Gene Panel Tumor Testing in Ovarian Cancer Patients Significantly Increases the Yield of Clinically Actionable Germline Variants beyond BRCA1/BRCA2
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
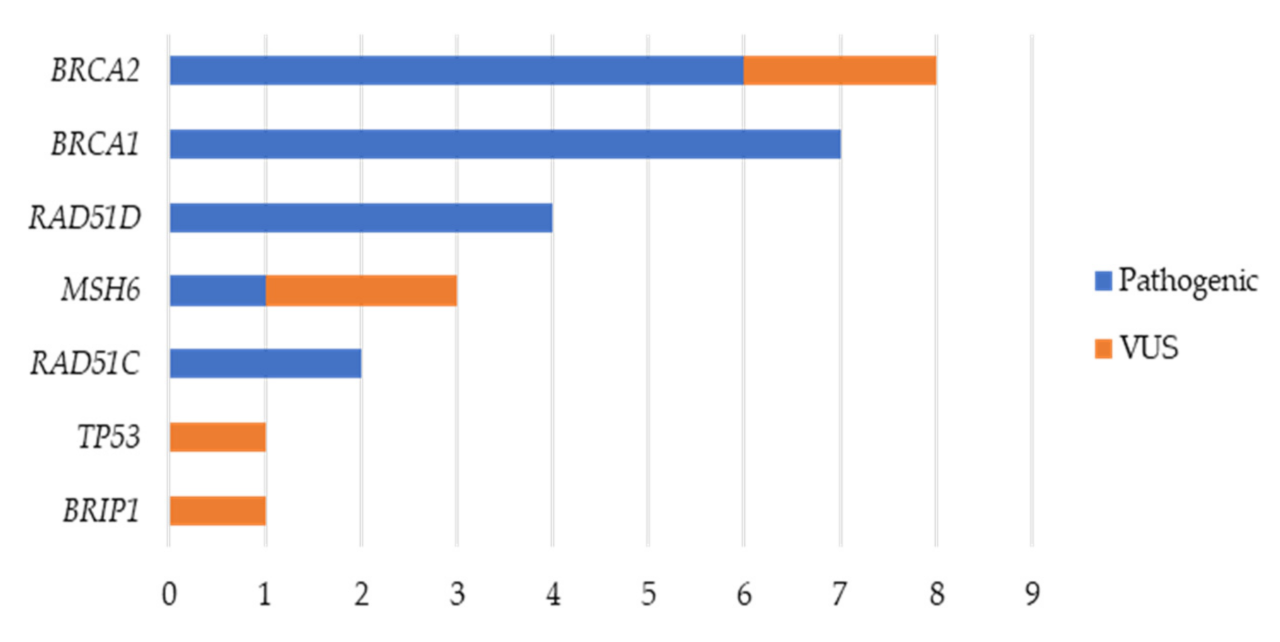
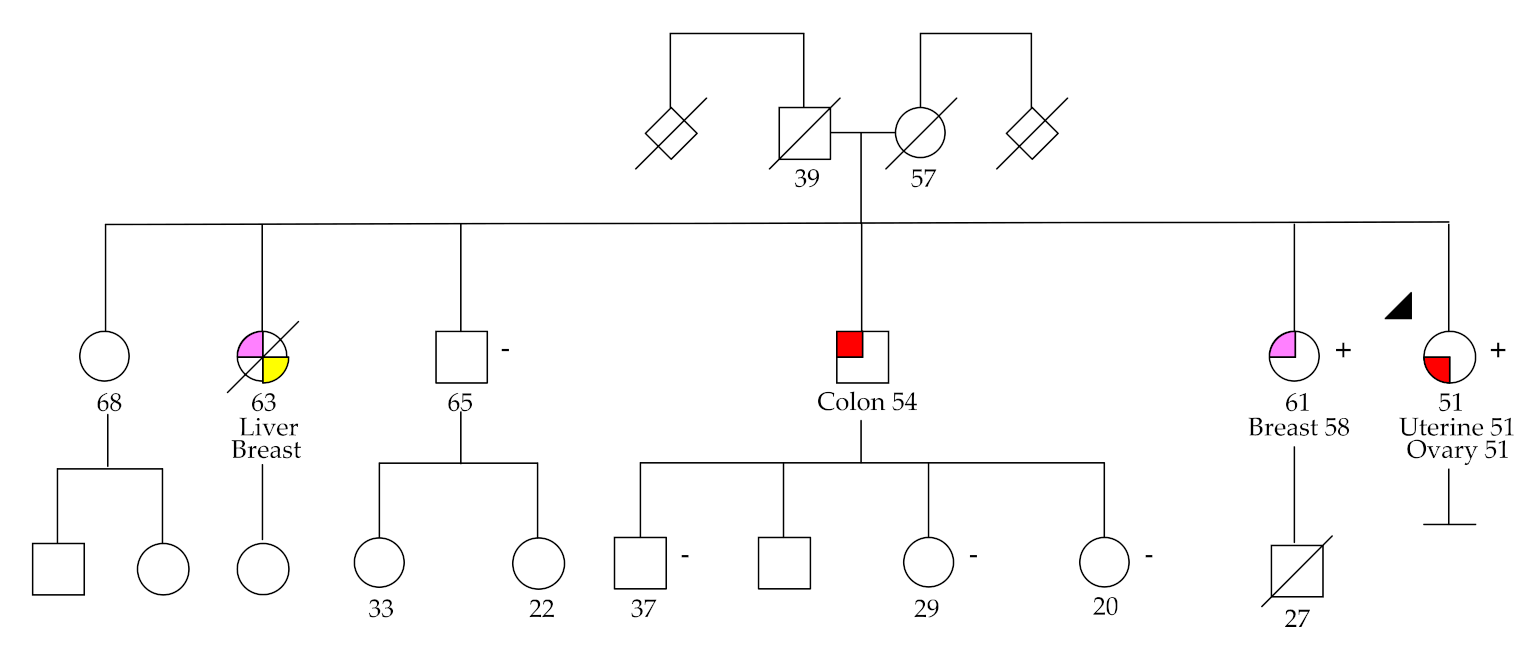
2.1. Variant Analysis
2.2. Germline Variants
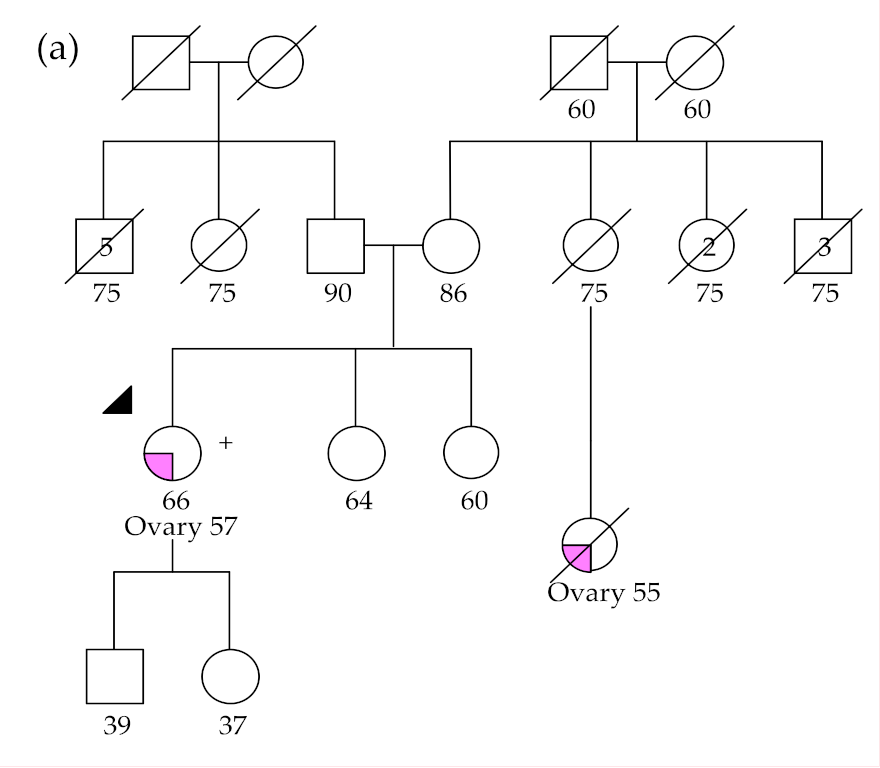
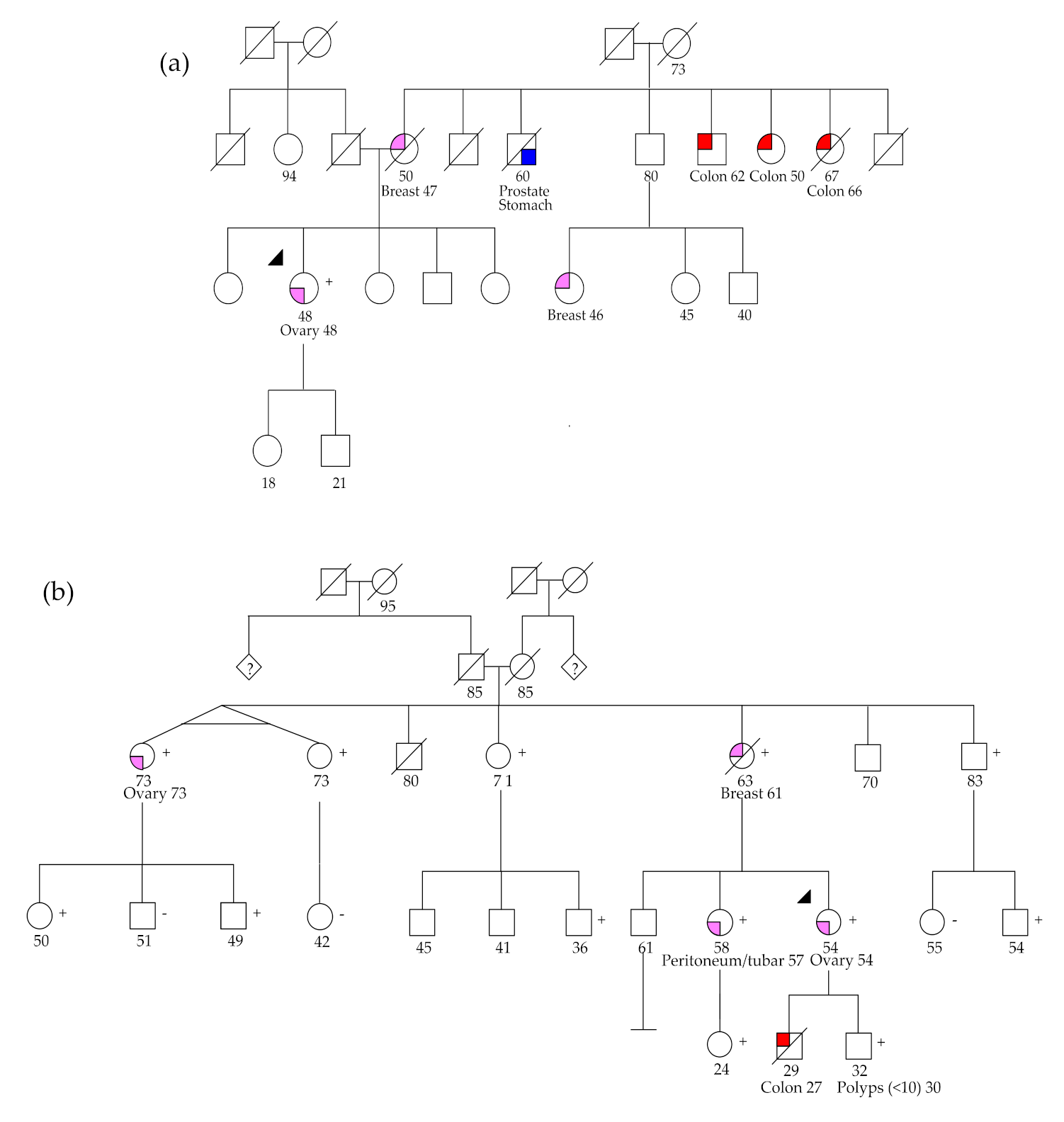
2.2.1. BRCA1 and BRCA2 Germline Variants
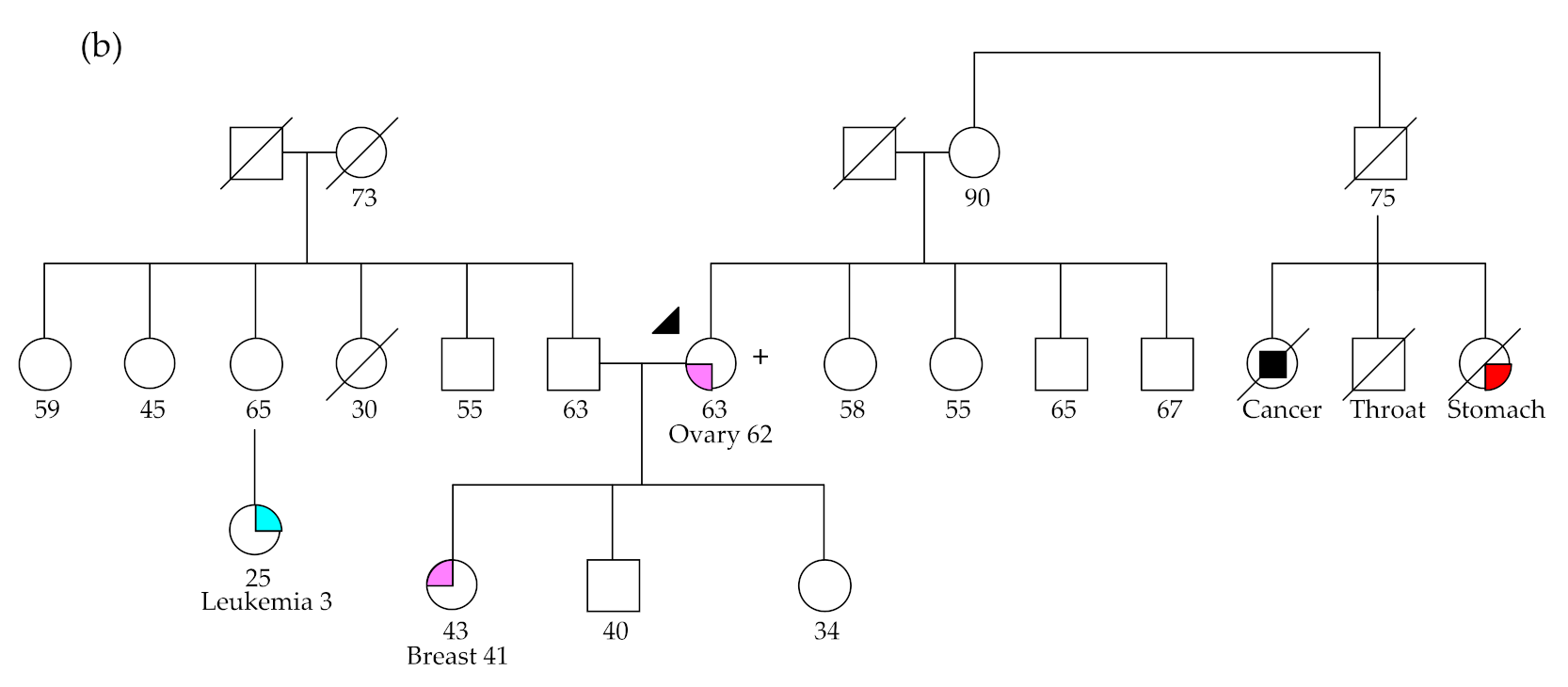
2.2.2. RAD51C and RAD51D Germline Variants
2.2.3. MSH6 Germline Variants
2.2.4. BRIP1 Germline Variant
2.2.5. TP53 Germline Variant
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. DNA Extraction from FFPE Tumor Samples
4.3. Next-Generation Sequencing and Bioinformatic Analyses
4.4. Variant Classification
4.5. Germline Variants Validation
4.6. Microsatellite Instability and Immunohistochemical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Guppy, A.; Nathan, P.; Rustin, G. Epithelial Ovarian Cancer: A Review of Current Management. Clin. Oncol. 2005, 17, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Santaballa, A.; Barretina, P.; Casado, A.; García, Y.G.; Martín, A.G.; Guerra, E.; Lainez, N.; Martínez, J.; Redondo, A.; Romero, I. SEOM Clinical Guideline in ovarian cancer. Clin. Transl. Oncol. 2016, 18, 1206–1212. [Google Scholar] [CrossRef]
- Morgan, R.J.; Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Behbakht, K.; Chen, L.-M.; Copeland, L.; Crispens, M.A.; DeRosa, M.; Dorigo, O.; et al. Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 1134–1163. [Google Scholar] [CrossRef]
- Pignata, S.; Cecere, S.C.; Du Bois, A.; Harter, P.; Heitz, F. Treatment of recurrent ovarian cancer. Ann. Oncol. 2017, 28, viii51–viii56. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Raja, F.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C. ESMO Guidelines Working Group Corrections to “Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up”. Ann. Oncol. 2018, 29, iv259. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A. A Synthetic Lethal Therapeutic Approach: Poly(ADP) Ribose Polymerase Inhibitors for the Treatment of Cancers Deficient in DNA Double-Strand Break Repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef]
