients with head and neck squamous cell carcinoma. Immun. Ageing 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet. Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- vom Steeg, L.G.; Klein, S.L. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef]
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex disparities in cancer incidence by period and age. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1174–1182. [Google Scholar] [CrossRef]
- Polanczyk, M.J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1). Int. Immunol. 2007, 19, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Cohen, J.H.; Danel, L.; Cordier, G.; Saez, S.; Revillard, J.P. Sex steroid receptors in peripheral T cells: Absence of androgen receptors and restriction of estrogen receptors to OKT8-positive cells. J. Immunol. 1983, 131, 2767–2771. [Google Scholar]
- Mor, G.; Sapi, E.; Abrahams, V.M.; Rutherford, T.; Song, J.; Hao, X.Y.; Muzaffar, S.; Kohen, F. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J. Immunol. 2003, 170, 114–122. [Google Scholar] [CrossRef]
- Laffont, S.; Seillet, C.; Guéry, J.C. Estrogen Receptor-Dependent Regulation of Dendritic Cell Development and Function. Front. Immunol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Butaney, M.; Satkunasivam, R.; Freedland, S.J.; Patel, S.P.; Hamid, O.; Pal, S.K.; Klaassen, Z. Association of Patient Sex with Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sirisinha, S. The potential impact of gut microbiota on your health:Current status and future challenges. Asian Pac. J. Allergy Immunol. 2016, 34, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Bigley, A.B.; Agha, N.; Hanley, P.J.; Bollard, C.M. Mobilizing Immune Cells with Exercise for Cancer Immunotherapy. Exerc. Sport Sci. Rev. 2017, 45, 163–172. [Google Scholar] [CrossRef]
- Lacey, J.; Lomax, A.J.; McNeil, C.; Marthick, M.; Levy, D.; Kao, S.; Nielsen, T.; Dhillon, H.M. A supportive care intervention for people with metastatic melanoma being treated with immunotherapy: A pilot study assessing feasibility, perceived benefit, and acceptability. Support. Care Cancer 2019, 27, 1497–1507. [Google Scholar] [CrossRef]
- Foy, J.P.; Bertolus, C.; Michallet, M.C.; Deneuve, S.; Incitti, R.; Bendriss-Vermare, N.; Albaret, M.A.; Ortiz-Cuaran, S.; Thomas, E.; Colombe, A.; et al. The immune microenvironment of HPV-negative oral squamous cell carcinoma from never-smokers and never-drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD-L1 blockade. Ann. Oncol. 2017, 28, 1934–1941. [Google Scholar] [CrossRef]
- Lin, Y.M.; Sung, W.W.; Hsieh, M.J.; Tsai, S.C.; Lai, H.W.; Yang, S.M.; Shen, K.H.; Chen, M.K.; Lee, H.; Yeh, K.T.; et al. High PD-L1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PLoS ONE 2015, 10, e0142656. [Google Scholar] [CrossRef]
- Orillion, A.; Damayanti, N.P.; Shen, L.; Adelaiye-Ogala, R.; Affronti, H.; Elbanna, M.; Chintala, S.; Ciesielski, M.; Fontana, L.; Kao, C.; et al. Dietary Protein Restriction Reprograms Tumor-Associated Macrophages and Enhances Immunotherapy. Clin. Cancer Res. 2018, 24, 6383–6395. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Wang, J.T.; Li, H.; Zhang, H.; Chen, Y.F.; Cao, Y.F.; Li, R.C.; Lin, C.; Wei, Y.C.; Xiang, X.N.; Fang, H.J.; et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann. Oncol. 2019, 30, 266–273. [Google Scholar] [CrossRef]
- Desrichard, A.; Kuo, F.; Chowell, D.; Lee, K.W.; Riaz, N.; Wong, R.J.; Chan, T.A.; Morris, L.G.T. Tobacco Smoking-Associated Alterations in the Immune Microenvironment of Squamous Cell Carcinomas. J. Natl. Cancer Inst. 2018, 110, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Reis, H.; Metzenmacher, M.; Goetz, M.; Savvidou, N.; Darwiche, K.; Aigner, C.; Herold, T.; Eberhardt, W.E.; Skiba, C.; Hense, J.; et al. MET Expression in Advanced Non-Small-Cell Lung Cancer: Effect on Clinical Outcomes of Chemotherapy, Targeted Therapy, and Immunotherapy. Clin. Lung Cancer 2018, 19, e441–e463. [Google Scholar] [CrossRef]
