1. Introduction
Family history has contributed greatly to understanding inherited diseases throughout the centuries, in particular familial and hereditary cancer syndromes. To assess the cancer risk for unaffected members and to identify a possible genetic cause, it is important to describe a detailed family history, including information about life status, gender, age at onset, affected members and the number of generations.
Therefore, a careful anamnesis focused on oncological data could lead to the diagnosis of familial and/or hereditary cancer. The definition of “familial” is a non-specific status and indicates only a positive family history. Rather, the diagnosis of hereditary cancer has to be confirmed by some specific genetic tests.
In 1964, Jones identified a Māori family with an exceptional frequency of gastric tumors: in a pedigree with 98 members, 28 were affected by primary gastric carcinoma, and within a period of 30 years, over 25 family members died from this disease [1]. Many years later, in 1998, Guilford et al. first identified three different CDH1 germline mutations in three Māori kindred from New Zealand with a strong familial cluster of diffuse gastric cancer (DGC). These Authors described a splice site (G1008T), a frameshift (2382–2386, C ins), and a premature termination (TAG, C2095T) germline mutation in the E-cadherin gene [2], respectively.
On the basis of clinical criteria, subsequently in 1999, the first International Gastric Cancer Linkage Consortium (IGCLC) defined families with the Hereditary Diffuse Gastric Cancer (HDGC) syndrome associated with CDH1 germline mutations as those fulfilling one of the following features [3]: (a) two or more documented cases of diffuse gastric cancer in first- or second-degree relatives, with at least one diagnosed before the age of 50; (b) three or more cases of documented diffuse gastric cancer in first- or second-degree relatives, independent of the age of onset.
However, due to the increase in the CDH1 germline mutation rate, those initial criteria have become insufficient.
2. Current Position
To date, it is assessed that about 80–90% of GC appears as sporadic form, 10–20% are within a familial setting, and only 1–3% are related to documented germline mutations. Specifically, for HDGC syndrome related to CDH1 germline mutations, the cumulative incidence of GC at age 80 years is about 70% for males and 56% for females. An increased risk of lobular breast cancer (BC) is also documented [4]. In this case, the risk of BC for females is about 42% [5].
Recently, novel international guidelines for CDH1 genetic screening have been published [6] as follows:
2.1. Family Criteria
- (a)
- ≥2 cases of gastric cancer in family regardless of age, with at least one DGC;
- (b)
- ≥1 case of DGC at any age and ≥1 case of lobular BC at age <70 years in different family members;
- (c)
- ≥2 cases of lobular BC in family members <50 years of age.
2.2. Individual Criteria
- (d)
- DGC at age <50 years;
- (e)
- DGC at any age in individuals of Māori ethnicity;
- (f)
- DGC at any age in individuals with a personal or family history (first-degree relative) of cleft lip or cleft palate;
- (g)
- History of DGC and lobular breast cancer, both diagnosed at age <70 years;
- (h)
- Bilateral lobular BC, diagnosed at age <70 years;
- (i)
- Gastric in situ signet ring cells or pagetoid spread of signet ring cells in individuals <50 years of age.
Prophylactic total gastrectomy is the only life-saving option in CDH1 asymptomatic mutation carriers fulfilling the abovementioned criteria, but probably not indicated in CDH1 incidental findings without a clear family history of GC [7].
Figure 1 shows briefly the historical overview of HDGC and the main discoveries since its early identification.
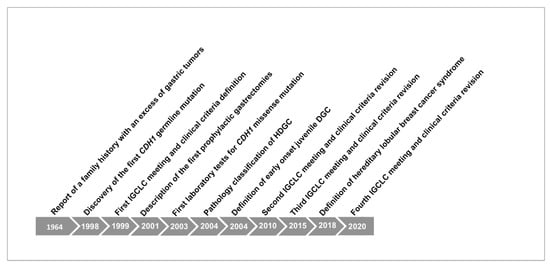
Figure 1.
Historical overview of Herditary Diffuse Gastric Cancer (HDGC) syndrome and CDH1 germline mutation.