- Capoluongo, E.; Ellison, G.; Lopez, J.A.-G.; Penault, F.-L.; Ligtenberg, M.J.L.; Banerjee, S.; Singer, C.; Friedman, E.; Markefka, B.; Schirmacher, P.; et al. Guidance Statement on BRCA1/2 Tumor Testing in Ovarian Cancer Patients. Semin. Oncol. 2017, 44, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Casadei, S.; Lee, M.K.; Pennil, C.C.; Nord, A.S.; Thornton, A.M.; Roeb, W.; Agnew, K.J.; Stray, S.M.; Wickramanayake, A.; et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 18032–18037. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; Van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Norquist, B.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Pinto, P.; Guerra, J.; Pinheiro, M.; Santos, C.; Pinto, C.; Santos, R.; Escudeiro, C.; Bartosch, C.; Canario, R.; et al. Tumor Testing for Somatic and Germline BRCA1/BRCA2 Variants in Ovarian Cancer Patients in the Context of Strong Founder Effects. Front. Oncol. 2020, 10, 1318. [Google Scholar] [CrossRef]
- Guidugli, L.; Shimelis, H.; Masica, D.L.; Pankratz, V.S.; Lipton, G.B.; Singh, N.; Hu, C.; Monteiro, A.N.A.; Lindor, N.M.; Goldgar, D.E.; et al. Assessment of the Clinical Relevance of BRCA2 Missense Variants by Functional and Computational Approaches. Am. J. Hum. Genet. 2018, 102, 233–248. [Google Scholar] [CrossRef]
- Parsons, M.T.; Tudini, E.; Li, H.; Hahnen, E.; Wappenschmidt, B.; Feliubadaló, L.; Aalfs, C.M.; Agata, S.; Aittomäki, K.; Alducci, E.; et al. Large scale multifactorial likelihood quantitative analysis of BRCA1 and BRCA2 variants: An ENIGMA resource to support clinical variant classification. Hum. Mutat. 2019, 40, 1557–1578. [Google Scholar] [CrossRef]
- Morgan, R.D.; Burghel, G.J.; Flaum, N.; Bulman, M.; Clamp, A.R.; Hasan, J.; Mitchell, C.L.; Schlecht, H.; Woodward, E.R.; Lallo, F.I.; et al. Prevalence of germline pathogenic BRCA1/2 variants in sequential epithelial ovarian cancer cases. J. Med. Genet. 2019, 56, 301–307. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Zhang, S.; Royer, R.; Li, S.; McLaughlin, J.R.; Rosen, B.; Risch, H.A.; Fan, I.; Bradley, L.; Shaw, P.A.; Narod, S.A. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol. Oncol. 2011, 121, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.; Casadei, S.; Nord, A.S.; et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2013, 20, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.M.; Cicek, M.S.; Larson, N.B.; Davila, J.; Wang, C.; Larson, M.C.; Song, H.; Dicks, E.M.; Harrington, P.; Wick, M.; et al. Clinical Characteristics of Ovarian Cancer Classified by BRCA1, BRCA2 and RAD51C Status. Sci. Rep. 2014, 4, 4026. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Chaudhuri, A.R.; Lopes, M.; Costanzo, V. Rad51 protects nascent DNA from Mre11-dependent degradation and promotes continuous DNA synthesis. Nat. Struct. Mol. Biol. 2010, 17, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Meindl, A.; Hellebrand, H.; Wiek, C.; Erven, V.; Wappenschmidt, B.; Niederacher, D.; Freund, M.; Lichtner, P.; Hartmann, L.; Schaal, H.; et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat. Genet. 2010, 42, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Loveday, C.; Breast Cancer Susceptibility Collaboration (UK); Turnbull, C.; Ramsay, E.; Hughes, D.; Ruark, E.; Frankum, J.R.; Bowden, G.; Kalmyrzaev, B.; Warren-Perry, M.; et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat. Genet. 2011, 43, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Dicks, E.; Ramus, S.J.; Tyrer, J.P.; Intermaggio, M.P.; Hayward, J.; Edlund, C.K.; Conti, D.; Harrington, P.; Fraser, L.; et al. Contribution of Germline Mutations in the RAD51B, RAD51C, and RAD51D Genes to Ovarian Cancer in the Population. J. Clin. Oncol. 2015, 33, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Hauke, J.; Heitz, F.; Reuss, A.; Kommoss, S.; Marme, F.; Heimbach, A.; Prieske, K.; Richtees, L.; Burges, A.; et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1). PLoS ONE 2017, 12, e0186043. [Google Scholar] [CrossRef]