- Lin, S.Y.; Yang, C.Y.; Liao, B.C.; Ho, C.C.; Liao, W.Y.; Chen, K.Y.; Tsai, T.H.; Hsu, C.L.; Hsu, W.H.; Su, K.Y.; et al. Tumor PD-L1 Expression and Clinical Outcomes in Advanced-stage Non-Small Cell Lung Cancer Patients Treated with Nivolumab or Pembrolizumab: Real-World Data in Taiwan. J. Cancer 2018, 9, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. Smoking and EGFR status may predict outcomes of advanced NSCLC treated with PD-(L)1 inhibitors beyond first line: A meta-analysis. Clin. Respir. J. 2018, 12, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Deng, W.; Zhu, S.; Zeng, L.; Liu, J.; Kang, R.; Yang, M.; Cao, L.; Wang, H.; Billiar, T.R.; Jiang, J.; et al. The Circadian Clock Controls Immune Checkpoint Pathway in Sepsis. Cell Rep. 2018, 24, 366–378. [Google Scholar] [CrossRef]
- Miller, A.H. Depression and immunity: A role for T cells? Brain Behav Immun. 2010, 24, 1–8. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef]
- Hodge, G.; Nairn, J.; Holmes, M.; Reynolds, P.N.; Hodge, S. Increased intracellular T helper 1 proinflammatory cytokine production in peripheral blood, bronchoalveolar lavage and intraepithelial T cells of COPD subjects. Clin. Exp. Immunol. 2007, 150, 22–29. [Google Scholar] [CrossRef]
- Hernandez, C.P.; Morrow, K.; Velasco, C.; Wyczechowska, D.D.; Naura, A.S.; Rodriguez, P.C. Effects of cigarette smoke extract on primary activated T cells. Cell. Immunol. 2013, 282, 38–43. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Norum, J.; Nieder, C. Tobacco smoking and cessation and PD-L1 inhibitors in non-small cell lung cancer (NSCLC): A review of the literature. ESMO Open 2018, 3, e000406. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Braicu, C.; Cojocneanu, R.; Magdo, L.; Onaciu, A.; Ciocan, C.; Mehterov, N.; Dudea, D.; Buduru, S.; Berindan-Neagoe, I. Differential Effect of Smoking on Gene Expression in Head and Neck Cancer Patients. Int. J. Environ. Res. Public Health 2018, 15, 1558. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, Y.C.; Gopalan, A.; Gao, D.; Chen, Y.; Joyner, A.L. Stromal hedgehog signaling maintains smooth muscle and hampers micro-invasive prostate cancer. Dis. Model. Mech. 2017, 10, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Woo, S.R.; Zha, Y.; Spaapen, R.; Zheng, Y.; Corrales, L.; Spranger, S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 268–276. [Google Scholar] [CrossRef]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef]
- Harlin, H.; Meng, Y.; Peterson, A.C.; Zha, Y.; Tretiakova, M.; Slingluff, C.; McKee, M.; Gajewski, T.F. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009, 69, 3077–3085. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef]
- Li, B.; Huang, X.; Fu, L. Impact of smoking on efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer patients: A meta-analysis. Onco Targets Ther. 2018, 11, 3691–3696. [Google Scholar] [CrossRef]