3. Future Perspectives
With the widespread introduction of MultiGene Panel Testing (MGPT) in clinical practice, we are observing an increased rate of CDH1 germline mutations in apparently healthy individuals and without any correlation with the classic HDGC syndrome [8]. The identification of unexpected CDH1 germline mutations in the absence of specific clinical criteria suggests that HDGC syndrome may be a more complex syndrome than the one originally defined [9]. A cross-sectional prevalence study from the University of Southern California, Los Angeles, included all patients who underwent MGPT between 2012–2014. A total of 27,254 individuals were identified, and 20 (0.07%) of these patients were selected as having a CDH1 mutation. However, if we considered only the clinic cohort, four (1.26%) of 318 had a pathogenic CDH1 mutation [8].
The unquestionable direction for the management of this complex inherited cancer predisposition syndrome is the multidisciplinary approach in high-specialized cancer centers. Opinions of pathologists, surgeons, biologists, geneticists, epidemiologists, and radiologists are required equally for a correct approach to the HDGC syndrome. Further, considering the possible impacts of the HDGC syndrome on the individuals and their lives, the psychological aspects should be taken into consideration [10,11,12].
Funding
This manuscript was supported by the Italian Ministry of Health (Project Code GR-2016-02361655) and partially supported by the Ricerca Corrente and 5 × 1000 funds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, E.G. Familial gastric cancer. N. Z. Med. J. 1964, 63, 287–296. [Google Scholar] [PubMed]
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Caldas, C.; Carneiro, F.; Lynch, H.T.; Yokota, J.; Wiesner, G.L.; Powell, S.M.; Lewis, F.R.; Huntsman, D.G.; Pharoah, P.D.; Jankowski, J.; et al. Familial gastric cancer: Overview and guidelines for management. J. Med. Genet. 1999, 36, 873–880. [Google Scholar] [PubMed]
- Corso, G.; Figueiredo, J.; La Vecchia, C.; Veronesi, P.; Pravettoni, G.; Macis, D.; Karam, R.; Gullo, R.L.; Provenzano, E.; Toesca, A.; et al. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J. Med. Genet. 2018, 55, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Ranola, J.M.O.; Marshall, M.L.; Susswein, L.R.; Graceffo, S.; Bohnert, K.; Tsai, G.; Klein, R.T.; Hruska, K.S.; Shirts, B.H. Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol. 2019, 5, 1325. [Google Scholar] [CrossRef] [PubMed]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’Addario, J.L.; van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; van der Post, R.S.; et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef]
- Corso, G. Is Prophylactic Total Gastrectomy Always Indicated in CDH1 Germline Mutant Carriers? J. Surg. Res. 2020, 255, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Lowstuter, K.; Espenschied, C.R.; Sturgeon, D.; Ricker, C.; Karam, R.; LaDuca, H.; Culver, J.O.; Dolinsky, J.S.; Chao, E.; Sturgeon, J.; et al. Unexpected CDH1 Mutations Identified on Multigene Panels Pose Clinical Management Challenges. JCO Precis. Oncol. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Corso, G.; Montagna, G.; Figueiredo, J.; La Vecchia, C.; Fumagalli Romario, U.; Fernandes, M.S.; Seixas, S.; Roviello, F.; Trovato, C.; Guerini Rocco, E.; et al. Hereditary Gastric and Breast Cancer Syndromes Related to CDH1 Germline Mutation: A Multidisciplinary Clinical Review. Cancers 2020, 12, 1598. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, C.; Riva, S.; Pravettoni, G. Who is a cancer survivor? A systematic review of published definitions. J. Cancer Educ. 2017, 32, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, S.; Masiero, M.; Arnaboldi, P.; Cutica, I.; Fioretti, C.; Pravettoni, G. Health orientation, knowledge, and attitudes toward genetic testing and personalized genomic services: Preliminary data from an italian sample. BioMed. Res. Int. 2016, 2016, 6824581. [Google Scholar] [CrossRef] [PubMed]
- Lucchiari, C.; Botturi, A.; Silvani, A.; Lamperti, E.; Gaviani, P.; Innocenti, A. Finocchiaro, C.Y.; Masiero, M.; Pravettoni, G. Cognitive strategies and quality of life of patients with high-grade glioma. Support. Care. Cancer. 2015, 23, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).