- Konstanta, I.; Fostira, F.F.; Apostolou, P.; Stratikos, E.; Kalfakakou, D.; Pampanos, A.; Kollia, P.; Papadimitriou, C.; Konstantopoulou, I.; Yannoukakos, D. Contribution of RAD51D germline mutations in breast and ovarian cancer in Greece. J. Hum. Genet. 2018, 63, 1149–1158. [Google Scholar] [CrossRef]
- Loveday, C.; Turnbull, C.; Ruark, E.; Xicola, R.M.M.; Ramsay, E.; Hughes, D.; Warren-Perry, M.; Snape, K.; Breast Cancer Susceptibility Collaboration (UK); Eccles, D.M.; et al. Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat. Genet. 2012, 44, 475–476. [Google Scholar] [CrossRef]
- Yang, X.; Song, H.; Leslie, G.; Engel, C.; Hahnen, E.; Auber, B.; Horváth, J.; Kast, K.; Niederacher, D.; Turnbull, C.; et al. Ovarian and Breast Cancer Risks Associated With Pathogenic Variants in RAD51C and RAD51D. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J. Natl. Compr. Cancer Netw. 2016, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Snyder, C.L.; Shaw, T.G.; Heinen, C.D.; Hitchins, M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer 2015, 15, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Vasen, H.F.A.; Mecklin, J.-P.; Bernstein, I.; Aarnio, M.; Järvinen, H.J.; Myrhøj, T.; Sunde, L.; Wijnen, J.T.; Lynch, H.T. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int. J. Cancer 2008, 123, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut 2017, 67, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Ramsoekh, D.; Wagner, A.; Van Leerdam, M.E.; Dooijes, D.; Tops, C.M.; Steyerberg, E.W.; Kuipers, E.J. Cancer risk in MLH1, MSH2 and MSH6 mutation carriers; different risk profiles may influence clinical management. Hered. Cancer Clin. Pract. 2009, 7, 17. [Google Scholar] [CrossRef]
- Bonadona, V.; Bonaiti, B.; Olschwang, S.; Grandjouan, S.; Huiart, L.; Longy, M.; Guimbaud, R.; Buecher, B.; Bignon, Y.-J.; Caron, O.; et al. Cancer Risks Associated With Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA 2011, 305, 2304–2310. [Google Scholar] [CrossRef]
- Bridge, W.L.; Vandenberg, C.J.; Franklin, R.J.; Hiom, K. The BRIP1 helicase functions independently of BRCA1 in the Fanconi anemia pathway for DNA crosslink repair. Nat. Genet. 2005, 37, 953–957. [Google Scholar] [CrossRef]