- Mo, J.; Hu, X.; Gu, L.; Chen, B.; Khadaroo, P.A.; Shen, Z.; Dong, L.; Lv, Y.; Chitumba, M.N.; Liu, J. Smokers or non-smokers: Who benefits more from immune checkpoint inhibitors in treatment of malignancies? An up-to-date meta-analysis. World J. Surg. Oncol. 2020, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Kerdidani, D.; Magkouta, S.; Chouvardas, P.; Karavana, V.; Glynos, K.; Roumelioti, F.; Zakynthinos, S.; Wauters, E.; Janssens, W.; Lambrechts, D.; et al. Cigarette Smoke-Induced Emphysema Exhausts Early Cytotoxic CD8(+) T Cell Responses against Nascent Lung Cancer Cells. J. Immunol. 2018, 201, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Zhang, L.; Zhao, X.C.; Gao, S.H.; Qu, L.W.; Yu, H.; Fang, W.F.; Zhou, Y.C.; Liang, F.; Zhang, C.; et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat. Commun. 2019, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.M.; Cho, C.H.; Shin, V.Y. Nicotine and gastrointestinal disorders: Its role in ulceration and cancer development. Curr. Pharm. Des. 2013, 19, 5–10. [Google Scholar] [CrossRef] [PubMed]
- McKaig, R.G.; Baric, R.S.; Olshan, A.F. Human papillomavirus and head and neck cancer: Epidemiology and molecular biology. Head Neck 1998, 20, 250–265. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Abizaid, A.; Rao, Y.; Salas, R.; DiLeone, R.J.; Gündisch, D.; Diano, S.; De Biasi, M.; Horvath, T.L.; Gao, X.B.; et al. Nicotine decreases food intake through activation of POMC neurons. Science 2011, 332, 1330–1332. [Google Scholar] [CrossRef]
- Chen, H.; Vlahos, R.; Bozinovski, S.; Jones, J.; Anderson, G.P.; Morris, M.J. Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology 2005, 30, 713–719. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Machiels, J.H.; Coulie, P.G. The promise of immunostimulatory antibodies in head and neck cancer. Lancet Oncol. 2016, 17, 856–857. [Google Scholar] [CrossRef]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bagby, G.J.; Happel, K.I.; Summer, W.R.; Nelson, S. Pulmonary host defenses and alcohol. Front. Biosci. 2002, 7, d1314–d1330. [Google Scholar] [PubMed]
- Atkinson, K.J.; Rao, R.K. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1280–G1288. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.E.; Happel, K.I.; Zhang, P.; Kolls, J.K.; Nelson, S. Focus on: Alcohol and the immune system. Alcohol. Res. Health 2010, 33, 97–108. [Google Scholar] [PubMed]
- Zhang, P.; Welsh, D.A.; Siggins, R.W., 2nd; Bagby, G.J.; Raasch, C.E.; Happel, K.I.; Nelson, S. Acute alcohol intoxication inhibits the lineage- c-kit+ Sca-1+ cell response to Escherichia coli bacteremia. J. Immunol. 2009, 182, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, L.E.; Young, L.S. Ethanol augments intracellular survival of Mycobacterium avium complex and impairs macrophage responses to cytokines. J. Infect. Dis. 1991, 163, 1286–1292. [Google Scholar] [CrossRef]
- Szabo, G.; Catalano, D.; White, B.; Mandrekar, P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol. Clin. Exp. Res. 2004, 28, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Eken, A.; Ortiz, V.; Wands, J.R. Ethanol inhibits antigen presentation by dendritic cells. Clin. Vaccine Immunol. 2011, 18, 1157–1166. [Google Scholar] [CrossRef]
- Mandrekar, P.; Bala, S.; Catalano, D.; Kodys, K.; Szabo, G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J. Immunol. 2009, 183, 1320–1327. [Google Scholar] [CrossRef]
- Pruett, S.B.; Zheng, Q.; Fan, R.; Matthews, K.; Schwab, C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol 2004, 33, 147–155. [Google Scholar] [CrossRef]
- Støy, S.; Dige, A.; Sandahl, T.D.; Laursen, T.L.; Buus, C.; Hokland, M.; Vilstrup, H. Cytotoxic T lymphocytes and natural killer cells display impaired cytotoxic functions and reduced activation in patients with alcoholic hepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G269–G276. [Google Scholar] [CrossRef] [PubMed]
- Laso, F.J.; Iglesias-Osma, C.; Ciudad, J.; López, A.; Pastor, I.; Torres, E.; Orfao, A. Alcoholic liver cirrhosis is associated with a decreased expression of the CD28 costimulatory molecule, a lower ability of T cells to bind exogenous IL-2, and increased soluble CD8 levels. Cytometry 2000, 42, 290–295. [Google Scholar] [CrossRef]
- Markwick, L.J.; Riva, A.; Ryan, J.M.; Cooksley, H.; Palma, E.; Tranah, T.H.; Manakkat Vijay, G.K.; Vergis, N.; Thursz, M.; Evans, A.; et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology 2015, 148, 590–602.e510. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Feeding the immune system. Proc. Nutr. Soc. 2013, 72, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Villano, D.V.; Roberts, S.A.; Cesqui, E.; Raguzzini, A.; Borges, G.; Crozier, A.; Catasta, G.; Toti, E.; Serafini, M. Consumption of mixed fruit-juice drink and vitamin C reduces postprandial stress induced by a high fat meal in healthy overweight subjects. Curr. Pharm. Des. 2014, 20, 1020–1024. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. Childhood obesity: Immune response and nutritional approaches. Front. Immunol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef]
- Yamazaki, N.; Kiyohara, Y.; Uhara, H.; Iizuka, H.; Uehara, J.; Otsuka, F.; Fujisawa, Y.; Takenouchi, T.; Isei, T.; Iwatsuki, K.; et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci. 2017, 108, 1022–1031. [Google Scholar] [CrossRef]
- Schülke, S. Induction of Interleukin-10 Producing Dendritic Cells as a Tool to Suppress Allergen-Specific T Helper 2 Responses. Front. Immunol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Kato, R.; Sumitomo, S.; Tsuchida, Y.; Tsuchiya, H.; Nakachi, S.; Sakurai, K.; Hanata, N.; Nagafuchi, Y.; Kubo, K.; Tateishi, S.; et al. CD4(+)CD25(+)LAG3(+) T Cells with a Feature of Th17 Cells Associated with Systemic Lupus Erythematosus Disease Activity. Front. Immunol. 2019, 10, 1619. [Google Scholar] [CrossRef]
- Delgado, L.; Fernandes, I.; González-Manzano, S.; de Freitas, V.; Mateus, N.; Santos-Buelga, C. Anti-proliferative effects of quercetin and catechin metabolites. Food Funct. 2014, 5, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, L.; An, N.; Mol, I.M.; Löwik, C.W.; Kaijzel, E.L. A new multicolor bioluminescence imaging platform to investigate NF-κB activity and apoptosis in human breast cancer cells. PLoS ONE 2014, 9, e85550. [Google Scholar] [CrossRef] [PubMed]
- Horia, E.; Watkins, B.A. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis 2007, 28, 809–815. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Smith, K.; McCoy, K.D.; Macpherson, A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 2007, 19, 59–69. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Martinic, M.M.; Harris, N. The functions of mucosal T cells in containing the indigenous commensal flora of the intestine. Cell Mol. Life Sci. 2002, 59, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Frutos Rde, L.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef] [PubMed]
- Sela, U.; Euler, C.W.; Correa da Rosa, J.; Fischetti, V.A. Strains of bacterial species induce a greatly varied acute adaptive immune response: The contribution of the accessory genome. PLoS Pathog. 2018, 14, e1006726. [Google Scholar] [CrossRef]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, Cd006145. [Google Scholar] [CrossRef]
- Carey, M.; Lambert, S.; Smits, R.; Paul, C.; Sanson-Fisher, R.; Clinton-McHarg, T. The unfulfilled promise: A systematic review of interventions to reduce the unmet supportive care needs of cancer patients. Supportive Care Cancer 2012, 20, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Harmer, S.L.; Panda, S.; Kay, S.A. Molecular bases of circadian rhythms. Ann. Rev. Cell Dev. Biol. 2001, 17, 215–253. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Ann. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Druzd, D.; Matveeva, O.; Ince, L.; Harrison, U.; He, W.; Schmal, C.; Herzel, H.; Tsang, A.H.; Kawakami, N.; Leliavski, A.; et al. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 2017, 46, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Nobis, C.C.; Dubeau Laramée, G.; Kervezee, L.; Maurice De Sousa, D.; Labrecque, N.; Cermakian, N. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc. Natl. Acad. Sci. USA 2019, 116, 20077–20086. [Google Scholar] [CrossRef]