- Rafnar, T.; Gudbjartsson, D.F.; Sulem, P.; Jonasdottir, A.; Sigurdsson, A.; Jonasdottir, A.; Besenbacher, S.; Lundin, P.; Stacey, S.N.; Gudmundsson, J.; et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat. Genet. 2011, 43, 1104–1107. [Google Scholar] [CrossRef]
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women With Ovarian Cancer. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Li, F.P.; Fraumeni, J.F. Soft-Tissue Sarcomas, Breast Cancer, and Other Neoplasms. Ann. Intern. Med. 1969, 71, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, A.D.; Espenschied, C.R.; Culver, J.O.; Weitzel, J.N. Tumor protein p53 (TP53) testing and Li-Fraumeni syndrome: Current status of clinical applications and future directions. Mol. Diagn. Ther. 2013, 17, 31–47. [Google Scholar] [CrossRef]
- Min, A.; Im, S.-A.; Yoon, Y.-K.; Song, S.-H.; Nam, H.-J.; Hur, H.-S.; Kim, H.-P.; Lee, K.-H.; Han, S.-W.; Oh, D.-Y.; et al. RAD51C-Deficient Cancer Cells Are Highly Sensitive to the PARP Inhibitor Olaparib. Mol. Cancer Ther. 2013, 12, 865–877. [Google Scholar] [CrossRef] [PubMed]
- AlHilli, M.M.; Becker, M.A.; Weroha, S.J.; Flatten, K.S.; Hurley, R.M.; Harrell, M.I.; Oberg, A.L.; Maurer, M.J.; Hawthorne, K.M.; Hou, X.; et al. In vivo anti-tumor activity of the PARP inhibitor niraparib in homologous recombination deficient and proficient ovarian carcinoma. Gynecol. Oncol. 2016, 143, 379–388. [Google Scholar] [CrossRef]
- McCabe, N.; Turner, N.C.; Lord, C.J.; Kluzek, K.; Bialkowska, A.; Swift, S.; Giavara, S.; O’Connor, M.J.; Tutt, A.; Zdzienicka, M.Z.; et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006, 66, 8109–8115. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Xu, C.; Gu, X.; Padmanabhan, R.; Wu, Z.; Peng, Q.; Dicarlo, J.; Wang, Y. smCounter2: An accurate low-frequency variant caller for targeted sequencing data with unique molecular identifiers. Bioinformatics 2018, 35, 1299–1309. [Google Scholar] [CrossRef]
- Dong, C.; Wei, P.; Jian, X.; Gibbs, R.; Boerwinkle, E.; Wang, K.; Liu, X. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 2014, 24, 2125–2137. [Google Scholar] [CrossRef]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2018, 47, D886–D894. [Google Scholar] [CrossRef] [PubMed]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, M.A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; A Rodriguez-Bigas, M.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar] [PubMed]