- Weninger, W.; Manjunath, N.; von Andrian, U.H. Migration and differentiation of CD8+ T cells. Immunol Rev. 2002, 186, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Fortier, E.E.; Rooney, J.; Dardente, H.; Hardy, M.P.; Labrecque, N.; Cermakian, N. Circadian variation of the response of T cells to antigen. J. Immunol 2011, 187, 6291–6300. [Google Scholar] [CrossRef]
- Kovacs, D.; Kovacs, P.; Eszlari, N.; Gonda, X.; Juhasz, G. Psychological side effects of immune therapies: Symptoms and pathomechanism. Curr. Opin. Pharm. 2016, 29, 97–103. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Heidelberger, V.; Goldwasser, F.; Kramkimel, N.; Jouinot, A.; Huillard, O.; Boudou-Rouquette, P.; Chanal, J.; Arrondeau, J.; Franck, N.; Alexandre, J.; et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Investig. New Drugs 2017, 35, 436–441. [Google Scholar] [CrossRef]
- Naik, G.S.; Waikar, S.S.; Johnson, A.E.W.; Buchbinder, E.I.; Haq, R.; Hodi, F.S.; Schoenfeld, J.D.; Ott, P.A. Complex inter-relationship of body mass index, gender and serum creatinine on survival: Exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J. Immunother. Cancer 2019, 7, 89. [Google Scholar] [CrossRef]
- Venetsanaki, V.; Boutis, A.; Chrisoulidou, A.; Papakotoulas, P. Diabetes mellitus secondary to treatment with immune checkpoint inhibitors. Curr. Oncol. 2019, 26, e111–e114. [Google Scholar] [CrossRef]
- Sakaguchi, C.; Ashida, K.; Yano, S.; Ohe, K.; Wada, N.; Hasuzawa, N.; Matsuda, Y.; Sakamoto, S.; Sakamoto, R.; Uchi, H.; et al. A case of nivolumab-induced acute-onset type 1 diabetes mellitus in melanoma. Curr. Oncol. 2019, 26, e115–e118. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, A.; Haddox, C.; Block, M.; Kudva, Y.C. Immune checkpoint inhibitors: An emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res. Care 2019, 7, e000591. [Google Scholar] [CrossRef]
- Martin-Liberal, J.; Furness, A.J.; Joshi, K.; Peggs, K.S.; Quezada, S.A.; Larkin, J. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: A case report. Cancer Immunol. Immunother. 2015, 64, 765–767. [Google Scholar] [CrossRef]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef]
- Hsieh, A.H.; Faithfull, S.; Brown, M.P. Risk of cumulative toxicity after complete melanoma response with pembrolizumab. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Dionisio de Sousa, I.J.; Ferreira, J.; Rodrigues, J.; Bonito, N.; Jacinto, P.; Marques, M.; Ribeiro, J.; Pais, A.; Gervasio, H. Association between bevacizumab-related hypertension and response to treatment in patients with metastatic colorectal cancer. ESMO Open 2016, 1, e000045. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Gootenberg, J.; Keegan, P.; Pazdur, R. FDA drug approval summary: Bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist 2007, 12, 356–361. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Tao, W.; Lagergren, J. Clinical management of obese patients with cancer. Nat. Rev. Clin. Oncol. 2013, 10, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Ann. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. (Lausanne) 2013, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Monjazeb, A.M.; Decock, J. The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer. Front. Immunol. 2019, 10, 1940. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Hakimi, A.A.; Xie, W.; McKay, R.R.; Simantov, R.; Lin, X.; Lee, J.L.; Rini, B.I.; Srinivas, S.; Bjarnason, G.A.; et al. Body Mass Index and Metastatic Renal Cell Carcinoma: Clinical and Biological Correlations. J. Clin. Oncol. 2016, 34, 3655–3663. [Google Scholar] [CrossRef]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef]
- Xu, H.; Cao, D.; He, A.; Ge, W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: A pooled analysis of 4090 cancer patients. Int. Immunopharmacol. 2019, 74, 105745. [Google Scholar] [CrossRef]
- Rogado, J.; Romero-Laorden, N.; Sanchez-Torres, J.M.; Ramos-Levi, A.M.; Pacheco-Barcia, V.; Ballesteros, A.I.; Arranz, R.; Lorenzo, A.; Gullon, P.; Garrido, A.; et al. Effect of excess weight and immune-related adverse events on the efficacy of cancer immunotherapy with anti-PD-1 antibodies. Oncoimmunology 2020, 9, 1751548. [Google Scholar] [CrossRef]
- Lewis, D.E.; Lysaght, J.; Wu, H. Editorial: T Cell Alterations in Adipose Tissue During Obesity, HIV, and Cancer. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kado, T.; Nawaz, A.; Takikawa, A.; Usui, I.; Tobe, K. Linkage of CD8+ T cell exhaustion with high-fat diet-induced tumourigenesis. Sci. Rep. 2019, 9, 12284. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Cocconi, G.; Bella, M.; Calabresi, F.; Tonato, M.; Canaletti, R.; Boni, C.; Buzzi, F.; Ceci, G.; Corgna, E.; Costa, P.; et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N. Engl. J. Med. 1992, 327, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Misra, A. Ethnic-Specific Criteria for Classification of Body Mass Index: A Perspective for Asian Indians and American Diabetes Association Position Statement. Diabetes Technol. 2015, 17, 667–671. [Google Scholar] [CrossRef]
- Deurenberg, P.; Yap, M.; van Staveren, W.A. Body mass index and percent body fat: A meta analysis among different ethnic groups. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1998, 22, 1164–1171. [Google Scholar] [CrossRef]
- McFarland, M.S.; Cripps, R. Diabetes mellitus and increased risk of cancer: Focus on metformin and the insulin analogs. Pharmacotherapy 2010, 30, 1159–1178. [Google Scholar] [CrossRef]
- Will, J.C.; Galuska, D.A.; Vinicor, F.; Calle, E.E. Colorectal Cancer: Another Complication of Diabetes Mellitus? Am. J. Epidemiol. 1998, 147, 816–825. [Google Scholar] [CrossRef]
- Yang, Y.X.; Hennessy, S.; Lewis, J.D. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology 2004, 127, 1044–1050. [Google Scholar] [CrossRef]
- Hemkens, L.G.; Grouven, U.; Bender, R.; Günster, C.; Gutschmidt, S.; Selke, G.W.; Sawicki, P.T. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: A cohort study. Diabetologia 2009, 52, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Eikawa, S.; Nishida, M.; Mizukami, S.; Yamazaki, C.; Nakayama, E.; Udono, H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc. Natl. Acad. Sci. USA 2015, 112, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Akturk, H.K.; Kahramangil, D.; Sarwal, A.; Hoffecker, L.; Murad, M.H.; Michels, A.W. Immune checkpoint inhibitor-induced Type 1 diabetes: A systematic review and meta-analysis. Diabet. Med. 2019, 36, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Gauci, M.L.; Laly, P.; Vidal-Trecan, T.; Baroudjian, B.; Gottlieb, J.; Madjlessi-Ezra, N.; Da Meda, L.; Madelaine-Chambrin, I.; Bagot, M.; Basset-Seguin, N.; et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: A case report and literature review. Cancer Immunol. Immunother. 2017, 66, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Vudattu, N.; Sznol, M.; Gettinger, S.; Kluger, H.; Lupsa, B.; Herold, K.C. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015, 38, e55–e57. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Gottlieb, P. Immunotherapy for the prevention and treatment of type 1 diabetes: Human trials and a look into the future. Diabetes Care 2009, 32, 1769–1782. [Google Scholar] [CrossRef]