- Pinheiro, M.; Veiga, I.; Pinto, C.; Afonso, L.; Sousa, O.; Fragoso, M.; Santos, L.L.; Lopes, P.; Pais, I.; Lopes, C.; et al. Mitochondrial genome alterations in rectal and sigmoid carcinomas. Cancer Lett. 2009, 280, 38–43. [Google Scholar] [CrossRef]
- Bartosch, C.; Pires-Luís, A.S.; Meireles, C.; Baptista, M.; Gouveia, A.; Pinto, C.; Shannon, K.M.; Jerónimo, C.; Teixeira, M.R.; Lopes, J.M.; et al. Pathologic Findings in Prophylactic and Nonprophylactic Hysterectomy Specimens of Patients With Lynch Syndrome. Am. J. Surg. Pathol. 2016, 40, 1177–1191. [Google Scholar] [CrossRef]
| Patient | Gene | HGVS Coding | HGVS Protein | VAF | ClinVar | MetaLR 1 | MetaSVM 1 | C-Score 1 | Varsome | Additional Tests | Personal History (Age 2) | Family History |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OC09 | BRCA1 | c.2037delinsCC | p.(Lys679AsnfsTer4) | 60% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (46) | 4 × OC, 2 × PaC, 1 × GC |
| OC11 | BRIP1 | c.2477A>G | p.(Asn826Ser) | 74% | VUS | D | D | 26.3 | VUS | N/A | OC (39) | 1 × CRC, 1 × L |
| OC12 | BRCA1 | c.211A>G | p.(Arg71Gly) | 81% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (58) | 2 × BC, 1 × PC |
| OC16 | RAD51C | c.709C>T | p.(Arg237Ter) | 81% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (57) | 1 × OC |
| OC17 * | RAD51D | c.748del | p.(His250ThrfsTer2) | 68% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (57) | 2 × OC, 2 × BC, 1 × CRC |
| OC19 | BRCA1 | c.3331_3334del | p.(Gln1111AsnfsTer5) | 81% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (61) | 4 × BC, 1 × OC |
| OC23 | BRCA1 | c.3817C>T | p.(Gln1273Ter) | 92% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (46) | 6 × BC, 1 × CRC |
| OC24 | MSH6 | c.3848_3862del | p.(Ile1283_Tyr1287del) | 45% | N/A | N/A | N/A | N/A | N/A | IHC: loss of expression | OC (51), UC (51) | 2 × BC, 1 × CRC,1 × LC |