- Kawabata, Y.; Ikegami, H.; Awata, T.; Imagawa, A.; Maruyama, T.; Kawasaki, E.; Tanaka, S.; Shimada, A.; Osawa, H.; Kobayashi, T.; et al. Differential association of HLA with three subtypes of type 1 diabetes: Fulminant, slowly progressive and acute-onset. Diabetologia 2009, 52, 2513–2521. [Google Scholar] [CrossRef]
- Matsumura, K.; Nagasawa, K.; Oshima, Y.; Kikuno, S.; Hayashi, K.; Nishimura, A.; Okubo, M.; Uruga, H.; Kishi, K.; Kobayashi, T.; et al. Aggravation of diabetes, and incompletely deficient insulin secretion in a case with type 1 diabetes-resistant human leukocyte antigen DRB1*15:02 treated with nivolumab. J. Diabetes Investig. 2018, 9, 438–441. [Google Scholar] [CrossRef]
- Lowe, J.R.; Perry, D.J.; Salama, A.K.; Mathews, C.E.; Moss, L.G.; Hanks, B.A. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J. Immunother. Cancer 2016, 4, 89. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Diaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, P.; Ye, S.; Weng, H.W.; Dai, Q.S. Hypertension as a predictive biomarker for efficacy of bevacizumab treatment in metastatic colorectal cancer: A meta-analysis. J. Balk. Union Oncol. 2014, 19, 917–924. [Google Scholar]



| Gene | Expression Profile | Modulators | Role in CPI Responsiveness | Predictive vs. Prognostic Value | Reference |
|---|---|---|---|---|---|
| PD-1 | TIL | PTEN, PI3K-Akt pathway, STAT3 | Inhibits T cell proliferation | Predictive and prognostic | [6,7] |
| PD-L1 | Tumor cells Macrophage, stroma cel | MAPK and PI3K or Akt pathways | On interaction with PD-1 inhibits T cell proliferation | Predictive and prognostic | [8,9] |
| TIM3 | CD4, CD8 memory T cells, DC, NK cells, monocytes | IL-2, TNF-α, IFN-γ | Promotes T cell dysfunction/exhaustion, Tim3+ Tregs correlated with metastasis disease | Prognostic | [10,11] |
| LAG-3 | CD4, CD8 T cells, NK cells, Treg cells | IL-10 and TGF-β1 | Promotes Treg mediated suppression Inhibits effector T cell proliferation | Prognostic | [12,13] |
| TIGIT | Tregs, TILs (CD8), DCs | IFN-γ and IL-17 | Suppresses anti-tumor immunity by dampening CD8 T cell function via Tregs | Prognostic | [14,15] |
| CTLA | Tregs, CD4 T cells | IL-2, PI3K pathway, Bcl-XL | Mediates immunosuppressive signaling by blocking co stimulatory CD28 receptor, inhibits T cell activation | Predictive and prognostic | [16,17] |
| Factor Influencing | Effect on Check Point Inhibitor Treatment | Mechanism Involved | Cancer Type | HR(CI), p-Value of CPI Response * | Reference |
|---|---|---|---|---|---|
| Sex/gender | No correlation | -- | Advanced Squamous-Cell Non-Small-Cell Lung Cancer | -- | [43] |
| For anti CTLA treatment PFS longer in males | Men have high CD8 T cells expression | NSCLC | HR Male: 0.77 (95% CI 0.63–0.94), p = 0.012 HR Female:0.89 (95% CI 0.76–1.05) p = 0.16 | [60] | |
| Longer survival in females for anti CTLA treatment | -- | Melanoma | HR Female: 0.80 (95% CI 0.68–0.94) p = 0.006 | [60] | |
| No correlation | -- | Advanced gastric and gastroesophageal junction adenocarcinoma | -- | [61] | |
| Race | AA have high response rate to Nivolumab | AA have higher mutational burden | Lung cancer | -- | [55] |
| Ageing | Adverse effects in elders in melanoma | Not clear | Melanoma, Metastatic melanoma | -- | [62,63] |
| No correlation | -- | NSCLC | -- | [64] | |
| Elders have less toxicity of CPI therapy | Not clear | Melanoma, non-small cell lung cancer, and renal cell carcinoma | -- | [65,66] |
| Factor Influencing | Effect on Check Point Inhibitor Treatment | Mechanism Involved | Cancer Type | HR(CI), p-Value of CPI Response * | Reference |
|---|---|---|---|---|---|
| Exercise | Improved response to immunotherapy | Exercise lowers the expression of PD-1 on T cells mobilizes more CD8 t cells | Blood cancer | -- | [94] |
| Low symptom burden | Not clear | Metastatic melanoma | -- | [95] | |
| Alcohol Consumption | Improved response to immunotherapy | High intratumoral T cell infiltrate, overexpression of PD-L1 in never drinkers | Oral squamous cell carcinoma | -- | [96] |
| No significant correlation between alcohol consumption and PD-L1 expression | -- | Oral squamous cell carcinoma | HR 1.2 (95% CI 0.91–1.71) p = 0.15 | [97] | |
| Diet | Enhanced immunotherapy effect | Enhanced anti-tumor capacity of TAM | Prostate and renal cell carcinoma | -- | [98] |
| Gut microbiome modifies host immunity | Melanoma | -- | [99] | ||
| High LAG-3 induced by IL-17 | Gastric cancer | -- | [100] | ||
| Smoking | Controversial reports in HNSC and LUSC | Increased mutation rate. Decreased immune cell infiltration and poorer survival in HNSC, reverse in LUSC | head and neck (HNSC) and lung (LUSC) squamous cell carcinoma | LUSC: HR 1.02 (95% CI 0.71–1.46) p = 0.92 | [101] |
| High responsiveness | High mutation rate | Lung adenocarcinoma, NSCLC | NSCLC: HR 0.86(p = 0.61) | [58] | |
| NSCLC: HR 0.81 (95% CI 0.27–2.43) p = 0.71 | [102] | ||||
| NSCLC: HR 0.45 (95% CI 0.22–0.92) p = 0.02 | [103] | ||||
| NSCLC: HR 0.71 (95% CI 0.63–0.82) p < 0.00001 | [104] | ||||
| NSCLC: HR 0.15 (95% CI: 0.06–0.39) p = 0.0001 | [105] | ||||
| Circadian rhythms | No direct evidence | Decreased Bimal-1 causes high PD-L1 expression | Not reported in cancer condition | -- | [106] |
| Psyco-emotional changes | No direct evidence | Depression and stress decreases proliferation, increases apoptosis in T cells | Not reported in cancer condition | -- | [107] |
| Factor Influencing | Effect on Check Point Inhibitor Treatment | Mechanism Involved | Cancer Type | Reference |
|---|---|---|---|---|
| Obesity | Improved survival | Not clear | Melanoma | [181] |
| High response rate | Leptin in obesity cases promoted PD-1 expression on T cells | Melanoma, colorectal cancer | [13,129] | |
| Acute limiting toxicity (ALT) | Low distribution of drug, high exposure | Melanoma | [182] | |
| Worst Survival | Lower creatinine levels in obese females | Melanoma | [183] | |
| Diabetes | Diabetes is secondary to immunotherapy | Endocrine toxicity, beta cell destruction | NSCLC Melanoma | [184,185,186,187] |
| Hypertension | Secondary to immunotherapy | Inhibition of blood vessel formation | Colorectal cancer, Melanoma, Endometrial cancer | [188,189,190,191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshpande, R.P.; Sharma, S.; Watabe, K. The Confounders of Cancer Immunotherapy: Roles of Lifestyle, Metabolic Disorders and Sociological Factors. Cancers 2020, 12, 2983. https://doi.org/10.3390/cancers12102983
Deshpande RP, Sharma S, Watabe K. The Confounders of Cancer Immunotherapy: Roles of Lifestyle, Metabolic Disorders and Sociological Factors. Cancers. 2020; 12(10):2983. https://doi.org/10.3390/cancers12102983
Chicago/Turabian StyleDeshpande, Ravindra Pramod, Sambad Sharma, and Kounosuke Watabe. 2020. "The Confounders of Cancer Immunotherapy: Roles of Lifestyle, Metabolic Disorders and Sociological Factors" Cancers 12, no. 10: 2983. https://doi.org/10.3390/cancers12102983
APA StyleDeshpande, R. P., Sharma, S., & Watabe, K. (2020). The Confounders of Cancer Immunotherapy: Roles of Lifestyle, Metabolic Disorders and Sociological Factors. Cancers, 12(10), 2983. https://doi.org/10.3390/cancers12102983