| OC29 | BRCA2 | c.5073dup | p.(Trp1692MetfsTer3) | 81% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (48), BC (48) | 3 × BC, 3 × PC, 3 × CRC |
| OC30 * | RAD51D | c.748del | p.(His250ThrfsTer2) | 81% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (73) | 2 × OC, 2 × BC, 1 × CRC |
| OC31 | RAD51C | c.709C>T | p.(Arg237Ter) | 77% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (62) | 1 × BC |
| OC36 | BRCA2 | c.8036A>G | p.(Asp2679Gly) | 36% | CIP | D | D | 31 | LP | N/A | OC (59) | 1 × CRC, 1 × |
| OC37 | MSH6 | c.1729C>T | p.(Arg577Cys) | 43% | VUS | D | D | 34 | VUS | IHC: normal; MSI: normal | OC (68) | 1 × LC |
| OC39 | BRCA1 | c.3331_3334del | p.(Gln1111AsnfsTer5) | 69% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (63) | 2 × BC, 1 × CRC |
| OC40 | BRCA1 | c.3331_3334del | p.(Gln1111AsnfsTer5) | 58% | Pathogenic | N/A | N/A | N/A | N/A | N/A | BC (49), OC (50) | --------- |
| OC50 | BRCA2 | c.9364G>C | p.(Ala3122Pro) | 88% | VUS | D | D | 31 | LP | N/A | OC (62) | 1 × CRC, 1 × BC, 1 × PC |
| OC52 | BRCA1 | c.2037_2038insC | p.(Lys680GlnfsTer3) | 54% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (52) | 2 × BC, 1 × PC |
| OC54 | RAD51D | c.748del | p.(His250ThrfsTer2) | 93% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (48) | 2 × BC, 1 × PC, 3 × CRC |
| OC55 * | RAD51D | c.748del | p.(His250ThrfsTer2) | 56% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (54) | 2 × OC, 2 × BC, 1 × CRC |
| OC60 | BRCA2 | c.7975A>G | p.(Arg2659Gly) | 62% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (56) | 3 × BC, 1 × PaC, 1 × CRC |
| OC72 | BRCA2 | c.5073dup | p.(Trp1692MetfsTer3) | 56% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (60) | 1 × PaC, 1 × PC |
| OC73 | BRCA2 | c.5073dup | p.(Trp1692MetfsTer3) | 86% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (44) | 2 × BC, 1 × CRC |
| OC79 | MSH6 | c.3182T>C | p.(Leu1061Pro) | 48% | VUS | D | D | 24.8 | VUS | IHC: loss of expression | OC (49) | 1 × CRC |
| OC88 | BRCA2 | c.2T>G | p.Met1? | 93% | Pathogenic | N/A | N/A | N/A | N/A | N/A | BC (59), OC (69) | 1 × BC, 1 × PC |
| OC91 | TP53 | c.845G>A | p.(Arg282Gln) | 47% | VUS | D | D | 35 | LP | N/A | OC (58), TC (60), PaC (60) | 1 × GC, 1 × CRC, BC |
| OC93 | BRCA2 | c.8488-1G>A | --------- | 57% | Pathogenic | N/A | N/A | N/A | N/A | N/A | OC (65) | 1 × BC, 1 × PaC |
| Clinical Features | N (%) | |
|---|---|---|
| Age, median (range years) | 57 (27–80) | |
| Figo Stage | Figo stage I | 14 (14.6) |
| Figo stage II | 7 (7.3) | |
| Figo stage III | 55 (57.3) | |
| Figo stage IV | 20 (20.8) | |
| Histological subtype | High-grade serous | 72 (71.9) |
| Low-grade serous | 8 (8.3) | |
| Clear cell | 6 (6.3) | |
| Endometrioid | 7 (7.3) | |
| Mucinous | 1 (1.0) | |
| Mixed | 3 (3.1) | |
| Carcinosarcoma | 2 (2.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, A.; Pinto, P.; Peixoto, A.; Guerra, J.; Pinto, C.; Santos, C.; Pinheiro, M.; Escudeiro, C.; Bartosch, C.; Silva, J.; et al. Gene Panel Tumor Testing in Ovarian Cancer Patients Significantly Increases the Yield of Clinically Actionable Germline Variants beyond BRCA1/BRCA2. Cancers 2020, 12, 2834. https://doi.org/10.3390/cancers12102834
Barbosa A, Pinto P, Peixoto A, Guerra J, Pinto C, Santos C, Pinheiro M, Escudeiro C, Bartosch C, Silva J, et al. Gene Panel Tumor Testing in Ovarian Cancer Patients Significantly Increases the Yield of Clinically Actionable Germline Variants beyond BRCA1/BRCA2. Cancers. 2020; 12(10):2834. https://doi.org/10.3390/cancers12102834
Chicago/Turabian StyleBarbosa, Ana, Pedro Pinto, Ana Peixoto, Joana Guerra, Carla Pinto, Catarina Santos, Manuela Pinheiro, Carla Escudeiro, Carla Bartosch, João Silva, and et al. 2020. "Gene Panel Tumor Testing in Ovarian Cancer Patients Significantly Increases the Yield of Clinically Actionable Germline Variants beyond BRCA1/BRCA2" Cancers 12, no. 10: 2834. https://doi.org/10.3390/cancers12102834
APA StyleBarbosa, A., Pinto, P., Peixoto, A., Guerra, J., Pinto, C., Santos, C., Pinheiro, M., Escudeiro, C., Bartosch, C., Silva, J., & Teixeira, M. R. (2020). Gene Panel Tumor Testing in Ovarian Cancer Patients Significantly Increases the Yield of Clinically Actionable Germline Variants beyond BRCA1/BRCA2. Cancers, 12(10), 2834. https://doi.org/10.3390/cancers12102834





